Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Ketoconazole is a potent inhibitor of the cytochrome P450 3A4 enzyme system.
Co-administration of ketoconazole and drugs primarily metabolized by the cytochrome P450 3A4 enzyme system may result in increased plasma concentrations of the drugs, which could increase or prolong both therapeutic and adverse effects.
Therefore, unless otherwise specified, appropriate dosage adjustments may be necessary.
WHAT THIS PAPER ADDS
The current study was conducted to determine the extent of interaction between the potent CYP3A inhibitor, ketoconazole, and the CYP 3A substrate, darunavir (given alone and with low-dose ritonavir).
This information provides data on the pharmacokinetic boosting ability of ketoconazole and serves as important guidance to HIV-infected patients and their treating physicians with regard to appropriate (co-)administration of darunavir/ritonavir and ketoconazole.
AIMS
To investigate the interaction between ketoconazole and darunavir (alone and in combination with low-dose ritonavir), in HIV–healthy volunteers.
Methods
Volunteers received darunavir 400 mg bid and darunavir 400 mg bid plus ketoconazole 200 mg bid, in two sessions (Panel 1), or darunavir/ritonavir 400/100 mg bid, ketoconazole 200 mg bid and darunavir/ritonavir 400/100 mg bid plus ketoconazole 200 mg bid, over three sessions (Panel 2). Treatments were administered with food for 6 days. Steady-state pharmacokinetics following the morning dose on day 7 were compared between treatments. Short-term safety and tolerability were assessed.
Results
Based on least square means ratios (90% confidence intervals),during darunavir and ketoconazole co-administration, darunavir area under the curve (AUC12h), maximum plasma concentration (Cmax) and minimum plasma concentration (Cmin) increased by 155% (80, 261), 78% (28, 147) and 179% (58, 393), respectively, compared with treatment with darunavir alone. Darunavir AUC12h, Cmax and Cmin increased by 42% (23, 65), 21% (4, 40) and 73% (39, 114), respectively, during darunavir/ritonavir and ketoconazole co-administration, relative to darunavir/ritonavir treatment. Ketoconazole pharmacokinetics was unchanged by co-administration with darunavir alone. Ketoconazole AUC12h, Cmax and Cmin increased by 212% (165, 268), 111% (81, 144) and 868% (544, 1355), respectively, during co-administration with darunavir/ritonavir compared with ketoconazole alone.
Conclusions
The increase in darunavir exposure by ketoconazole was lower than that observed previously with ritonavir. A maximum ketoconazole dose of 200 mg day−1 is recommended if used concomitantly with darunavir/ritonavir, with no dose adjustments for darunavir/ritonavir.
Keywords: darunavir, ketoconazole, pharmacokinetic interaction, ritonavir, TMC114
Introduction
As a consequence of the increase in transmission of HIV strains with resistance to currently available antiretroviral therapy including protease inhibitors (PIs) [1], limitations of treatment options exist [2].
Darunavir (TMC114) is a new HIV PI, with high levels of antiviral activity against wild-type virus and strains with phenotypic resistance to other currently approved PIs [3]. In common with other PIs, the metabolism of darunavir is cytochrome P450 (CYP) 3A4-dependent [4], and in clinical practice darunavir is co-administered with low-dose ritonavir [5, 6], an inhibitor of CYP3A4. After 48 weeks of treatment in two Phase IIb clinical trials (POWER 1 and 2), HIV-infected treatment-experienced patients receiving ritonavir-boosted darunavir had statistically significantly higher virological and immunological responses than those receiving investigator-selected, currently available PIs, and darunavir/ritonavir was associated with a favourable safety and tolerability profile [7]. Darunavir/ritonavir at a dose of 600/100 mg twice daily (bid) has recently been approved in several countries, including the USA [6] and Europe [8]. Darunavir 800/100 mg once daily (qd) is currently being evaluated in treatment-naive HIV-naive patients (clinicaltrials.gov reference NCT00258557).
