Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Benzodiazepine use increases the risk of fracture in the elderly.
It is controversial which conditions of use are most risky, e.g. use of short- or long-acting benzodiazepines, dose and duration of use.
The well-known Beers criteria include statements about inappropriate benzodiazepine use in elderly and the risk of fracture, but their clinical value has never been tested in an outcome study.
WHAT THIS STUDY ADDS
Inappropriate benzodiazepine use according to the Beers criteria is not associated with an increased risk of fracture.
Daily dose and duration of use is associated with higher risk of fracture, not the type of benzodiazepine prescribed as the Beers criteria state.
AIMS
The Beers criteria for prescribing in elderly are well known and used for many drug utilization studies. We investigated the clinical value of the Beers criteria for benzodiazepine use, notably the association between inappropriate use and risk of fracture.
METHODS
We performed a nested case–control study within the Rotterdam Study, a population-based cohort study in 7983 elderly. The proportion of ‘inappropriate’ benzodiazepine use according to the Beers criteria was compared between fracture patients and controls. ‘Inappropriate’ use for elderly implies use of some long-acting benzodiazepines and some intermediate/short-acting ones exceeding a suggested maximum daily dose. Also, alternative criteria were applied to compare the risk of fracture. Cases were defined as persons with incident fracture between 1991 and 2002 who were current benzodiazepine users on the fracture date. Controls were matched on fracture date and were also current benzodiazepine users.
RESULTS
The risk of fracture in ‘inappropriate’ benzodiazepine users according to the Beers criteria was not significantly different from ‘appropriate’ users [odds ratio (OR) 1.07, 95% confidence interval (CI) 0.72, 1.60]. However, a significantly higher risk of fracture was found in ‘high dose’ users and a longer duration of use (14–90 days), irrespective of the type of benzodiazepine (OR 3.45, 95% CI 1.38, 8.59).
CONCLUSIONS
These findings suggest that inappropriate benzodiazepine use according to the Beers criteria is not associated with increased risk of fracture. Daily dose and longer duration of use (>14 days) is associated with higher risk of fracture, irrespective of the type of benzodiazepine prescribed.
Keywords: Beers criteria, benzodiazepines, elderly, fractures, inappropriate use
Introduction
Inappropriate medication use is a major concern, especially for the growing older population, because it increases the risk of adverse drug reactions [1]. Inappropriate medication use for older adults is defined as medication use for which the potential harm outweighs the potential benefit and for which a good alternative is available [2].
In 1997, Beers and colleagues developed a comprehensive set of explicit criteria for potentially inappropriate drug use in the ambulatory elderly, aged ≥65 years [2]. Drugs were classified as inappropriately prescribed when they fulfilled one of the following criteria: (i) drugs that generally should be avoided in older adults, (ii) drugs that exceed a maximum recommended daily dose and (iii) drugs to be avoided in combination with specific co-morbidity. These criteria were developed with the intent to be applicable to any population of persons >65 years old, regardless of their level of frailty or their place of residence. In 2003, Beers et al. presented the results of the updated 1997 criteria by a US consensus panel of experts, based on scientific literature and new insights [3].
The Beers criteria have been criticized, since they do not identify all causes of potentially inappropriate prescribing and sometimes define appropriate prescribing as inappropriate [4]. Moreover, the relevance of using these criteria in clinical practice is unproven, because they still have not been properly validated in patient outcome studies.
The Beers criteria contain statements about use of benzodiazepines. In short, some long-acting benzodiazepines should not be prescribed to older adults, and some intermediate/short-acting benzodiazepines should preferably not exceed a proposed maximum daily dose. The potential adverse effects of this inappropriate prescribing are prolonged sedation and increased incidence of falls and fractures [3].
A review in 2003 of observational studies assessing the association between use of benzodiazepines and risk of hip fracture concluded that use of benzodiazepines by older people increases their risk of hip fracture by ≥50% [5]. However, it is controversial whether the risk of hip fracture differs between short- and long-acting benzodiazepine users, and the authors concluded that there is more evidence that people using higher doses of benzodiazepines and those having recently started are at highest risk, regardless of the type of benzodiazepine [5].
Therefore, we performed a nested case–control study in a cohort of benzodiazepine users ≥65 years old, to compare the risk of fracture by applying the Beers criteria and by applying alternative criteria based on daily dose, elimination half-life and duration of use, respectively.
