Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Circadian variations of tacrolimus pharmacokinetics are controversial.
Also, the pharmacokinetics has time-dependent variability, such as a decrease in oral clearance and increase in the dose-adjusted AUC after transplantation.
Although the CYP3A5 polymorphism is associated with tacrolimus pharmacokinetics, differences in the influence of this gene on the pharmacokinetics between the early and maintenance stages have not yet been clarified.
WHAT THIS STUDY ADDS
Tacrolimus pharmacokinetics did not show circadian variation in either the early or maintenance stage with our designated-time administration strategy.
Based on previous results and our own findings, the interval between food consumption and tacrolimus administration might influence the interindividual and interinstitutional variability of tacrolimus chronopharmacokinetics.
The CYP3A5 polymorphism may be associated with the time-dependent changes in tacrolimus oral clearance.
AIMS
We investigated whether tacrolimus pharmacokinetics shows circadian variation and the influence of the CYP3A5 A6986G polymorphism on the pharmacokinetics in both the early and maintenance stages after renal transplantation.
METHODS
Tacrolimus was administered twice daily at specified times (09.00 and 21.00 h) throughout the pre- and post-transplant period according to the trough-targeting strategy. Fifty recipients with stable graft function were studied on day 28 and beyond 1-year post transplantation. Whole blood samples were collected prior to and 1, 2, 3, 4, 6, 9 and 12 h after both the morning and evening doses during hospitalization.
RESULTS
Tacrolimus pharmacokinetics did not show circadian variation in either the early or maintenance stage [AUC0–12 197.1 (95% confidence interval 182.9, 212.3) in daytime vs. 203.6 ng h ml−1 (189.8, 217.4) in the night-time at day 28, 102.0 (92.1, 111.9) vs. 107.7 (97.9, 117.5) at 1 year, respectively]. In CYP3A5 *1 allele carriers (CYP3A5 expressers), body weight-adjusted oral clearance was markedly decreased from the early stage to the maintenance stage [0.622 (0.534, 0.709) to 0.369 l h−1 kg−1 (0.314, 0425)] compared with a smaller decrease [0.368 (0.305, 0.430) to 0.305 (0.217, 0.393)] in CYP3A5 non-expressers; however, the CYP3A5 genetic variation did not influence tacrolimus chronopharmacokinetics.
CONCLUSION
Equivalent daytime and night-time tacrolimus pharmacokinetics were achieved during both the early and maintenance stages with our specified-time administration strategy. The CYP3A5 polymorphism may be associated with the time-dependent changes in the oral clearance of tacrolimus, suggesting that genotyping of this polymorphism is useful for determining the appropriate dose of tacrolimus in both the early and maintenance stages after renal transplantation.
Keywords: circadian pharmacokinetics, CYP3A5 polymorphism, maintenance stage, renal transplantation, tacrolimus
Introduction
A calcineurin inhibitor, either tacrolimus or ciclosporin, remains the main immunosuppressive drug administered during both the early and maintenance stages in most renal transplant recipients. A number of studies on the pharmacokinetics and pharmacogenetics of tacrolimus have been reported.
Tacrolimus is generally administered in two equally divided doses every 12 h, and the concentrations of tacrolimus are routinely measured and the administered doses adjusted according to the target trough level [1–4]. Transplant clinicians generally assume that the equivalent peak concentrations and area under the concentration–time curves (AUCs) are obtained after each dose of tacrolimus [1]. However, circadian variations in the pharmacokinetics of tacrolimus are controversial [1–4]. Moreover, there are no available reports regarding differences in the circadian pharmacokinetics of tacrolimus between the early stage and maintenance stage beyond 1 year after transplantation with the same designated-time administration strategy.
Tacrolimus is oxidatively metabolized by cytochrome P450 (CYP) 3A4 and 3A5, which are encoded by the CYP3A4 and CYP3A5 genes, respectively [5, 6]. Although the CYP3A4 polymorphism in the 5'-untranslated region (A-392G) is associated with the trough level of tacrolimus [7], the frequency of polymorphisms in CYP3A4 is quite low in Asian populations [8]. However, the CYP3A5 polymorphism is associated with tacrolimus pharmacokinetics in Japanese subjects [6]. A higher CYP3A5 protein concentration in the liver was reportedly associated with the CYP3A5 *1 allele [9]. CYP3A5 is also the major enzyme for tacrolimus in the small intestine, and its expression is recognized as responsible for decreased tacrolimus bioavailability [10]. Therefore, kidney transplant recipients with the CYP3A5 *1 allele (CYP3A5 expresser) require a higher daily tacrolimus dose than those with the CYP3A5 *3/*3 genotype (CYP3A5 non-expresser) in order to maintain both the target trough level and AUC [6]. However, differences in the influence of this gene on tacrolimus pharmacokinetics between the early and maintenance stages have not yet been clarified in Japanese subjects.