Given the length of time over which HIV-infected patients receive antiretroviral therapy and the range of concomitant morbidities they may develop, investigation into potential interactions between antiretroviral drugs and other medications is essential for appropriate recommendations to be made in clinical practice.
The azole-based antifungal ketoconazole is commonly co-administered with antiretroviral therapy in HIV-infected patients. Ketoconazole is prescribed at an oral dosage of 200–400 mg day−1, for the treatment of severe systemic fungal infections, such as Candida albicans and histoplasmosis. Ketoconazole competitively inhibits the CYP3A4 isoenzyme [9] and has been shown to increase the plasma concentrations of drugs with CYP3A4-dependent metabolism, such as saquinavir [10] and midazolam [11]. Ketoconazole has also been reported to increase steady-state concentrations of both ritonavir and saquinavir, despite the strong inhibitory effect of ritonavir on CYP3A4 metabolism [12], which may suggest that ketoconazole and ritonavir increase exposure to certain drugs by independent mechanisms. In addition, ketoconazole is a substrate for CYP3A4 metabolism, and co-administration with CYP3A4 inhibitors can increase exposure to ketoconazole [13].
This study was designed to assess the potential for interaction between darunavir (with and without low-dose ritonavir) and ketoconazole in HIV–, healthy volunteers.
Methods
Study design
HIV–, healthy men and postmenopausal women aged between 18 and 55 years were eligible for this Phase I, open-label, controlled, randomized, crossover pharmacokinetic interaction study. Individuals testing positive for HIV at screening and those suffering from a clinically significant medical condition were excluded. Concomitant medications were not permitted, with the exception of paracetamol. The study protocol was conducted at C&T Paradigm (Antwerp, Belgium), reviewed and approved by the institutional ethics committee, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers.
Volunteers were randomized into two panels. Panel 1 (n = 8) received darunavir 400 mg bid, and darunavir 400 mg bid plus ketoconazole 200 mg bid, over two separate sessions. Panel 2 (n = 18) received darunavir/ritonavir 400/100 mg bid, ketoconazole 200 mg bid and darunavir/ritonavir 400/100 mg bid plus ketoconazole 200 mg bid, over three separate sessions. Each session lasted for 6 days with a morning dose on day 7, prior to pharmacokinetic assessment. Since the presence of food increases exposure to darunavir, all treatments were administered with food at the clinical trial unit for the first dose and either at home or at the trial unit for subsequent doses [15]. The standard breakfast consisted of four slices of bread, one slice of ham, one slice of cheese, butter, jelly and two cups of coffee or tea with milk and/or sugar. Each session was separated by a wash-out period of at least 7 days. Drug intake was directly observed and timed at the clinical trial unit, with adherence to therapy at home monitored using pill diaries and pill counts. The net effect of co-administration of low-dose ritonavir with darunavir is CYP3A inhibition, and no inductive effects were expected following the ritonavir dosing regimen used.
No formal sample size calculation was performed; the first part of the trial (involving Panel 1) was exploratory, and therefore fewer volunteers were selected than for the second part of the trial (involving Panel 2), since the results of the second part were intended to be used for the dose recommendations for darunavir/ritonavir and ketoconazole co-administration. The darunavir/ritonavir 400/100 mg bid dose was selected for use in this study as this dose is generally considered to be safe and well tolerated in healthy volunteers based on a pharmacokinetic study at a range of darunavir/ritonavir doses [14].
