Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The absorption of valacyclovir presents a highly negative correlation with the level of P-glycoprotein expression.
It has been confirmed that a polymorphism of the MDR1 gene in exon 26 is related to the level of P-glycoprotein expression in intestine.
This study was conducted to find the relationship between polymorphism of MDR1 gene and absorption of valacyclovir.
WHAT THIS STUDY ADDS
Linkage disequilibrium exists between G2677T/A in exon 21 and C3435T in exon 26, between C1236T in exon 12 and C3435T, but not between C1236T and G2677T/A of MDR1 gene in the Chinese Han ethnic population.
Three single nucleotide polymorphisms of MDR1 gene do not influence the absorption of valacyclovir in the healthy Chinese Han ethnic population.
AIMS
To investigate the influence of three single nucleotide polymorphisms (SNPs) in exon 12 (C1236T), exon 21 (G2677T/A) and exon 26 (C3435T) of MDR1 gene on the absorption of valacyclovir after a single oral administration in the Chinese Han ethnic population.
METHODS
Two hundred healthy Chinese subjects were genotyped for the SNPs of C1236T, G2677T/A and C3435T in the MDR1 gene using allele-specific polymerase chain reaction. Linkage disequilibrium (LD) was analysed. Twenty-four subjects derived from a large random sample (n = 200) received a single oral dose of 600 mg valacyclovir. Plasma concentrations of acyclovir were determined up to 14 h after administration to obtain a pharmacokinetic profile.
RESULTS
LD existed between G2677T/A in exon 21 and C3435T in exon 26 (P < 0.001), between C1236T in exon 12 and C3435T (P < 0.001), but not between C1236T and G2677T/A (P > 0.05). Cmax, AUC0–1.5 h and AUC0–∞ were used as indices of valacyclovir absorption. AUC0–∞ for the 2677TA genotype was 17.45 ± 2.40 µg × h/ml, which was much higher compared with the 2677GG, GA and TT genotypes of 10.44 ± 1.00, 11.84 ± 2.83, 11.34 ± 2.32 µg × h/ml, respectively (P < 0.05). Similarly, a statistically significant difference of AUC0–∞ was also observed for different linked genotypes at position 2677 vs. 3435, and 1236 vs. 3435 (P < 0.05). However, there was no significant difference in valacyclovir absorptive pharmacokinetics between carriers and noncarriers of different haplotypes (P > 0.05).
CONCLUSIONS
Three SNPs of MDR1 gene did not influence the absorption of a single oral dose of 600 mg valacyclovir in healthy Chinese Han ethnic subjects.
Keywords: absorption, genotype, haplotype, MDR1, P-glycoprotein, valacyclovir
Introduction
P-glycoprotein (P-gp) encoded by the human multidrug resistance gene (MDR1) is an integral membrane protein, a member of the ATP-binding cassette (ABC) superfamily of transporters, acting as an efflux pump. P-gp can transport many structurally unrelated compounds, including anticancer agents, cardiac drugs, HIV protease inhibitors, antibiotics, calcium channel blockers and H1 antihistamines.
P-gp is expressed not only in tumour cells but also in many normal human tissues (small intestine, liver and kidney) and at various blood–tissue barriers (blood–brain barrier, blood–testis barrier and placenta) [1–4]. In these tissues, P-gp locates on the apical or luminal surface of the epithelial cells, which results in limited drug absorption from the gastrointestinal tract, promotes drug elimination into bile and urine and impedes drug penetration into the brain, testis and fetus [5]. As mentioned above, P-gp plays a very import role in the process of absorption, distribution, metabolism and excretion of its various substrates, and is also associated with drug–drug interaction due to its inhibition and induction. Moreover, P-gp provides additional protection for some sensitive tissues, including the brain, testis and fetus.
Many pharmacogenetics and pharmacogenomics studies have revealed that some single nucleotide polymorphisms (SNPs) of the MDR1 result in changes in P-gp expression and function among different ethnicities and subjects [6–9]. In recent years, 50 SNPs and three insertion/deletion polymorphisms have been reported in the MDR1 gene [10]. Among them, most attention has been focused on a synonymous mutation at position 3435 in exon 26 (C3435T). Although it does not change the encoded amino acid sequence, it is associated with altered protein expression. The mechanism by which C3435T affects P-gp expression may be in linkage disequilibrium (LD) with one or more unidentified variants in other regions of the MDR1 gene that control expression. It has been shown recently that this synonymous SNP in exon 26 (C3435T) is linked to the nonsynonymous exon 21 (G2677T/A) polymorphism, which results in Ala893Ser/Thr exchange, as well as another synonymous exon 12 (C1236T) mutation [8, 11, 12]. It is now widely held that haplotype-based approaches will offer greater ability to predict changes in phenotype than SNP-based approaches [13–15].