The involvement of ATP-binding cassette B1 (ABCB1) polymorphisms in tacrolimus pharmacokinetics is controversial. Although some studies have shown an association of the ABCB1 C3435T or G2677T/A polymorphism with the pharmacokinetics [11–13], Hessenlink et al. [7] and our previous report [6] have not identified any effect of the ABCB1 polymorphisms on tacrolimus pharmacokinetics.
The present study investigated whether the pharmacokinetics of tacrolimus shows circadian variation with the same designated-time administration strategy and also compared the influences of CYP3A5 polymorphisms on the pharmacokinetics in the maintenance stage (beyond 1 year) to those in the early stage (day 28). In addition, we investigated the association of ABCB1 polymorphisms with tacrolimus pharmacokinetics.
Methods
Subjects
The circadian pharmacokinetics and pharmacogenetics of tacrolimus were studied in 50 recipients (31 men, 19 women) with a mean age of 43.8 years [95% confidence interval (CI) 40.3, 47.3] at day 28 and 45.8 years (42.5, 49.0) beyond 1 year after transplantation. All subjects had received a kidney graft after January 2001. The mean follow-up period after transplantation was 26.7 months (range 12–58). The eligibility criteria in this study were: (i) living-donor transplantation, (ii) identical maintenance immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil (MMF) and prednisolone, (iii) no withdrawal of prednisolone in the study period, (iv) absence of remarkable clinical episodes of chronic rejection, and (v) patients who gave informed consent to participate. The ethics committee of Akita University School of Medicine approved the study protocol. The causes of end-stage renal disease were chronic glomerulonephritis, including IgA nephropathy in 37 patients, Alport syndrome in two, systemic lupus erythematosus in two, polycystic kidney in two, diabetes mellitus in one, Wegener's granulomatosis in one, and others including unknown cause in five.
Immunosuppressive regimen
Patients initially received combination immunosuppressive therapy consisting of tacrolimus and MMF starting 2 (ABO compatible) or 7 days (ABO incompatible or second transplantation) prior to surgery. An initial oral dose (0.15 mg kg−1) of tacrolimus was given in two equally divided fractions every 12 h at 09.00 and 21.00 h. The daily tacrolimus dose was adjusted according to the clinical state of the patient, the whole blood trough target level being 15–20 ng ml−1 up to 2 weeks, 10–15 ng ml−1 up to 4 weeks and <10 ng ml−1 thereafter. Beyond 1 year post transplantation, the blood target level was between 3 and 8 ng ml−1. Trough levels were measured when recipients visited our outpatient unit each month. The initial oral dose of 1.5 or 2 g day−1 of MMF was also given twice a day at 09.00 and 21.00 h. The daily doses of tacrolimus and MMF continued to be equally divided into morning (09.00 h) and night-time (21.00 h) fractions throughout the pre- and post-transplant periods. Methylprednisolone was given concomitantly: a dose of 500 mg on the day of surgery, tapered to 40 mg day−1 during the first week, 20 mg day−1 of prednisolone in the second week, 15 mg day−1 of prednisolone in the third week, and 10 mg day−1 thereafter. In the maintenance stage, the dose of prednisolone ranged from 2.5 to 12.5 mg day−1 based on individual immunosuppressive states such as recurrence of nephritis or original autoimmune disease. Of 50 patients, nine ABO incompatible or second renal transplant recipients received additional treatments including plasma exchanges before transplantation and splenectomy. Oral prednisolone was given after breakfast at around 08.00 h. During hospitalization, patients received a controlled hospital diet served at 07.30, 12.30 and 18.00 h daily.
Sample collection and analysis
On day 28 and beyond 1 year after renal transplantation, whole blood samples were collected in ethylenediamine tetraaceticacid–2Na tubes just prior to and 1, 2, 3, 4, 6, 9 and 12 h after both the morning and evening doses during hospitalization. The doses of drugs were unchanged for at least 3 days prior to sampling. A total of 15 blood samples during a 24-h period were collected from each patient. Blood samples were stored at 4°C for no more than 7 days prior to analyses. The blood concentration of tacrolimus was determined by a microparticle enzyme immunoassay (IMx; Abbott Laboratories, Abbott Park, IL, USA) as previously reported [4]. The quantitative limit of this assay was 1.5 ng ml−1, and the within-run coefficients of variation of whole-blood tacrolimus obtained using 5 ng ml−1, 11 ng ml−1 and 22 ng ml−1 in the present study were 18.4, 12.6 and 10.7%, respectively.
Pharmacokinetic analysis
The pharmacokinetic parameters for tacrolimus were estimated by a standard noncompartmental analytical method using Win-nonlin Standard Edition, version 4.0.1 software (Pharsight Co., Mountain View, CA, USA). The trough level as the minimal concentration just prior to administration (C0), peak tacrolimus concentration (Cmax), and time required to reach the peak (tmax) were obtained directly from the raw data. The AUC0–12 was calculated using linear trapezoidal rules from 0 to 12 h. CL/F was determined by dividing the dose by AUC0–12, where F indicates the absolute bioavailability. These pharmacokinetic parameters were calculated for both daytime (from 09.00 to 21.00 h) and night-time (from 21.00 to 9.00 h).