Pharmacokinetic blood sampling and bioanalysis
Venous blood samples (5 ml) were collected predose and 1, 2, 3, 4, 5, 6, 9 and 12 h post dose on day 7 of drug intake for all treatments. Blood was centrifuged (2000 g; 15 min) as soon as possible after collection, and plasma was isolated and stored (−18°C) until analysis. Plasma concentrations of darunavir, ritonavir and ketoconazole were determined by validated liquid chromatography mass spectrometry/mass spectrometry methods. The lower limits of quantification were 10.0, 5.0 and 20.0 ng ml−1 for darunavir, ritonavir and ketoconazole, respectively [17]. The precision and accuracy of the analytical method for plasma darunavir and ritonavir were within the acceptable limit of 15%, with coefficients of variation for the low-, medium- and high-quality control samples being <12%. The precision and accuracy of the analytical method for plasma ketoconazole was within the acceptable limit of 15%, with coefficients of variation for the low-, medium- and high-quality control samples being <5.5%.
Safety and tolerability
Short-term safety and tolerability were monitored throughout the study. All laboratory abnormalities were graded according to the AIDS Clinical Trials Group (ACTG) severity grading scale; World Health Organization toxicity grades were used for the tests for which no ACTG grades existed. Clinical adverse events, whether related to study therapy or not, were monitored and recorded over the study duration. Electrocardiogram parameters were assessed at screening, on days 1 and 7 of each session and at follow-up.
Statistical methods
Descriptive statistics were calculated for the plasma concentrations of darunavir, ritonavir and ketoconazole. The pharmacokinetic parameters calculated were: minimum (Cmin) and maximum (Cmax) plasma concentrations, area under the curve (AUC, calculated by linear trapezoidal summation) from administration until 12 h post dose (AUC12h) and time to maximum plasma concentration (tmax), using WinNonlin Professional(tm) (version 3.3; Pharsight Corporation, Mountain View, CA, USA). Comparison of the pharmacokinetic parameters Cmin, Cmax, AUC12h of darunavir alone and darunavir/ritonavir, with and without concomitant ketoconazole, and of ketoconazole with and without concomitant darunavir alone or darunavir/ritonavir, was performed using linear mixed-effect modelling. The least squares mean (LSM) ratios were calculated individually and 90% confidence intervals around the LSM ratio were generated, with treatment of darunavir alone, darunavir/ritonavir alone, or ketoconazole alone as references. Subjects with paired data were included in appropriate pair-wise comparisons. The nonparametric t-test (Koch) was used to compare tmax values.
Results
Study participants
A total of 39 volunteers were screened; 26 were enrolled in the study, with eight assigned to Panel 1 and 18 assigned to Panel 2. The median age of the enrolled volunteers was 41 years (range 27–55 years); the majority (73%) were male and all were of Caucasian origin. Twenty-one of the 26 volunteers completed the study. One volunteer tested positive for hepatitis C virus and benzodiazepine use at screening; these were considered major protocol violations and the volunteer was excluded from the pharmacokinetic analysis.
Pharmacokinetics
Based on LSM ratios, when ketoconazole was added to treatment with darunavir alone, exposure to darunavir (AUC12h) increased by 155% (Table 1; Figure 1). Darunavir Cmax and Cmin increased by 78 and 179%, respectively, compared with treatment with darunavir alone. Tmax ranged from 2.0 to 5.0 h for treatment with darunavir alone and from 2.0 to 4.0 h following addition of ketoconazole.
Table 1.
Mean darunavir pharmacokinetic parameters on day 7 following treatment with darunavir 400 mg bid alone and in combination with ketoconazole 200 mg bid
| Parameter (mean ± SD) n | Darunavir (reference) 7 | Darunavir + ketoconazole (test) 6 | LSM ratio (90% CI) 6 |
|---|---|---|---|
| Cmin (ng ml−1) | 68.2 ± 26.8 | 235 ± 154 | 2.79 (1.58, 4.93) |
| Cmax (ng ml−1) | 1808 ± 871 | 2 788 ± 912 | 1.78 (1.28, 2.47) |
| AUC12h(ng h−1 ml−1) | 6478 ± 3341 | 15 505 ± 7 589 | 2.55 (1.80, 3.61) |
| tmax (h)* | 2.0 (2.0–5.0) | 3.0 (2.0–4.0) | – |
median (range).