Valacyclovir is an effective antiviral drug and is prodrug of acyclovir. Studies have demonstrated that the absorption of valacyclovir and acyclovir presents a highly negative correlation with the level of P-gp expression [16, 17]. However, the influence of polymorphism of MDR1 gene on their absorption has not been reported. In this study, the LD of mutations in exon 12 (C1236T), exon 21 (G2677T/A) and exon 26 (C3435T) was evaluated, and the effects of one SNP, common linked genotypes and haplotypes on pharmacokinetic parameters after oral valacyclovir were also explored.
Methods
Human subjects
Two hundred healthy unrelated male Chinese subjects living in Chengdu city had been determined for the MDR1 genotypes at positions 3435, 2677 and 1236. Twenty-four subjects were enrolled in pharmacokinetic studies of oral valacyclovir. To avoid bias by ethnicity, every individual was of Han ethnic group. The subjects ranged in age from 21 to 28 years (median 24 years) and in body weight from 58 to 70 kg (median 64 kg). All were in good health as judged by their medical histories, physical examination, electrocardiogram, urine analysis and routine tests of biochemistry, hepatitis B and C. The subjects refrained from alcohol, coffee, tea or any fruit juice and did not take any drug during the entire pharmacokinetic study period. All subjects provided written informed consent before participating. The investigation was approved by the local ethics committee of Sichuan University.
MDR1 genotyping
Genomic DNA was extracted from leucocytes from peripheral venous blood samples using the Gentra Genomic DNA purification kit (D4000; Gentra Systems, Minneapolis, MN, USA). The allele-specific polymerase chain reaction (AS-PCR) method was used to determine the MDR1 C3435T, G2677T/A and C1236T genotypes according to the method of Plassmann et al., with minor modifications [18]. This single-tube PCR technique relies on allele-specific primers that differ in length by 8–10 bp for each SNP and results in PCR products of different sizes. The PCR products were separated for 1 h at 100 V on 2% agarose gels using 10 mg ml−1 ethidium bromide as stain and visualized under Gel Imaging system (GenoSens1200, Shanghai, China). The accuracy and reliability of the AS-PCR method were confirmed by DNA sequencing by TakaRa Biotechnology Corporation (Dalian, China).
Haplotype analysis
LD was analysed between the different pairs of three SNPs after all 200 healthy subjects had been genotyped. Haplotype analysis would be completed next if LD had been confirmed between two SNPs. Each genotype was appointed to a haplotype pair [19, 20]. The haplotype pair could be appointed unambiguously for those individuals who were homozygous at both variants or who were heterozygous at only one variant. However, for those individuals who were heterozygous at both variants, such as genotype 11, two haplotype pairs, 11/22 and 12/21, were possible (Table 4). At this time, haplotype pairs were determined by haplotype frequencies that had been calculated for the sample of 200 subjects. With the assumption that each haplotype is inherited dominantly, comparisons were performed between carriers and noncarriers of each particular haplotype.
Table 4.
Four haplotypes and nine genotypes of MDR1deduced from single nucleotide polymorphism (SNP) C1236T (exon 12) and C3435T (exon 26)
| Genotype | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 00 | 01 | 02 | 10 | 11 | 12 | 20 | 21 | 22 | ||||||||||
| Pos 1236 | T | T | T | T | T | T | C | T | C | T | C | T | C | C | C | C | C | C |
| Pos 3435 | C | C | C | T | T | T | C | C | C | T | T | T | C | C | C | T | T | T |
| Haplotype | 11 | 11 | 11 | 12 | 12 | 12 | 21 | 11 | 21 | 12 | 22 | 12 | 21 | 21 | 21 | 22 | 22 | 22 |
| or | ||||||||||||||||||
| Pos 1236 | C | T | ||||||||||||||||
| Pos 3435 | T | C | ||||||||||||||||
| Haplotype | 22 | 11 | ||||||||||||||||
| n | 1 | 4 | 4 | 6 | 6 | 0 | 3 | 0 | 0 | |||||||||
Genotype coding: 0, homozygous for nucleotides identical with the reference sequence (1236TT, 3435CC); 1, heterozygous (1236CT, 3435CT); 2, homozygous for nucleotides different from the reference sequence (1236CC, 3435TT). The first digit refers to the exon 12 SNP, and the second refers to the exon 26. Haplotype coding is a follow: 1, identical to the reference sequence (variant in exon 12, 1236T, variant in exon 26, 3435C); 2, different from the reference sequence (variant in exon 12, 1236C, variant in exon 26, 3435T).