Genotyping of genetic polymorphisms
DNA was extracted from a peripheral blood sample using a QIAamp Blood kit (Qiagen, Hilden, Germany) and stored at −4 °C prior to analysis. Primer sequences and polymerase chain reaction (PCR) conditions for analyses of CYP3A5 A6986G, ABCB1 C3435T and G2677T/A were performed according to our previous report. Briefly, to genotype CYP3A5 A6986G, ABCB1 C3435T and G2677T/A, the PCR-restriction fragment length polymorphism method was used [6, 14].
Statistical procedures
The data were expressed as the mean with the 95% CI in parentheses, and differences at P < 0.05 were considered significant. The concentration–time profile of tacrolimus was drawn using Microsoft Excel X software for Macintosh (Microsoft Asia Ltd., Tokyo, Japan). Paired values in the different stages (daytime vs. night-time and the early vs. maintenance stages) were compared using a nonparametrical test in paired series (Wilcoxon), and unpaired values between two genotypes (CYP3A5 expressers vs. non-expressers and ABCB1 C3435T CC vs. CT+TT) were compared using the Mann–Whitney U-test, because these variables were not normally distributed. To determine the independent and combined effects of the ABCB1 G2677T/A and CYP3A5 polymorphisms on the pharmacokinetics of tacrolimus, comparisons between groups were performed with a two-way analysis of variance (anova). The analysis was performed using SPSS version 15.0 statistical software (SPSS Japan Inc., Tokyo, Japan). To test the population homogeneity of the subjects, the genotype frequencies of the CYP3A5 and ABCB1 polymorphisms were tested against Hardy–Weinberg equilibrium with the χ2 test.
Results
The mean age, body weight and laboratory data in the early (day 28) and maintenance (beyond 1 year) stages after transplantation are shown in Table 1. The serum creatinine levels and the creatinine clearance levels calculated by the Cockcroft and Gault method [15] did not change, the platelet number was decreased, and the levels of total protein, albumin and haematocrit were increased beyond 1 year after transplantation compared with those on day 28.
Table 1.
Comparison of laboratory data between the early and maintenance stages after renal transplantation in 50 recipients
| Early | Maintenance | P | ||
|---|---|---|---|---|
| Age | (year) | 43.8 | 45.8 | <0.001 |
| (95% CI) | (40.3, 47.3) | (42.5, 49.0) | ||
| BW | (kg) | 57.4 | 60.3 | 0.002 |
| (95% CI) | (35.6, 61.1)) | (55.9, 64.5) | ||
| SCr | (mg dl−1) | 1.27 | 1.17 | 0.240 |
| (95% CI) | (1.15, 1.39) | (1.08, 1.25) | ||
| Ccr | (ml min−1) | 61.4 | 64.3 | 0.220 |
| (95% CI) | (54.6, 68.2) | (55.2, 69.1) | ||
| TP | (g dl−1) | 6.1 | 6.7 | <0.001 |
| (95% CI) | (6.0, 6.3) | (6.5, 6.9) | ||
| Alb | (g dl−1) | 4.1 | 4.5 | <0.001 |
| (95% CI) | (4.0, 4.3) | (4.4, 4.5) | ||
| GPT | (U dl−1) | 19.5 | 17.7 | 0.852 |
| (95% CI) | (14.3, 24.6) | (14.4, 21.1) | ||
| Hct | (%) | 30.1 | 40.3 | <0.001 |
| (95% CI) | (28.9, 31.3) | (38.6, 41.9) | ||
| Plt | (×10 000 mm−3) | 28.2 | 20.6 | <0.001 |
| (95% CI) | (22.9, 33.5) | (19.0, 22.1) | ||
Values are expressed as means. 95% CIs are shown in parentheses. Alb, albumin; BW, body weight; Ccr, calculated creatinine clearance; Early, early stage (day 28); GPT, glutamic pyruvic transaminase; Hct, haematocrit; Plt, platelet; Maintenance, maintenance stage (beyond 1 year) after transplantation; SCr, serum creatinine; TP, total protein.
The daily dose of tacrolimus was equally divided into two fractions given every 12 h at a designated time (09.00 and 21.00 h). The blood concentration–time profiles of tacrolimus in 50 recipients in the early and maintenance stages after transplantation are shown in Figure 1, and the daytime and night-time pharmacokinetic parameters are shown in Table 2. Most of the parameters did not differ significantly between daytime and night-time in the early or maintenance stage. Since the dose of tacrolimus in the maintenance stage was significantly decreased compared with that in the early stage, both daytime and night-time AUCs0–12 were smaller in the maintenance than early stage. Daytime and night-time dose- and body weight-adjusted (dose-adjusted) AUCs0–12, dose-adjusted trough levels and dose-adjusted Cmax increased, and body weight-adjusted oral clearances decreased in the maintenance stage compared with the early stage.
Figure 1.