Figure 1.

(a) Panel 1. Darunavir plasma profiles following darunavir (DRV) 400 mg bid (▴), and DRV 400 mg bid + ketoconazole (KTZ) 200 mg bid, (♦). (b) Panel 2. Darunavir plasma profiles following DRV/r 400/100 mg bid (•), and DRV/r 400/100 mg bid + KTZ 200 mg bid, (▪)
Co-administration of darunavir/ritonavir and ketoconazole resulted in increases in darunavir AUC12h, Cmax and Cmin of 42, 21 and 73%, respectively, relative to treatment with darunavir/ritonavir alone (Table 2; Figure 1). Tmax ranged from 1.0 to 5.0 h for treatment with darunavir/ritonavir alone and 0.0 to 0.6 h following addition of ketoconazole.
Table 2.
Mean darunavir pharmacokinetic parameters on day 7 following treatment with darunavir/ritonavir 400/100 mg bid alone and in combination with ketoconazole 200 mg bid
| Parameter (mean ± SD) n | Darunavir/ritonavir (reference) 14 | Darunavir/ritonavir + ketoconazole (test) 17 | LSM ratio (90% CI) 14 |
|---|---|---|---|
| Cmin (ng ml−1) | 2 209 ± 1 143 | 3 582 ± 966 | 1.73 (1.39, 2.14) |
| Cmax (ng ml−1) | 5 209 ± 1 625 | 6 934 ± 1 445 | 1.21 (1.04, 1.40) |
| AUC12h(ng h−1 ml−1) | 42 707 ± 16 234 | 60 690 ± 14 174 | 1.42 (1.23, 1.65) |
| tmax (h)* | 2.5 (1.0–5.0) | 3.0 (0.0–6.0) | – |
median (range).
When co-administered with darunavir alone, the pharmacokinetic parameters of ketoconazole were unchanged (Table 3; Figure 2). However, when ketoconazole was administered concurrently with darunavir/ritonavir, ketoconazole AUC12h, Cmax and Cmin increased by 212, 111 and 868%, respectively, relative to treatment with ketoconazole alone.
Table 3.
Mean ketoconazole pharmacokinetic parameters on day 7 following treatment with ketoconazole 200 mg bid alone and in combination with darunavir 400 mg bid and darunavir/ritonavir 400/100 mg bid
| Parameter (mean ± SD) n | Ketoconazole + darunavir 6 | Ketoconazole (reference) 15 | Ketoconazole + darunavir/ritonavir (test) 17 | LSM ratio: ketoconazole + darunavir/ritonavir vs. ketoconazole alone (90% CI) 15 |
|---|---|---|---|---|
| Cmin (ng ml−1) | 692 ± 565 | 831 ± 794 | 5 354 ± 1 627 | 9.68 (6.44, 14.55) |
| Cmax (ng ml−1) | 5 458 ± 747 | 5 311 ± 2 018 | 10 501 ± 2 524 | 2.11 (1.81, 2.44) |
| AUC12h (ng h−1 ml−1) | 35 292 ± 7 209 | 33 098 ± 16 014 | 94 343 ± 25 129 | 3.12 (2.65, 3.68) |
| tmax (h)* | 3.0 (2.0–3.0) | 3.0 (1.0–4.0) | 3.0 (1.0–5.0) | – |
median (range).
Figure 2.