Pharmacokinetic analysis
After overnight fasting, each subject received a single oral dose of 600 mg valacyclovir with 100 ml water. They were allowed eat a standardized meal 3 h later after administration. Venous blood samples were collected through an indwelling cannula before and at 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 8.0, 12.0 and 14.0 h after administration, and serum samples were prepared and immediately stored in polypropylene tubes at –70 °C. Acyclovir serum concentration was determined by sensitive and specific high-performance liquid chromatography with ultraviolet detection. The method was routinely validated to confirm accuracy and precision. The average recovery of acyclovir ranged from 94 to 102%. Coefficients of intra- and interday variation for acyclovir were 1.0 and 3.9%, respectively. The limit of quantification in this assay was 6 ng ml−1.
Mean values of duplicate measurements were used for further calculations. The absorption of valacyclovir was characterized by peak plasma concentration Cmax, area under the plasma concentration–time curve from time zero to 1.5 h (AUC0–1.5 h), and AUC0–∞ from zero to infinity of acyclovir. The Cmax was estimated directly from the observed plasma concentration vs. time data curve. The AUC0–1.5 h was calculated by use of the linear trapezoidal rule. The AUC0–∞ was calculated as follows: AUC0–∞ = AUC0–14 h + C14/ke, where C14 is the plasma concentration measured 14 h after drug administration and the elimination rate constant (ke) was estimated from the least-squares regression slope of the terminal plasma concentrations.
Statistical analysis
Data were presented as mean ± SD. Genotype and allele frequencies of three SNPs were assessed for deviation from Hardy–Weinberg equilibrium using the χ2 test. Classical statistic Lewontin's coefficient D′ and χ2 tests were used for analysis of LD between SNPs at position 1236, 2677 and 3435. The Mann–Whitney U-test was used for evaluation of the significance of differences in pharmacokinetic parameters between the two genotypic groups. Data from three or more different genotypic groups were compared by Kruskal–Wallis H-test. Statistical analysis was performed using SPSS software (version 10.0; SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
MDR1 genotype distribution in the Chinese Han ethnic group
The genotypic and allelic frequencies of the C1236T, G2677T/A and C3435T SNPs in 200 Chinese Han subjects are shown in Table 1. C1236T, G2677T and C3435T mutations largely appeared with variant allele frequencies of 34.3% [confidence interval (CI) 29.6, 39.0], 45.0% (CI 40.1, 49.9) and 43.3% (CI 38.4, 48.2), respectively, whereas G2677A mutation occurred with a frequency of 13.3% (CI 10.0, 16.6). Age and weight had no effect on the genotype or allele distribution. The results were in good agreement with those published in other studies [11, 14] and did not show significant deviation from Hardy–Weinberg equilibrium.
Table 1.
Position, genotype and allele frequencies of MDR1 SNPs in 200 Han subjects of Chinese
| SNP | Exon | Genotype frequency (%) | Allele frequency (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1236T | 12 | CC | CT | TT | C | T | ||||
| 10.5 | 47.5 | 42.0 | 34.3 | 65.7 | ||||||
| (29.6, 39.0) | (61.0, 70.4) | |||||||||
| G2677T/A | 21 | GG | GT | GA | TA | TT | AA | G | T | A |
| 17.5 | 37.5 | 11.0 | 10.5 | 21.0 | 2.5 | 41.7 | 45.0 | 13.3 | ||
| (36.9, 46.5) | (40.1, 49.9) | (10.0, 16.6) | ||||||||
| C3435T | 26 | CC | CT | TT | C | T | ||||
| 30.0 | 53.5 | 16.5 | 56.7 | 43.3 | ||||||
| (51.8, 61.6) | (38.4, 48.2) | |||||||||
In three single nucleotide polymorphisms (SNPs), no effects of age and weight were presented on the genotype and allele frequencies. Allele frequency was calculated on the basis of the Hardy–Weinberg distribution.