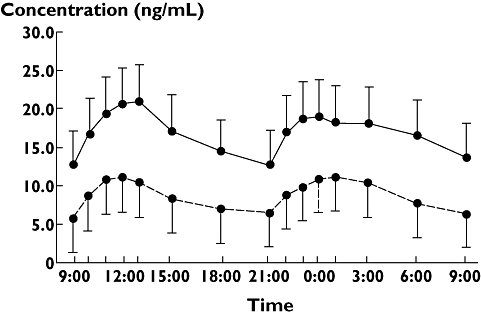
Blood concentration–time profiles of tacrolimus in 50 renal transplant recipients on day 28 and >1 year postoperatively. Each point and bar represents the mean + SD on day 28 and the mean − SD >1 year after transplantation. Day 28 after transplant, ( ); more than 1 year after transplant, (
); more than 1 year after transplant, ( )
)
Table 2.
Comparison of daytime and night-time pharmacokinetic parameters of tacrolimus in both the early and maintenance stages after transplantation in 50 recipients
| Daytime | Night-time | P | ||
|---|---|---|---|---|
| Single dose (mg) | E | 5.6 (range 2–16) | same | – |
| M | 1.8* (range 1–3.5) | same | – | |
| AUC0–12 (ng h ml−1) | E | 197 (183, 212) | 204 (190, 217) | 0.272 |
| M | 102* (92, 112) | 108* (98, 118) | 0.081 | |
| Dose-adjusted AUC0–12 (ng h ml−1 mg−1 kg−1) | E | 0.75 (0.65, 0.85) | 0.77 (0.68, 0.86) | 0.273 |
| M | 1.10* (0.90, 1.29) | 1.15* (0.97, 1.33) | 0.076 | |
| Dose-adjusted trough level (ng ml−1 mg−1 kg−1) | E | 0.05 (0.04, 0.06) | 0.05 (0.05, 0.06) | 0.054 |
| M | 0.07* (0.07, 0.08) | 0.07* (0.06, 0.08) | 0.051 | |
| Dose-adjusted Cmax (ng ml−1 mg−1 kg−1) | E | 0.09 (0.08, 0.100) | 0.08 (0.07, 0.09) | 0.347 |
| M | 0.13* (0.11, 0.16) | 0.14* (0.12, 0.16) | 0.357 | |
| BW adjusted CL/F (l h−1 kg−1) | E | 0.50 (0.43, 0.57) | 0.50 (0.44, 0.56) | 0.443 |
| M | 0.36* (0.31, 0.41) | 0.34* (0.29, 0.39) | 0.067 | |
Values are expressed as means. 95% CIs are shown in parentheses. AUC, area under the concentration–time curve; BW, body weight; dose adjusted, dose- and BW-adjusted; Cmax, maximal concentration of tacrolimus; CL/F, apparent oral clearance. E, early state (day 28); M, maintenance (beyond 1 year) after transplantation.
P < 0.05 compared with the early stage.
The CYP3A5 *1/*1, *1/*3 and *3/*3 genotype was detected in three (6%), 23 (46%) and 24 (48%) of the 50 recipients, respectively, and the genotype distribution was in Hardy–Weinberg equilibrium (P = 0.578). The subjects were divided into two genotype groups, i.e. CYP3A5 expressers (CYP3A5 *1/*1 + *1/*3) and CYP3A5 non-expressers (CYP3A5 *3/*3). The distribution of sex, age and body weight did not differ significantly between the two groups (P > 0.248). The blood concentration–time profiles of tacrolimus in each group in the early and maintenance stages after transplantation are plotted in Figure 2, and the daytime and night-time pharmacokinetic parameters are shown in Table 3. There were no significant differences between the daytime and night-time AUC0–12 of each CYP3A5 genotype group in either the early or maintenance stages.
Figure 2.
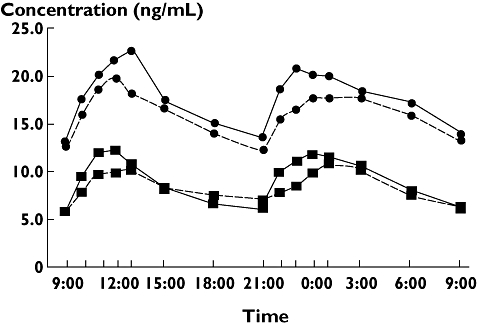
Blood concentration–time profiles of tacrolimus in CYP3A5 expressers and non-expressers on day 28 and >1 year postoperatively. Each point represents the mean on day 28 and >1 year after transplantation. CYP3A5 expresser at day 28, ( ); CYP3A5 nonexpresser at day 28, (
); CYP3A5 nonexpresser at day 28, ( ); CYP3A5 expresser more than 1 year, (
); CYP3A5 expresser more than 1 year, ( ); CYP3A5 nonexpresser more than 1 year, (
); CYP3A5 nonexpresser more than 1 year, ( )
)
Table 3.