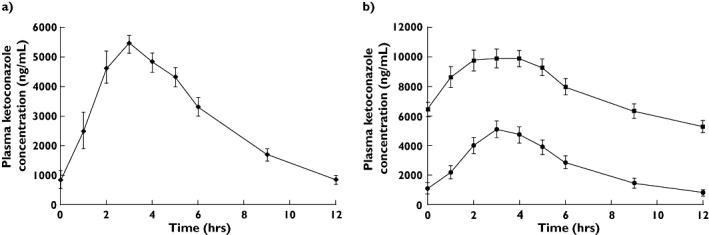
(a) Panel 1. Ketoconazole plasma profile following darunavir (DRV) 400 mg bid + ketoconazole (KTZ) 200 mg bid, (♦). (b) Panel 2. Ketoconazole plasma profile following KTZ 200 mg bid (•), and DRV/r 400/100 mg bid + KTZ 200 mg bid, (▪)
Plasma concentration–time profiles of ritonavir were comparable between treatment with darunavir alone and darunavir in combination with ketoconazole, indicating that systemic exposure to ritonavir was similar between treatments. Mean ritonavir AUC12h, Cmax and Cmin during co-administration with darunavir alone were 7556 ng h−1 ml−1, 1232 ng ml−1 and 215 ng ml−1, respectively. When ketoconazole was added to darunavir/ritonavir treatment, mean ritonavir AUC12h, Cmax and Cmin were 8515 ng h−1 ml−1, 1368 ng ml−1 and 287 ng ml−1, respectively. Median tmax was 4.0 h for both treatments.
Safety and tolerability
The incidence of adverse events was comparable between treatments, and the majority were grade 1 or 2 in severity.
Four volunteers discontinued the study due to adverse events, including one case each of grade 4 maculopapular rash with an oral mucosal disorder (reported 2 days after the last intake of darunavir in Panel 1), grade 3 increased pancreatic enzymes (reported during screening), grade 2 localized urticaria and grade 3 increased lipase (both reported after 7 days of combined darunavir/ritonavir and ketoconazole treatment in Panel 2). Except for the case of a grade 3 increase in pancreatic enzymes reported during screening, all adverse events leading to discontinuation were considered possibly related to study medication. All four cases resolved without sequelae. Rash and lipase changes, although not treatment-limiting, have been observed previously in patient studies using these doses of darunavir/ritonavir. No clinically relevant changes in laboratory or cardiovascular parameters were observed over the study duration.
Discussion
Co-administration of ketoconazole with darunavir alone increased exposure to darunavir. The magnitude of the increase in exposure to darunavir by ketoconazole (1.55-fold) was lower than that previously observed with low-dose ritonavir (14-fold) [16]. Co-administration of ketoconazole and darunavir alone did not affect ketoconazole pharmacokinetics.
The addition of ketoconazole to darunavir/ritonavir treatment also increased darunavir exposure (LSM ratio of 1.42) relative to treatment with darunavir/ritonavir alone. Co-administration of darunavir/ritonavir with ketoconazole increased exposure to ketoconazole relative to treatment with ketoconazole alone (LSM ratio of 3.12). Systemic exposure to ritonavir was comparable between treatments.
Increases in exposure to ketoconazole have also been observed after co-administration with either ritonavir alone (3.4-fold increase in ketoconazole AUC) [18] or other ritonavir-boosted HIV PIs such as lopinavir/ritonavir (ketoconazole LSM ratio for AUC was 3.04 after co-administration) [13]. Smaller increases in ketoconazole exposure have also been reported with unboosted PIs, such as fosamprenavir (ketoconazole AUC LSM ratio of 2.69 after co-administration) [19]. This indicates that the ritonavir component of the PI combination is likely to be the main factor in increased ketoconazole exposure. Of the currently available PIs, ritonavir has the strongest inhibitory effect on the CYP3A4 isoenzyme and considerably affects the pharmacokinetics of other CYP3A4-metabolized drugs [20].
In this study, ketoconazole increased exposure to darunavir by 42%, which is comparable to its effects on indinavir (62%) [21] and saquinavir/ritonavir (37%) [12]. Other azole drugs, such as itraconazole and fluconazole, have also demonstrated effects on saquinavir exposure [22, 23]. However, little or no effect of ketoconazole co-administration has been reported on exposure to ritonavir [18], lopinavir/ritonavir [13], atazanavir [24] or amprenavir [19]. In light of the increase in ketoconazole exposure in the presence of ritonavir-boosted darunavir, when these drugs are co-administered, no more than 200 mg day−1 of ketoconazole is recommended. This recommendation is consistent with dosing recommendations for ketoconazole in the presence of other ritonavir-boosted PIs such as lopinavir/ritonavir.