Genetic linkage of MDR1 SNPs
Distribution of combined genotypes and the results of analysed LD between the different pairs of three MDR1 SNPs are shown in Table 2. Because the variant 2677A in exon 21 occurred with a lower allele frequency, variant 2677A was counted together with 2677T when χ2 values were calculated. According to the results of D′ value and χ2 test, LD existed between G2677T/A in exon 21 and C3435T in exon 26 (P < 0.001), C1236T in exon 12 and C3435T (P < 0.001), but not between C1236T and G2677T/A (P > 0.05). D′ value ranged from 0 to 1. A D′ value of 0 denotes complete linkage equilibrium, whereas a value 1 denotes complete LD.
Table 2.
Influence of individual SNPs on valacyclovir pharmacokinetics
The results of the investigated genetic variants in a small sample for pharmacokinetic study were consistent with previous findings in a large (n = 200) sample, including analyses of genotypic and allelic frequencies of three SNPs, Hardy–Weinberg equilibrium and LD. Volunteers were grouped according to SNP genotype (Table 3) to explore the influence of three SNPs on valacyclovir pharmacokinetics of the absorptive phase. After administration of a single oral dose of 600 mg valacyclovir, a significant difference of AUC0–∞ was observed among different genotypes at position 2677. AUC0–∞ for the 2677TA genotype was 17.45 ± 2.40 µg × h/ml, which was much higher compared with the 2677GG, GA and TT genotypes of 10.44 ± 1.00, 11.84 ± 2.83 and 11.34 ± 2.32 µg × h/ml, respectively (P < 0.05). Figure 1 also shows the difference in AUC0–∞ between different genotypes at position 2677. The group with 2677AA was not investigated due to its low frequency.
Table 3.
Effect of individual single nucleotide polymorphisms on valacyclovir pharmacokinetic parameters in 24 healthy male volunteers
| Cmax (µg ml−1) | AUC0–1.5 h (µg × h/ml) | AUC0–∞ (µg × h/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| Location | Genotype | n | Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences |
| Exon12 (C1236T) | CC | 3 | 2.86 ± 1.44 | 2.41 ± 1.51 | 10.98 ± 3.35 | |||
| CT | 12 | 4.13 ± 1.51 | (−0.48, 3.00) | 3.59 ± 1.43 | (−0.44, 2.79) | 13.82 ± 3.84 | (−1.69, 7.37) | |
| TT | 9 | 3.12 ± 0.86 | (−1.54, 2.05) | 2.71 ± 0.66 | (−1.38, 1.96) | 11.75 ± 2.61 | (−3.91, 5.45) | |
| Exon21 (G2677T/A) | GG | 3 | 2.96 ± 0.37 | 2.81 ± 0.60 | 10.44 ± 1.00** | |||
| GT | 9 | 3.87 ± 1.77 | (−0.97, 2.79) | 3.34 ± 1.54 | (−1.24, 2.30) | 13.20 ± 3.73 | (−1.49, 7.00) | |
| GA | 4 | 3.92 ± 1.07 | (−1.19, 3.12) | 3.20 ± 1.12 | (−1.64, 2.41) | 11.84 ± 2.83** | (−3.47, 6.26) | |
| TT | 4 | 2.92 ± 0.67 | (−2.20, 2.12) | 2.47 ± 0.74 | (−2.37, 1.68) | 11.34 ± 2.32** | (−3.97, 5.76) | |
| TA | 3 | 4.47 ± 1.10 | (−0.80, 3.82) | 3.99 ± 1.36 | (−0.99, 3.35) | 17.45 ± 2.40 | (1.81, 12.20) | |
| AA | 1 | 1.78 | – | 1.51 | – | 9.50 | – | |
| Exon26 (C3435T) | CC | 10 | 3.37 ± 0.95 | 2.88 ± 0.94 | 11.85 ± 2.60 | |||
| CT | 10 | 4.09 ± 1.76 | (−0.53, 1.96) | 3.60 ± 1.56 | (−0.42, 1.86) | 14.08 ± 4.22 | (−0.89, 5.36) | |
| TT | 4 | 2.92 ± 0.67 | (−2.10, 1.19) | 2.47 ± 0.74 | (−1.92, 1.09) | 11.34 ± 2.32 | (−4.64, 3.63) | |
Cmax, peak plasma concentration; AUC0–1.5 h, area under the serum concentration–time curve from time zero to 1.5 h; AUC0–∞, area under serum concentration–time curve from time zero to infinity; n, number of subjects; 95% CI of differences, compared with wild-type groups (1236CC, 2677GG, 3435CC). Data are expressed as arithmetic mean ± standard deviation (SD). **Statistically significant difference (P < 0.05) compared with 2677TA group.
Figure 1.