Comparison of tacrolimus pharmacokinetic parameters between the CYP3A5 genotype groups in daytime and night-time and the early and maintenance stages after transplantation
| CYP3A5 (number, male:female) | *1/*1 + *1/*3 (n = 26, 17 : 9) | *3/*3 (n = 24, 14 : 10) | P | |
|---|---|---|---|---|
| Daytime | ||||
| AUC0–12 (ng h ml−1) | E | 204.2 (194.4, 233.3) | 189.2 (172.4, 212.5) | 0.207 |
| M | 101.4* (96.5, 125.8) | 102.5* (90.1, 117.6) | 0.600 | |
| Dose-adjusted AUC (ng h ml−1 mg−1 kg−1) | E | 0.584 (0.507, 0.661) | 0.928 (0.772, 1.084) | 0.001 |
| M | 0.907* (0.726, 1.088) | 1.302* (0.951, 1.653) | 0.038 | |
| Dose-adjusted trough level (ng ml−1 mg−1 kg−1) | E | 0.038 (0.031, 0.046) | 0.059 (0.051, 0.067) | <0.001 |
| M | 0.054* (0.044, 0.061) | 0.066 (0.054, 0.075) | 0.028 | |
| Dose-adjusted Cmax (ng ml−1 mg−1 kg−1) | E | 0.070 (0.060, 0.081) | 0.107 (0.086, 0.128) | 0.006 |
| M | 0.124* (0.101, 0.146) | 0.145 (0.104, 0.186) | 0.573 | |
| BW-adjusted CL/F (l h−1 kg−1) | E | 0.619 (0.516, 0.722) | 0.375 (0.310, 0.441) | <0.001 |
| M | 0.399* (0.327, 0.471) | 0.319* (0.239, 0.400) | 0.052 | |
| Night-time | ||||
| AUC0–12 (ng h ml−1) | E | 213.8 (194.4, 233.3) | 192.4 (172.4, 212.5) | 0.103 |
| M | 111.2* (96.5, 125.8) | 103.9* (90.1, 117.6) | 0.455 | |
| Dose-adjusted AUC (ng h ml−1 mg−1 kg−1) | E | 0.614 (0.527, 0.700) | 0.937 (0.796, 1.078) | <0.001 |
| M | 0.991* (0.791, 1.191) | 1.322* (1.011, 1.632) | 0.045 | |
| Dose-adjusted trough level (ng ml−1 mg−1 kg−1) | E | 0.042 (0.035, 0.049) | 0.066 (0.055, 0.076) | <0.001 |
| M | 0.056* (0.042, 0.070) | 0.080 (0.060, 0.099) | 0.027 | |
| Dose-adjusted Cmax (ng ml−1 mg−1 kg−1) | E | 0.068 (0.057, 0.080) | 0.101 (0.085, 0.116) | 0.001 |
| M | 0.122* (0.099, 0.144) | 0.156* (0.120, 0.192) | 0.103 | |
| BW-adjusted CL/F (l h−1 kg−1) | E | 0.622 (0.534, 0.709) | 0.368 (0.305, 0.430) | <0.001 |
| M | 0.369* (0.314, 0.425) | 0.305* (0.217, 0.393) | 0.064 | |
Values are expressed as means. 95% CIs are shown in parentheses. AUC, area under the concentration–time curve; BW, body weight; dose adjusted, dose- and BW-adjusted; Cmax, maximal concentration of tacrolimus; CL/F, apparent oral clearance. E, Early stage; M, maintenance stage after transplantation.
P < 0.05 compared with the early stage. There were no differences between daytime and night-time parameters in either the early or maintenance stage.
The dose-adjusted AUC0–12 and dose-adjusted trough levels in both the early and maintenance stages were significantly smaller and lower in CYP3A5 expressers than in non-expressers, respectively. The dose-adjusted AUC0–12 increased, and body weight-adjusted oral clearance decreased from the early stage to the maintenance stage after transplantation in the two groups. Notably, the body weight-adjusted oral clearance markedly decreased from the early to maintenance stage in CYP3A5 expressers. Although the body weight-adjusted oral clearance was higher and dose-adjusted Cmax was lower in CYP3A5 expressers than in non-expressers in the early stage, these pharmacokinetic differences between the two groups disappeared in the maintenance stage.
The ABCB1 C3435T CC, CT, and TT genotypes were detected in 20 (40%), 21 (42%) and nine (18%) of the 50 recipients, respectively, and the genotype distribution was in Hardy–Weinberg equilibrium (P = 0.638). For the G2677(A/T) polymorphism, GG, GA, GT, AT, TT and AA genotypes were detected in nine (18%), 11 (22%), 12 (24%), seven (14%), 11 (22%) and 0 (0%) of the 50 recipients, respectively. The distributions of age and body weight did not significantly differ among each ABCB1 genotype group (P > 0.214). None of the dose-adjusted pharmacokinetic parameters showed a significant difference among each genotype group of ABCB1 C3435T or G2677A/T polymorphism in either the early or maintenance stage after transplantation. Moreover, there were no significant differences in any dose-adjusted kinetic parameters of tacrolimus as they relate to the ABCB1 C3435T and C2677(A/T) polymorphisms in the CYP3A5 expressers and non-expressers (data not shown). These single nucleotide polymorphisms did not affect the pharmacokinetics of tacrolimus in Japanese subjects.