Methods
Setting
This study was conducted as part of the Rotterdam Study, a prospective population-based cohort study on the occurrence and determinants of disease and disability in elderly persons [6]. In 1990, all inhabitants of Ommoord, a suburb of Rotterdam in the Netherlands, who were ≥55 years old and had lived in the district for at least 1 year were invited to participate in the study. Of the 10 275 eligible persons, 7983 (78%) participated. Participants gave informed consent and permission to retrieve information from medical records. At baseline, trained interviewers administered an extensive questionnaire covering socio-economic background and medical history, among other topics, during a home interview. During subsequent visits to the study centre, additional interviewing, laboratory assessments and clinical examinations were performed. Information on vital status was obtained at regular time intervals from the municipal authorities in Rotterdam. The Medical Ethics Committee of the Erasmus Medical Centre, Rotterdam, the Netherlands, approved the study.
Case and control definition
The study participants' general practitioners report all fatal and nonfatal events, such as fractures, through a computerized system. These data cover approximately 80% of the study sample. For participants who were not covered by this system, research physicians performed annual checks on the complete medical records of all general practitioners in the Rotterdam Study.
Two research physicians independently coded all fractures that occurred during the study period using the International Classification of Diseases, 10th revision (ICD-10) [7]. A medical expert in the field, who was unaware of the patients' history and medication use, reviewed all coded events for a final classification. For the present study, all participants were followed from 1 January 1991 until they had an incident fracture, died, or reached the end of the study at 31 December 2001, whichever came first. Pathological fractures (M84.4), vertebral fractures (S22.0, S22.1 and S32.0) and fractures in prosthetic hips (M96.6) were excluded. Accordingly, 1224 incident fractures were detected.
The date of fracture occurrence was defined as the index date. Cases were defined as persons with an incident fracture between 1 January 1991 and 31 December 2001 who were current benzodiazepine users and aged ≥65 years on the index date. From the 1224 incident fracture patients, 200 cases were identified. To each case, we matched all persons aged ≥65 years who were current benzodiazepine users on the index date of the corresponding case and who were at risk for fracture. Consequently, cases and controls had to have been current benzodiazepine users for at least 1 day before the index date. The case–control analysis is visualized and explained in Figure 1.
Figure 1.
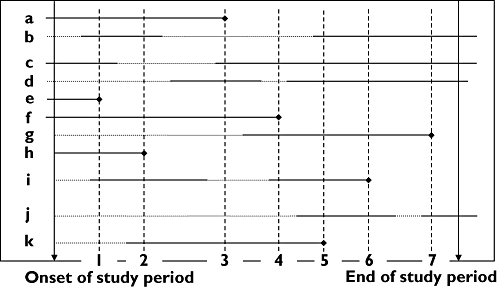
Analysis of the nested case–control study (basic scheme). The horizontal solid lines represent the exposed time to benzodiazepines and the dotted lines the non-exposed time. The vertical dotted lines show that every time on an index date of a case, the exposure (e.g. high/low dose) of the case is compared with the exposure of all persons in the cohort that are alive and at risk to become a case. For example: on the index date of case 1 (person e), all persons in the cohort who are at risk to become a case and who are current benzodiazepine users are used as controls (a, b, c, f, h and i). On the index date of case 2 (person h), the persons a, b, f, i and k are used as controls. On the index date of case 3 (person a), the persons c, d, f and k are used as controls. Consequently, persons can be used multiple times as control and cases are censored on the index date
Exposure definition
In the research area, there are seven fully computerized pharmacies that are linked to one network. During the study, all participants filled their prescriptions in one of these seven pharmacies. Data on all dispensed drugs since 1 January 1991 are available in computerized format on a day-to-day basis. The data include the date of prescribing, the total amount of drug units per prescription, the prescribed daily number of units, and product name.
Current use of benzodiazepines at the index date was retrieved from these prescription records. The exposure of interest included current use of the following benzodiazepines: lorazepam, oxazepam, alprazolam, temazepam, triazolam, diazepam, flurazepam, clorazepate, chlordiazepoxide, medazepam, quazepam, bromazepam, clobazam, ketazolam, prazepam, nitrazepam, flunitrazepam, lormetazepam, midazolam, brotizolam and loprazolam. These benzodiazepines were prescribed at least once to any of the study participants during the study period.