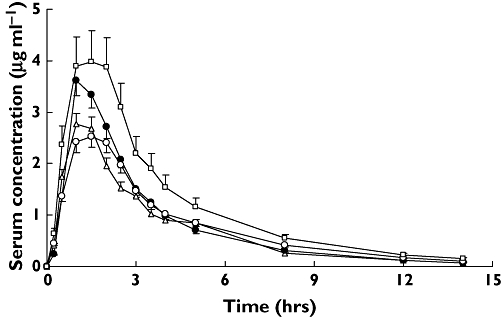
Comparison of serum concentration–time profiles of acyclovir after a single oral dose of 600 mg valacyclovir between groups with difference genotype in exon 21 G2677T/A of MDR1 gene. Values are mean ± SD. GG, (▵); GA, (•); TT, (○); TA, (□)
Influence of MDR1 genotypes and haplotypes on valacyclovir pharmacokinetics
Analysis was expanded on linked genotypes and haplotypes on the basis of the result of study of LD. Different allelic combinations of both variants of SNPs 1236 and 3435 can result in four possible haplotypes and nine possible genotypes (Table 4). Three haplotypes and six genotypes were detected in the 24 healthy male subjects. Comparisons among the five most common genotypes of our study demonstrated statistically significant differences in the AUC0–∞ of pharmacokinetic parameters of the absorptive phase (Table 5). AUC0–∞ ranged between 10.98 ± 3.35 and 16.07 ± 4.09 µg × h/ml (1.5-fold). Figure 2 demonstrates such a difference in terms of plasma concentration–time profiles of acyclovir. In addition, no significant differences were found for Cmax, AUC0–1.5 h or AUC0–∞ between carriers and noncarriers of different MDR1 haplotypes (P > 0.05, Table 6). Likewise, seven of 18 theoretically possible linked genotypes were found between exon 21 (G2677T/A) and exon 26 (C3435T). AUC0–∞ for the 2677TA/3435CT genotype was 17.45 ± 2.40 µg × h/ml, which was much higher compared with the 2677GA/3435CC, 2677GG/3435CC, 2677GT/3435CT and 2677TT/3435TT genotypes of 11.84 ± 2.83, 10.44 ± 1.00, 12.64 ± 4.09 and 11.34 ± 2.32 µg × h/ml, respectively (P < 0.05, Table 5). Figure 3 also describes the above difference. Moreover, haplotype analyses showed no statistical difference in pharmacokinetic parameters of absorptive phase between carriers and noncarriers of haplotype 11 (2677G/3435C), 22 (2677T/3435T) and 31 (2677A/3435C) (data not shown).
Table 5.
Comparison of pharmacokinetic parameters after a single oral administration of valacyclovir between groups with different linked genotype at positions 2677 vs. 3435 and 1236 vs. 3435 of MDR1 gene
| Cmax (µg ml−1) | AUC0–1.5 h (µg × h/ml) | AUC0–∞ (µg × h/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined genotype | n | Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences | ||
| 2677 vs.3435 | GG | CC | 3 | 2.96 ± 0.37 | 2.81 ± 0.60 | 10.44 ± 1.00* | |||
| AA | CC | 1 | 1.78 | – | 1.51 | – | 9.50 | – | |
| GA | CC | 4 | 3.92 ± 1.07 | (−1.34, 3.27) | 3.20 ± 1.12 | (−1.73, 2.50) | 11.84 ± 2.83* | (−.3.63, 6.42) | |
| GT | CC | 2 | 3.67 ± 0.10 | – | 3.04 ± 1.08 | – | 15.14 ± 1.27 | – | |
| GT | CT | 7 | 3.92 ± 2.04 | (−1.12, 3.05) | 3.43 ± 1.70 | (−1.29, 2.53) | 12.64 ± 4.09* | (−2.34, 6.74) | |
| TA | CT | 3 | 4.46 ± 1.10 | (−0.95, 3.97) | 3.99 ± 1.36 | (−1.08, 3.44) | 17.45 ± 2.40 | (1.64, 12.37) | |
| TT | TT | 4 | 2.92 ± 0.67 | (−2.34, 2.27) | 2.46 ± 0.74 | (−2.46, 1.77) | 11.34 ± 2.32* | (−4.13, 5.92) | |
| 1236 vs. 3435 | CC | CC | 3 | 2.86 ± 1.44 | 2.41 ± 1.51 | 10.98 ± 3.35** | |||
| CT | CC | 6 | 3.56 ± 0.76 | (−1.25, 2.63) | 2.96 ± 0.62 | (−1.18, 2.27) | 11.58 ± 1.90** | (−3.80, 4.99) | |
| CT | CT | 6 | 4.70 ± 1.92 | (−0.11, 3.78) | 4.22 ± 1.77 | (0.08, 3.53) | 16.07 ± 4.09 | (0.69, 9.48) | |
| TT | CC | 1 | 3.75 | – | 3.80 | – | 16.04 | – | |
| TT | CT | 4 | 3.17 ± 1.15 | (−1.79, 2.40) | 2.67 ± 0.38 | (−1.60, 2.12) | 11.10 ± 2.41** | (−4.63, 4.86) | |
| TT | TT | 4 | 2.92 ± 0.67 | (−2.05, 2.15) | 2.46 ± 0.74 | (−1.81, 1.91) | 11.34 ± 2.32** | (−4.40, 5.10) | |
95%CI of differences, compared with 2677GG/3435CC group, 1236CC/3435CC group. Data are expressed as arithmetic mean ± standard deviation (SD).