Discussion
A few studies have reported that tacrolimus pharmacokinetics showed circadian variation [1–3]. Min et al. [2] and Iwahori et al. [3] have reported that the AUC0–12 of tacrolimus was significantly greater, Cmax was higher and tmax was shorter after the morning dose than after the evening dose in 12 stable liver and 11 kidney allograft recipients, respectively, in the early stage after transplantation. In the maintenance state, Hardinger et al. [1] also showed a greater tacrolimus AUC0–12 (117 vs. 97 ng h ml−1) and twofold higher Cmax (17.8 vs. 8.4 ng ml−1) after the morning dose than after the evening dose. However, our previous study in 16 kidney allograft recipients has demonstrated that tacrolimus pharmacokinetics did not show circadian variation on day 28 after transplantation [4], and the present study involved 12 of the 16 recipients. Tacrolimus concentration–time profiles in the night-time closely resembled those in the daytime.
These circadian pharmacokinetic differences in each study might result from the interval between tacrolimus administration and meal consumption, because the tacrolimus AUC was smaller after meals than during fasting [16]. Hardinger et al. [1] designed their study so that food was available from 2.5 to 3 h prior to the evening dose, and fasting occurred for 10 h prior to the morning dose. In that study, breakfast was provided 2 h after the morning dose of tacrolimus at 10.00 h, lunch at noon, and dinner at 17.00 h. In the present study, morning doses were given 1.5 h after breakfast, whereas night-time doses were given 3 h after the evening meal during both the early and maintenance stages after transplantation. Breakfast was provided at 07.30 h, lunch at noon and dinner at 18.00 h. Based on previous findings as well as our own study, the interval between the consumption of food and administration of tacrolimus may play a role in the circadian variation of tacrolimus pharmacokinetics [1, 4, 16].
We hypothesized that night-time immunosuppression for allograft recipients may be important to prevent rejection because of enhanced immunocompetence during nocturnal sleep [17, 18]. Indeed, we have previously shown that less night-time exposure to mycophenolic acid, the pharmacologically active metabolite of MMF, was associated with the occurrence of acute rejection [19]. Therefore, all of our recipients took tacrolimus at the same designated times (09.00 and 21.00 h) to lessen the influence of meals throughout the pre- and post-transplant periods. Although the clinical relevance of the circadian variation in the pharmacokinetics of tacrolimus is not yet clear, there was no such variation with our designated-time administration strategy.
In the present study, dose-adjusted parameters of tacrolimus pharmacokinetics differed significantly between the early and maintenance stages. The dose-adjusted AUC0–12 was greater, and the dose-adjusted trough level and Cmax were higher in the maintenance stage than on day 28 post transplant. The body weight-adjusted oral clearance of tacrolimus decreased by approximately 70% between the early and maintenance stages, probably resulting in a significant increase in the dose-adjusted AUC0–12 during the maintenance stage in the present study. These findings were comparable to those of previous reports showing time-dependent variability in tacrolimus pharmacokinetics after transplantation [20–22].
Several factors, such as CYP3A and P-glycoprotein activities, serum albumin, haematocrit, and corticosteroid, have been suggested to be associated with the bioavailability and clearance of tacrolimus [21, 23]. Tacrolimus exposure increased after the withdrawal of steroids [24]. Corticosteroids interfere with tacrolimus exposure because a corticosteroid-induced increase in CYP3A activity would result in increased clearance [21, 23]. Therefore, the oral clearance of tacrolimus may greatly increase and the AUC decrease with co-administration of prednisolone in CYP3A5 expressers rather than in non-expressers. In the present study, mean prednisolone dose per body weight on day 28 and beyond 1 year post transplantation was 0.185 (0.174, 0.196) and 0.124 mg kg−1 (0.101, 0.140) (P < 0.001), respectively. This progressive reduction in the dose of prednisolone might have played a role in reducing the oral clearance of tacrolimus, resulting in an increase in the AUC0–12 during the maintenance stage, especially in CYP3A5 expressers.
Haematocrit may also affect whole blood tacrolimus concentrations. Akbas et al. [25] found a significant negative correlation between the haematocrit value and tacrolimus concentration in patients with haematocrit values of ≤25% using the microparticle immunoassay technique. Therefore, time-dependent parameters of the tacrolimus pharmacokinetics in the present study might be affected because the mean haematocrit value increased 1.34-fold from the early stage to the maintenance stage (30.1 to 40.3%). However, we did not find a significant negative correlation between the haematocrit value and tacrolimus concentration.