To compare the proportion of ‘inappropriate’ benzodiazepine use according to the Beers criteria between cases of fracture and controls, all current benzodiazepine users at the index date were defined as ‘inappropriate’ or ‘appropriate’ users. ‘Inappropriate’ users fulfilled the 2003 Beers criteria for inappropriate benzodiazepine use [3] (Table 1). Users who did not fulfil the Beers criteria for inappropriate benzodiazepine use were defined as ‘appropriate’.
Table 1.
Beers criteria for inappropriate benzodiazepine use in older adults*
| Type of benzodiazepine† | Daily dose |
|---|---|
| Short-acting | |
| Lorazepam | >3 mg |
| Oxazepam | >60 mg |
| Alprazolam | >2 mg |
| Temazepam | >15 mg |
| Triazolam | >0.25 mg |
| Long-acting | |
| Diazepam | Do not prescribe |
| Flurazepam | Do not prescribe |
| Clorazepate | Do not prescribe |
| Chlordiazepoxide | Do not prescribe |
| Quazepam | Do not prescribe |
| Halazepam‡ | Do not prescribe |
| Chlordiazepoxide-amitriptyline‡ | Do not prescribe |
| Clidinium-chlordiazepoxide‡ | Do not prescribe |
In a second analysis, the effect of dosage (high vs. low) on the risk of fracture was studied, regardless of what type of benzodiazepine was prescribed. Benzodiazepine users with a daily dose >10 mg of diazepam dose equivalents were defined as ‘high dose’ and those with ≤10 mg of diazepam dose equivalents as ‘low dose’ users.
Subsequently, we studied the effect of elimination half-life (long vs. short) on the risk of fracture. For this analysis, users of benzodiazepines with an elimination half-life >24 h were defined as ‘long-acting’ and ≤24 h as ‘short-acting’ benzodiazepine users [8, 9]. Consequently, diazepam, flurazepam, clorazepate, chlordiazepoxide, quazepam, clobazam, ketazolam and prazepam were judged to be long-acting benzodiazepines. The other benzodiazepines prescribed to the study participants were judged as ‘short-acting’.
The effect of duration of use on the risk of fracture was assessed by dividing current use into three mutually exclusive groups: ≤14 days (short-term use), use between 14 and 90 days (intermediate-term use) and use >90 days (long-term or chronic use).
Cofactors
The following patient characteristics, all determined by interview or during the visit to the examination centre, were individually assessed as potential confounders: age, sex, use of a walking aid, any fracture in the 5 years before baseline, rheumatoid arthritis, frequency of falling (once or more per month), current smoking, intake of alcohol (g day−1), dizziness, visual impairment, Parkinson's disease, hypertension, diabetes mellitus, presence of peripheral arterial disease and body mass index (BMI). For assessment of prevalent dementia at baseline, participants were cognitively screened. Screen-positives underwent further cognitive testing, which has been described in detail elsewhere [10]. Lower-limb disability was assessed by using a modified version of the Stanford Health Assessment Questionnaire [11] and by calculating the mean score of answers to questions about rising, walking, bending and getting in and out of a car [12]. The score ranges from 0 (no disability) to 3 (severe disability). Bone mineral density (BMD) of the femoral neck was measured by using dual-energy X-ray absorptiometry (DPX-L densitometer; Lunar Corp., Madison, WI, USA), as described elsewhere [13].
Data on use of other medications on the index date, such as antidepressants, anticonvulsants, antihistamines, antipsychotics, opioids, barbiturates, antacids, diuretics, β-blockers, corticosteroids, statins and the amount of prescribed medications, were obtained from pharmacy records and analysed as a potential confounder. While applying the alternative criteria to investigate the association between benzodiazepine use and fractures, daily dose, elimination half-life and duration of use of the benzodiazepines were also analysed as potential confounder in the different exposure models.
Statistical analysis
The incidence rate of fracture in benzodiazepine users was calculated using the number of cases as the numerator and the total number of person-years of benzodiazepine use during the study period as denominator.
Conditional logistic regression analyses were performed to estimate the crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). To adjust for potential confounders, cofactors associated with the occurrence of fracture were included one-by-one in the age- and sex-adjusted model. Cofactors that changed the OR for fracture during use of benzodiazepines by >5% or that were biologically plausible were maintained in the final model. In an extra analysis, we used missing value indicators for missing values of cofactors to prevent exclusion of cases with incomplete data and to evaluate potential confounding by missing values. Cofactors with missing values were therefore categorized, with a separate category for missing values. Accordingly, missing values were incorporated in the statistical model to investigate whether the missing status confounded the association of interest.