*Statistically significant difference (P < 0.05) compared with 2677TA/3435CT group.
**Statistically significant difference (P < 0.05) compared with 1236CT/3435CT group.
Figure 2.
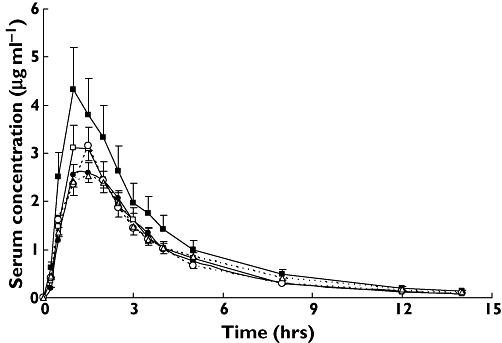
Comparison of serum concentration–time profiles of acyclovir after a single oral dose of 600 mg valacyclovir between groups with different linked genotype at positions 1236 and 3435 of MDR1 gene. Values are mean ± SD. 1236CC/3435CC, (•); 1236CT/3435CC, (□); 1236CT/3435CT, (▪); 1236TT/3435CT, (○); 1236TT/3435TT, (▵)
Table 6.
Comparisons of valacyclovir pharmacokinetic parameters between subjects grouped according to MDR1 haplotype 11, 12 and 21 deduced from single nucleotide polymorphism C1236T and C3435T
| Cmax (µg ml−1) | AUC0–1.5 h (µg × h/ml) | AUC0–∞ (µg × h/ml) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences | Mean ± SD | 95% CI of differences | |
| Haplotype 11 | ||||||
| Carrier (n = 11) | 3.43 ± 0.86 | 2.93 ± 0.58 | 11.81 ± 2.36 | |||
| Noncarrier (n = 13) | 3.73 ± 1.69 | (−1.46, 0.88) | 3.26 ± 1.63 | (−1.41, 0.75) | 13.44 ± 4.08 | (−4.53, 1.26) |
| Haplotype 12 | ||||||
| Carrier (n = 14) | 3.75 ± 1.60 | 3.27 ± 1.44 | 13.30 ± 3.90 | |||
| Noncarrier (n = 10) | 3.37 ± 0.95 | (−0.79, 1.56) | 2.88 ± 0.94 | (−0.69, 1.48) | 11.85 ± 2.60 | (−1.49, 4.40) |
| Haplotype 21 | ||||||
| Carrier (n = 15) | 3.87 ± 1.54 | 3.35 ± 1.47 | 13.26 ± 3.82 | |||
| Noncarrier (n = 9) | 3.12 ± 0.86 | (−0.41, 1.92) | 2.71 ± 0.66 | (−0.43, 1.73) | 11.75 ± 2.61 | (−1.49, 4.50) |
Carriers are endowed with at least one respective haplotype. Data are means ± SD. Mann–Whitney test was used for comparisons within haplotype groups.
Figure 3.