Recently, a number of studies have shown that CYP3A5 expressers needed a larger dose of tacrolimus to reach the target trough level than did non-expressers [6, 7, 13, 26–32]. The present study has shown that the dose of tacrolimus was about 1.8-fold higher, dose per body weight was 2.0-fold higher, body weight-adjusted oral clearance was 1.7-fold higher, and dose-adjusted AUC0–12 and dose-adjusted trough level were 63% lower in CYP3A5 expressers than in non-expressers on day 28 after transplantation. Beyond 1 year after transplantation, the single dose of tacrolimus was still about 1.4-fold higher, dose per body weight was 1.5-fold higher, and body weight-adjusted oral clearance was 1.3-fold higher, and the dose-adjusted AUC0–12 and dose-adjusted trough level were 69% lower in CYP3A5 expressers than in non-expressers. The body weight-adjusted oral clearance markedly decreased in CYP3A5 expressers from the early stage to the maintenance stage. The CYP3A5 polymorphism was associated with the time-dependent change in the oral clearance of tacrolimus. We speculated that drug–drug interaction between tacrolimus and corticosteroids occurred in the CYP3A5 expressers probably because the corticosteroids induced an increase in CYP3A activity [25].
The influence of the ABCB1 polymorphisms on tacrolimus pharmacokinetics is controversial. In our previous report, as well as in other reports, there was no association of ABCB1 polymorphisms [6, 7, 29–32] with any pharmacokinetic parameters of tacrolimus. There was no association of ABCB1 C3435T or C2677T/A polymorphisms with the tacrolimus pharmacokinetics in either CYP3A5 expressers or non-expressers [32]. However, some studies have shown either a significant or slight association of ABCB1 polymorphisms with tacrolimus pharmacokinetics [11–13, 27, 28, 33]. Small sample sizes, inaccurate analyses of single nucleotide polymorphisms, the masking of any effects of ABCB1 genotypes by strong effects of CYP3A5 polymorphisms, or other factors may explain the discrepancy in the reported effects of ABCB1 polymorphisms on tacrolimus pharmacokinetics. Furthermore, although the ABCB1 C3435T CC genotype is associated with high expression of P-glycoprotein, Nakamura et al. [34] have reported that the C allele is associated with a significantly reduced intestinal ABCB1 messenger RNA concentration in Japanese subjects in comparison with those demonstrating the TT genotype. Moreover, the C allele and T allele of the C3435T polymorphism were strongly linked to the G and T or A allele of the G2677A/T polymorphism, respectively, in our subjects (data not shown). The ABCB1 C3435T polymorphism might be a marker in linkage disequilibrium with other genes truly producing P-glycoprotein in different ethnic groups [13]. We did not find any association of the ABCB1 polymorphisms with tacrolimus pharmacokinetic parameters in our cohort.
In conclusion, the pharmacokinetics of tacrolimus did not show circadian variation in either the early or maintenance stage with our designated-time administration strategy. Based on previous results and our own findings, the interval between the consumption of food and administration of tacrolimus might influence the interindividual and interinstitutional variability of tacrolimus chronopharmacokinetics. The CYP3A5 polymorphism may be associated with time-dependent changes in the oral clearance of tacrolimus, suggesting that genotyping of this polymorphism is useful for determining the appropriate dose of tacrolimus in both the early and maintenance stages after renal transplantation.
Acknowledgments
This study was supported by Akita University and Departments of Urology and Pharmaceutical Science.
Competing interests: None declared.
REFERENCES
- 1.Hardinger KL, Park JM, Schnitzler MA, Koch MJ, Miller BW, Brennan DC. Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. Am J Transplant. 2004;4:621–5. doi: 10.1111/j.1600-6143.2004.00383.x. [DOI] [PubMed] [Google Scholar]
- 2.Min DI, Chen HY, Lee MK, Ashton K, Martin MF. Time-dependent disposition of tacrolimus and its effect on endothelin-1 in liver allograft recipients. Pharmacotherapy. 1997;17:457–63. [PubMed] [Google Scholar]
- 3.Iwahori T, Takeuchi H, Matsuno N, Johjima Y, Konno O, Nakamura Y, Hama K, Uchiyama M, Ashizawa T, Okuyama K, Nagao T, Abudoshukur M, Hirano T, Oka K. Pharmacokinetic differences between morning and evening administration of cyclosporine and tacrolimus therapy. Transplant Proc. 2005;37:1739–40. doi: 10.1016/j.transproceed.2005.02.104. [DOI] [PubMed] [Google Scholar]
- 4.Tada H, Satoh S, Iinuma M, Shimoda N, Murakami M, Hayase Y, Kato T, Suzuki T. Chronopharmacokinetics of tacrolimus in kidney transplant recipients: occurrence of acute rejection. J Clin Pharmacol. 2003;43:859–65. doi: 10.1177/0091270003254797. [DOI] [PubMed] [Google Scholar]
- 5.Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27:201–14. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, Suzuki T, Habuchi T, Kato T. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182–7. doi: 10.1097/01.tp.0000137789.58694.b4. [DOI] [PubMed] [Google Scholar]
- 7.Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 8.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–94. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thumme K, Boguski MS, Schuetz E. Sequence diversity in CYP3A5 promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 10.Lampen A, Christians U, Guengerich FP, Watkins PB, Kolars JC, Bader A, Gonschior AK, Dralle H, Hackbarth I, Sewing KF. Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variability. Drug Metab Dispos. 1995;23:1315–24. [PubMed] [Google Scholar]