To test for effect modification by cofactors such as age, sex, alcohol use, BMI, elimination half-life, daily dose and duration of use, interaction terms were introduced in the statistical model and separate analyses were performed in the different categories. All statistical analyses were performed using SPSS-PC version 11.0 (SPSS Inc., Chicago, IL, USA; 1989–2001).
Results
The incidence rate of fracture in benzodiazepine users during the study period was 2.9 per 100 person-years. Baseline characteristics of cases and controls are shown in Table 2. There were 200 cases and 2678 controls. Of the cases, 70 patients had a hip fracture, 43 a wrist fracture and 87 patients ‘other’ fractures, such as tibia, hand, foot and skull. At baseline, the proportion of women and mean age were higher in cases than in controls. Cases were more likely to have dementia at baseline, and had a relatively lower BMD, adjusted for age and sex.
Table 2.
Baseline characteristics of the study population
| Characteristic | Cases (n = 200) | Controls (n = 2678) | OR* (95% CI) | Subjects with missing values (%) |
|---|---|---|---|---|
| Sex | ||||
| Women | 174 (87%) | 1884 (70%) | 1.68 (1.11, 2.54) | 0 |
| Age, year† | 76.6 ± 8.51 | 74.9 ± 8.04 | 1.04 (1.02, 1.06) | 0 |
| BMI, kg m−2† | 26.3 ± 3.90 | 26.5 ± 3.89 | 0.97 (0.94, 1.01) | 14 |
| Femoral neck BMD† | 0.74 ± 0.14 | 0.81 ± 0.14 | 0.04 (0.01, 0.15) | 29 |
| History of fracture (in 5 years before baseline) | 37 (19%) | 374 (14%) | 1.14 (0.79, 1.63) | 8 |
| Falling ≥1 × /month | 8 (4%) | 76 (3%) | 1.68 (0.83, 3.43) | 4 |
| Current smoking | 41 (21%) | 509 (19%) | 1.26 (0.87, 1.83) | 5 |
| Alcohol intake >2 g day−1 | 57 (29%) | 830 (31%) | 1.16 (0.81, 1.67) | 35 |
| Dementia | 18 (14%) | 186 (7%) | 1.77 (1.05, 2.00) | 6 |
| Parkinsonism‡ | 9 (5%) | 68 (3%) | 1.76 (0.90, 3.44) | <1 |
| Lower-limb disability score† | 0.83 ± 0.79 | 0.63 ± 0.74 | 1.12 (0.91, 2.55) | 3 |
| Use of walking aid | 42 (21%) | 430 (16%) | 0.92 (0.80, 1.07) | 9 |
OR for fracture, age- and sex-adjusted.
Mean ± SD.
Includes Parkinson's disease. BMD, bone mineral density; BMI, body mass index.
‘Inappropriate’ benzodiazepine use according to the Beers criteria was similar in cases and controls (OR 1.02, 95% CI 0.75, 1.38) (Table 3). In subsequent analyses, alternative criteria for ‘inappropriate’ use were used based on the existing literature. The risk of fracture in ‘high dose’ users was significantly higher than the risk in ‘low dose’ users (OR 1.57, 95% CI 1.11, 2.18) (Table 3). After additional adjustments for age, sex, dementia, alcohol intake, BMI and BMD (the final model), the risk of fracture was still statistically significantly different (OR 1.80, 95% CI 1.16, 2.78). Studying the effect of use of long- vs. short-acting benzodiazepines, no statistically significant difference in the risk of fracture was found (OR 1.23, 95% CI 0.73, 2.08). However, when assessing the duration of use, people using between 14 and 90 days had a significantly higher risk of fracture than those using ≤14 days (OR 2.15, 95% CI 1.14, 4.08) (Table 3).
Table 3.