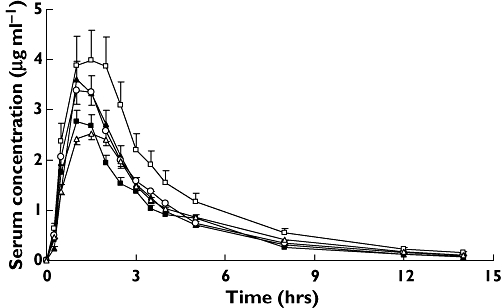
Comparison of serum concentration–time profiles of acyclovir after a single oral dose of 600 mg valacyclovir between groups with different linked genotype at positions 2677 and 3435 of MDR1 gene. Values are mean ± SD. 2677GA/3435CC, (▴); 2677GG/3435CC, (▪); 2677GT/3435CT, (○); 2677TA/3435CT, (□); 2677TT/3435TT, (▵)
Discussion
Valacyclovir is the prodrug of acyclovir and is rapidly and extensively converted to acyclovir after oral administration. The resulting acyclovir bioavailability is three to five times more than that of oral acyclovir [21, 22]. Accordingly, the plasma concentration of acyclovir was determined during the pharmacokinetic study of valacyclovir. Since acyclovir is primarily eliminated by the kidney, plasma concentration exceeding therapeutic range may lead to serious neurological toxicity and impaired renal function, whereas low plasma concentration may cause failure of treatment of patients with herpes simplex and herpes zoster [23, 24]. Pharmacokinetic tests have revealed significant individual differences in human [16]. Therefore, it is important to find the factors causing such a difference, which may promote individualized treatment of valacyclovir and enhance safety and efficacy.
In this study, pharmacokinetic parameters of absorptive phase were chosen to assess primarily the influence of MDR1 SNPs on absorption after a single oral dose of valacyclovir, including Cmax, AUC0–1.5 h and AUC0–∞. The result of the present study indicates that SNP at position 2677 leads to a significant difference of AUC0–∞. Meanwhile, it was demonstrated that such a significant difference of AUC0–∞ was observed during analyses based on linked genotypes at position 2677 vs. 3435 and 1236 vs. 3435, whereas haplotype analyses showed no statistical difference in any pharmacokinetic parameter between carriers and noncarriers. For the study of drug absorption, the use of AUC0–∞ can give erroneous and exaggerated results because the area also includes the distribution phase, elimination phase or recycling besides the absorption phase. Likewise, it is well known that Cmax also has serious shortcomings as an indirect measure of rate of drug absorption. However, it has been reported that partial AUC from zero to tmax of the test or reference formulation (AUCp) had greater statistical power than Cmax and AUC0–∞ at detecting the difference in rate of absorption [25, 26]. Thus, this study could not draw any conclusion on the influence of individual SNPs at position 2677 and linked genotypes between G2677T/A, C1236T and C3435T on absorption of valacyclovir on the basis of a single statistical difference of AUC0–∞.
In contrast to previously reported results [16], our results also indicate that there was a large variation in valacyclovir pharmacokinetics within every subject. Coefficient of variation of Cmax, AUC0–1.5 h and AUC0–∞ were 38%, 40% and 27%, respectively. High variability showed that additional factors may influence the absorption of valacyclovir. Christopher et al. [16] have studied the correlation of pharmacokinetic parameters following oral valacyclovir or acyclovir administration with expression levels of intestinal genes in human. They observed highly positive and significant correlations with 4F2hc (activator of dibasic and neutral amino acid transport) and human oligopeptide transporter (HPT1) and that the highly negative correlations were observed with MRP2, CYP3A subfamily. Therefore, this high variability may be related to other transporters and metabolic enzyme. In order to determine the real reason for this individual difference of valacyclovir, more studies are necessary to understand the degree of impact of various known and unknown genes and their variants on absorption of valacyclovir.
In summary, this present study has indicated significant linkage between the MDR1 SNPs at positions 2677 or 1236 and 3435 in the Chinese Han ethnic population and no significant effect of MDR1 SNPs in exon 12 (C1236T), exon 21 (G2677T/A) or exon 26 (C3435T) on absorption of valacyclovir.
Acknowledgments
The authors thank Bin Long and Ling Zhang at West China School of Forensic Medicine Sichuan University for their directions in experimental technique.
Competing interest: None to declare.