- 11.Akbas SH, Bilgen T, Keser I, Tuncer M, Yucetin L, Tosun O, Gultekin M, Luleci G. The effect of MDR1 (ABCB1) polymorphism on the pharmacokinetics of tacrolimus in Turkish renal transplant recipients. Transplant Proc. 2006;38:1290–2. doi: 10.1016/j.transproceed.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, Op den Buijsch RA, Wong KM, Chan HW, Chau KF, Li CS, Leung KT, Kwan TH, de Vrie JE, Wijnen PA, van Dieijens-Visser MP, Bekers O. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmcogenomics. 2006;7:563–74. doi: 10.2217/14622416.7.4.563. [DOI] [PubMed] [Google Scholar]
- 13.Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659–65. doi: 10.1097/01.fpc.0000220571.20961.dd. [DOI] [PubMed] [Google Scholar]
- 14.Miura M, Satoh S, Tada H, Saito M, Kagaya H, Inoue K, Sagae Y, Kanno S, Ishikawa M, Habuchi T, Suzuki T. Influence of ABCB1 C3435T polymorphism on the pharmacokinetics of lansoprazole and gastroesophageal symptoms in Japanese renal transplant recipients classified as CYP2C19 extensive metabolizers and treated with tacrolimus. Int J Clin Pharm Ther. 2006;44:605–13. doi: 10.5414/cpp44605. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Bekersky I, Dressler D, Mekki Q. Effect of time of meal consumption on bioavailability of a single oral 5 mg tacrolimus dose. J Clin Pharmacol. 2001;41:289–97. doi: 10.1177/00912700122010104. [DOI] [PubMed] [Google Scholar]
- 17.Chacon F, Cano P, Lopez-Varela S, Jimenez V, Marcos A, Esquifino AL. Chronobiological features of the immune system. Effect of calorie restriction. Eur J Clin Nutr. 2002;56:S69, 72. doi: 10.1038/sj.ejcn.1601491. [DOI] [PubMed] [Google Scholar]
- 18.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 19.Satoh S, Tada H, Murakami M, Tsuchiya N, Li Z, Numakura K, Saito M, Inoue T, Miura M, Hayase Y, Suzuki T, Habuchi T. Circadian pharmacokinetics of mycophenolic acid and implication of genetic polymorphisms for early clinical events in renal transplant recipients. Transplantation. 2006;82:486–93. doi: 10.1097/01.tp.0000231874.53240.ba. [DOI] [PubMed] [Google Scholar]
- 20.Kuypers DRJ, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75:434–47. doi: 10.1016/j.clpt.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Kuypers DRJ, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids. Clin Pharmacokinet. 2004;43:741–62. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- 22.Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, den Hartigh J, Paul LC, de Fijter JW. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67:2440–7. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 23.Antignac M, Barrou B, Farinotti R, Lechat P, Urien S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol. 2007;64:750–7. doi: 10.1111/j.1365-2125.2007.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Duijnhoven EM, Boots JM, Christiaans MH, Stolk LM, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16:721–5. doi: 10.1007/s00147-003-0615-1. [DOI] [PubMed] [Google Scholar]
- 25.Akbas SH, Ozdem S, Caglar S, Tuncer M, Gurkan A, Yucetin L, Senol Y, Demirbas A, Gultekin M, Ersoy FF, Akaydin M. Effects of some hematological parameters on whole blood tacrolimus concentration measured by two immunoassay-based analytical methods. Clin Biochem. 2005;38:552–7. doi: 10.1016/j.clinbiochem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, Legendre C, Daly AK. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233–5. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H, Webber S, Zeevi A, Schuetz E, Zhanh J, Bowman P, Boyle G, Law Y, Miller S, Lamba J, Burckart GJ. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3:477–83. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP, Webber S, Ristich J, Dauber J, Iacono A, Grgurich W, Zaldonis D, McDade K, Zhang J, Burckart GJ. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol. 2004;44:135–40. doi: 10.1177/0091270003262108. [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Masuda S, Kiuchi T, Ogura Y, Oike F, Okuda M, Tanaka K, Inui K. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 2004;14:471–8. doi: 10.1097/01.fpc.0000114747.08559.49. [DOI] [PubMed] [Google Scholar]
- 30.Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–54. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Liu ZH, Zheng JM, Chen ZH, Tang Z, Chen JS, Li LS. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after transplantation. Clin Transpl. 2005;19:638–43. doi: 10.1111/j.1399-0012.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 32.Tada H, Tsuchiya N, Satoh S, Kagaya H, Li Z, Sato K, Miura M, Suzuki T, Kato T, Habuchi T. Impact of CYP3A5 and MDR1 (ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc. 2005;37:1730–2. doi: 10.1016/j.transproceed.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 33.Fredericks S, Moreton M, Reboux S, Carter ND, Goldberg L, Holts DW, Macphee IA. Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation. 2006;82:705–8. doi: 10.1097/01.tp.0000234942.78716.c0. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, Matsuo M, Kasuga M, Okumura K. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther. 2002;71:297–303. doi: 10.1067/mcp.2002.122055. [DOI] [PubMed] [Google Scholar]