Odds ratio (OR) for fracture based on different criteria for benzodiazepine use
| Exposure on index date | Cases (n = 200) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|
| Beers criteria | |||
| Appropriate, n (%) | 140 (70%) | 1.00 (reference) | 1.00 (reference) |
| Inappropriate, n (%) | 60 (30%) | 1.02 (0.75, 1.38) | 1.07 (0.72, 1.60) |
| Daily dose† | |||
| Low, n (%) | 157 (78%) | 1.00 (reference) | 1.00 (reference) |
| High, n (%) | 43 (22%) | 1.57 (1.11, 2.18) | 1.80 (1.16, 2.78) |
| Type (half-life) | |||
| Short-acting (≤24 h) | 176 (88%) | 1.00 (reference) | 1.00 (reference) |
| Long-acting (>24 h)‡ | 24 (12%) | 0.90 (0.59, 1.38) | 1.23 (0.73, 2.08) |
| Duration of use | |||
| ≤14 days | 25 (13%) | 1.00 (reference) | 1.00 (reference) |
| 14–90 days | 82 (41%) | 1.72 (1.10, 2.69) | 2.15 (1.14, 4.08) |
| 90 days | 93 (46%) | 1.19 (0.77, 1.85) | 1.70 (0.91, 3.20) |
Adjusted for age, sex, dementia, alcohol intake, body mass index and bone mineral density.
High: > 10 mg of diazepam equivalents.
Diazepam, flurazepam, clorazepate, chlordiazepoxide, quazepam, clobazam, ketazolam and prazepam.
In additional analyses in different categories, the highest OR for the risk of fracture was found in the group of persons with a high daily dose and a duration of use between 14 and 90 days compared with persons with a low daily dose and duration of use of ≤14 days (OR 3.45, 95% CI 1.38, 8.59) (Table 4).
Table 4.
Odds ratio (95% CI)* for fracture in different categories of benzodiazepine users: interaction of daily dose and duration of use
| Daily dose† | ||
|---|---|---|
| Duration of use | Low | High |
| ≤14 days | 1.00 (reference) | 0.96 (0.12, 7.42) |
| (24)‡ | (1)‡ | |
| 14–90 days | 1.98 (1.01, 3.89) | 3.45 (1.38, 8.59) |
| (70)‡ | (12)‡ | |
| 90 days | 1.42 (0.72, 2.80) | 2.79 (1.31, 5.93) |
| (63)‡ | (30)‡ | |
Adjusted for age, sex, dementia, alcohol intake, body mass index and bone mineral density.
High: > 10 mg of diazepam equivalents.
Number of cases.
BMI appeared to be an effect modifier by dividing the study sample at the median observation (26 kg m−2). When assessing the risk of fracture in high- vs. low-dose benzodiazepine users, a statistically significant effect was found only in people with a relatively low BMI (<26 kg m−2), adjusted for age, sex, dementia, alcohol intake and BMD (OR 2.44, 95% CI 1.40, 4.24).
After adjustment for missing values, the risk of fracture in ‘high dose’ users was somewhat lower, but still significant, with an OR of 1.69 (95% CI 1.20, 2.38). Similarly, for duration of use between 14 and 90 days the OR was 1.67 (95% CI 1.07, 2.62). The OR in persons with a high daily dose and duration of use between 14 and 90 days was 2.33 (95% CI 1.16, 4.67).
Discussion
In this population-based study of community-dwelling elderly, the risk of fracture was not different between persons with ‘inappropriate’ benzodiazepine use, according to the Beers criteria, and those with ‘appropriate’ benzodiazepine use. This finding suggests that the Beers criteria for benzodiazepine use are not clinically relevant regarding the risk of fracture. However, using other criteria based on more recent insights in the literature, i.e. dose and duration of use being more important than type of benzodiazepine [5, 9, 14, 15], we found that the risk of fracture significantly increases in persons with a high daily dose and longer duration of use compared with those on a low daily dose and short duration of use (≤14 days), regardless of the type of benzodiazepine prescribed (OR 3.45, 95% CI 1.38, 8.59). These results seem to indicate that dosage and duration of use are more important criteria for appropriate benzodiazepine use in clinical practice than the Beers criteria, which are only focusing on elimination half-life and dosage of specific benzodiazepines.