REFERENCES
- 1.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product p-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–87. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- 3.Borst P, Schinkel AH, Smit JJ, Wagenaar E, Van Deemter L, Smith AJ, Eijdems EW, Baas F, Zaman GJ. Classical and novel forms of multidrug resistance and the physiological functions of P-glycoprotein in mammals. Pharmacol Ther. 1993;60:289–99. doi: 10.1016/0163-7258(93)90011-2. [DOI] [PubMed] [Google Scholar]
- 4.MacFarland A, Abramovich DR, Ewen SW, Pearson CK. Stage-specific distribution of P-glycoprotein in first-trimester and full-term human placenta. Histochem J. 1994;26:417–23. doi: 10.1007/BF00160054. [DOI] [PubMed] [Google Scholar]
- 5.Fromm MF. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol Sci. 2004;25:423–9. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmeyer S, Burk O, Von-Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameyaw MM, Regateiro F, Li T, Liu XH, Tariq M, Mobarek A, Thornton N, Folayan GO, Githang'a J, Indalo A, Ofori-Adjei D, Price-Evans DA, Mcleod HL. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–21. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 9.Balram C, Sharma A, Sivathasan C, Edmund J, Lee D. Frequency of C3435T single nucleotide MDR1 genetic polymorphism in an Asian population: phenotypic–genotypic correlates. Br J Clin Pharmacol. 2003;56:78–83. doi: 10.1046/j.1365-2125.2003.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–85. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 11.Tang K, Ngoi SM, Gwee PC, Chua JM, Lee EJ, Chong SS, Lee CG. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 2002;12:437–50. doi: 10.1097/00008571-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, Young JD, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, Cha IJ, Shin JG. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005;78:191–201. doi: 10.1016/j.clpt.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WX, Chen GL, Zhang W, Tan ZR, Liu J, Zhou G, Hu DL, Zhou HH. MDR1 genotype does not influence the absorption of a single oral dose of 100 mg talinolol in healthy Chinese males. Clin Chim Acta. 2005;359:46–52. doi: 10.1016/j.cccn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234:4–33. doi: 10.1016/j.canlet.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Landowski CP, Sun D, Foster DR, Menon SS, Barnett JL, Welage LS, Ramachandran C, Amidon GL. Gene expression in the human intestine and correlation with oral valacyclovir pharmacokinetic parameters. J Pharmacol Exp Ther. 2003;306:778–86. doi: 10.1124/jpet.103.051011. [DOI] [PubMed] [Google Scholar]
- 17.Palmberger TF, Hombach J, Bernkop-Schnürch A. Thiolated chitosan: development and in vitro evaluation of an oral delivery system for acyclovir. Int J Pharm. 2008;348:54–60. doi: 10.1016/j.ijpharm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Sunder-Plassmann R, Rieger S, Endler G, Brunner M, Müller M, Mannhalter C. Simultaneous analysis of MDR1 C3435T, G2677T/A, and C1236T genotypes by multiplexed mutagenically separated PCR. Clin Chem Lab Med. 2005;43:192–4. doi: 10.1515/CCLM.2005.032. [DOI] [PubMed] [Google Scholar]
- 19.Johne A, Köpke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, Eichelbaum M, Brockmöller J, Cascorbi I, Roots I. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–94. doi: 10.1067/mcp.2002.129196. [DOI] [PubMed] [Google Scholar]
- 20.Mai I, Perloff ES, Bauer S, Goldammer M, Johne A, Filler G, Budde K, Roots L. MDR1 haplotypes derived from exons 21 and 26 do not affect the steady-state pharmacokinetics of tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2004;58:548–53. doi: 10.1111/j.1365-2125.2004.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beutner KR. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antivir Res. 1995;28:281–90. doi: 10.1016/0166-3542(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 22.Miserocchi E, Modorati G, Galli L, Rama P. Efficacy of valacyclovir vs acyclovir for the prevention of recurrent herpes simplex virus eye disease: a pilot study. Am J Ophthalmol. 2007;144:547–51. doi: 10.1016/j.ajo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Peyrière H, Branger B, Bengler C, Vécina F, Pinzani V, Hillaire-Buys D, Blayac JP. Neurotoxicity induced by valacyclovir in three patients with impaired renal function, overdose related to the improvement of the bioavailability of the drug? Rev Med Intern. 2001;22:297–33. doi: 10.1016/s0248-8663(00)00332-5. [DOI] [PubMed] [Google Scholar]
- 24.Bardin C, Hamel A, Pichard A, Havard L. Relation between concentration and toxicity of the anti-herpesvirus drugs. Pharmacocinétique des médicaments anti-infectieux. 2004;365:53–7. [Google Scholar]
- 25.Duquesnoy C, Lacey LF, Keene ON, Bye A. Evaluation of different partial AUCs as indirect measures of rate of drug absorption in comparative pharmacokinetic studies. Eur J Pharm Sci. 1998;6:259–63. doi: 10.1016/s0928-0987(97)10023-9. [DOI] [PubMed] [Google Scholar]
- 26.Endrenyi L, Csizmadia F, Tothfalusi L, Chen ML. Metrics comparing simulated early concentration profile for the determination of bioequivalence. Pharm Res. 1998;15:1292–9. doi: 10.1023/a:1011912512966. [DOI] [PubMed] [Google Scholar]