We were not able to confirm the findings from earlier studies [9, 14, 15] and published clinical information that adverse effects usually occur during the first few days of benzodiazepine use. Possibly, users of benzodiazepines have to accumulate the drug or active metabolites up to a certain level at receptor sites before adverse effects such as prolonged sedation occur. Alternatively, patients may still be alert during the first 2 weeks of use because they have been warned against central nervous system effects by their doctor or pharmacist. After a couple of weeks, they might become less attentive and develop an increased risk of falling. However, we found a trend of decreasing risk again in chronic users (>90 days of use, Table 3), possibly because these people get less sensitive to the sedation effects (receptor down-regulation) or it may be the consequence of ‘depletion of susceptibles’ (‘healthy user effect’).
Regarding effect modification, we found that especially in elderly with a lower BMI (<26 kg m−2) the effect of daily dose on the risk of fracture was high. This is not surprising, because these persons have a higher risk of fracture when falling (greater impact of force on bone) compared with those with a higher BMI, and this group is more sensitive to higher doses of drugs.
Several aspects of validity need to be discussed. Selection bias is unlikely because cases and controls were derived from a prospective population-based cohort study, and controls came from the same study base as cases. Information bias is unlikely as data on drug use were prospectively gathered. As benzodiazepines are available only on prescription, pharmacy records provide complete coverage. Although we do not know if the patients really took the benzodiazepines as prescribed and dispensed, compliance with benzodiazepines is usually high. Misclassification of fractures is unlikely and would be nondifferential, because the outcome was assessed independently of the exposure. Hence, this could not lead to overestimation of the risk.
As far as we know, this is the first study to investigate the effects of dose and duration within benzodiazepine users, which means that confounding (by indication) is less likely. Other observational studies that also investigated dose and duration effects of benzodiazepine use on the risk of fracture almost always used non-users as the reference group. In these studies, confounding (by indication) is a potential problem: the amount and differences in patient characteristics related to exposure and outcome are probably greater between users vs. non-users, and thus more difficult to adjust for. In our study, it is unlikely that confounding explains our results, because we were able to adjust for many potential confounders. To take into account potential confounding by missing value status of cofactors, we performed an extra analysis using missing value indicators in the adjusted model. In this analysis, the associations were less strong, but still statistically significant. As we suspect that missing value status is nonrandom in this population, we did not perform (multiple) imputation of missing values. People who are more ill and less mobile tended to have more missing values, e.g. because they were not able to come to the research centre where several measurements had to be performed. This might be a reason that taking into account missing status slightly affected the results.
In conclusion, we have been unable to demonstrate that the Beers criteria for benzodiazepine use are clinically relevant regarding the risk of fracture. However, based on the findings in this study, we may conclude that, when prescribing benzodiazepines to the elderly, a high daily dose and longer duration of use (>14 days) are ‘inappropriate’ regarding the risk of fracture, irrespective of the type of benzodiazepine prescribed. Consequently, if a benzodiazepine is indicated for an older person, physicians should prescribe a lower (geriatric) dose and for a time period as short as possible to reduce the risk of fracture.
Competing interests
None to declare.
REFERENCES
- 1.Willcox SM, Himmelstein DU, Woolhandler S. Inappropriate drug prescribing for the community-dwelling elderly. JAMA. 1994;272:292–6. [PubMed] [Google Scholar]
- 2.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157:1531–6. [PubMed] [Google Scholar]
- 3.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH, Rochon P. Improving the quality of medication use in elderly patients: a not-so-simple prescription. Arch Intern Med. 2002;162:1670–2. doi: 10.1001/archinte.162.15.1670. [DOI] [PubMed] [Google Scholar]
- 5.Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17:825–37. doi: 10.2165/00023210-200317110-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 7.WHO. International Statistical Classification of Diseases and Related Health Problems. Vol. 1. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 8.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–7. [PubMed] [Google Scholar]
- 9.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures. Dosage more important than elimination half-life. Arch Intern Med. 1995;155:1801–7. [PubMed] [Google Scholar]
- 10.Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147:574–80. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 11.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 12.Burger H, de Laet CE, van Daele PL. Risk factors for increased bone loss in an elderly population: the Rotterdam Study. Am J Epidemiol. 1998;147:871–9. doi: 10.1093/oxfordjournals.aje.a009541. [DOI] [PubMed] [Google Scholar]
- 13.Burger H, van Daele PL, Algra D. The association between age and bone mineral density in men and women aged 55 years and over: the Rotterdam Study. Bone Miner. 1994;25:1–13. doi: 10.1016/s0169-6009(08)80203-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158:892–8. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- 15.Wagner AK, Zhang F, Soumerai SB. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–72. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]