Azole antifungals are known to inhibit cytochrome P450 enzymes at hepatic and extrahepatic sites, thus decreasing the first-pass metabolism and clearance of HIV PIs. Furthermore, both ketoconazole and PIs are substrates of multidrug-transporter proteins, such as P-glycoprotein (P-gp) [25]. Therefore, the mechanisms by which ketoconazole could increase the exposure to darunavir (and vice versa) are inhibition of hepatic CYP3A resulting in reduction in systemic metabolism, inhibition of gastrointestinal CYP3A resulting in increased absorption, and inhibition of P-gp resulting in increased systemic bioavailability. The half-life of darunavir is 15 h in the presence of low-dose ritonavir [8]; however, as this study was not designed to allow accurate determination of darunavir half-life over the 12-h dosing interval, the observed pharmacokinetic interaction between darunavir/ritonavir and ketoconazole could result from a combination of all three mechanisms or from an effect on an individual mechanism.
Similar findings to those of this study are expected for the approved darunavir/ritonavir 600/100 mg bid dose and the 800/100 mg qd dose being evaluated in treatment-naive patients, as there is significant overlap in plasma darunavir concentrations at the 400/100 mg bid and 600/100 mg bid doses and a lack of dose proportionality in darunavir pharmacokinetics [4].
Based on the pharmacokinetic data demonstrated in this study, we conclude that, similar to the recommendations for other PIs, if ketoconazole is used in combination with darunavir/ritonavir, a maximum daily ketoconazole dose of 200 mg is recommended, with no dose adjustment for darunavir/ritonavir.
Acknowledgments
Some of the data were presented at the 44th Annual Meeting of the Infectious Diseases Society of America, 12–15 October 2006, Toronto, Ontario, Canada (Poster 960). Medical writing services were provided by Emily de Looze (Medical Writer), Gardiner-Caldwell Communications, UK. Financial assistance for this support was provided by Tibotec.
Competing interests
All authors are full-time employees of Tibotec or Janssen-Cilag (E.L.).
REFERENCES
- 1.Geretti AM. Epidemiology of antiretroviral drug resistance in drug-naive persons. Curr Opin Infect Dis. 2007;20:22–32. doi: 10.1097/QCO.0b013e328013caff. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, Thompson MA, Carpenter CC, Fischl MA, Gazzard BG, Gatell JM, Hirsch MS, Katzenstein DA, Richman DD, Vella S, Yeni PG, Volberding PA, International AIDS Soceity-USA panel Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. J Am Med Assoc. 2006;296:827–43. [Google Scholar]
- 3.De Meyer S, Azijn H, Surleraux D, Jochmans D, Tahri A, Pauwels R, Wigerinck P, de Béthune MP. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49:2314–21. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekar V, Spinosa-Guzman S, Lefebvre E, Hoetelmans R. Clinical pharmacology of TMC114 – a new HIV protease inhibitor. abstract TUPE0083]. 16th International AIDS Conference, Toronto, Canada.
- 5.Hoetelmans R, Van der Sandt I, De Pauw M, Struble K, Peeters M, Van der Geest R. TMC114, a next generation HIV protease inhibitor: pharmacokinetics and safety following oral administration of multiple doses with and without low doses of ritonavir in healthy volunteers. abstract 549]. 10th Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA.
- 6.Tibotec Inc. PREZISTATM (darunavir) Prescribing Information. October. Available at http://www.prezista.com (last accessed: 14 March 2007.
- 7.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, Wöhrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E, POWER 1 and 2 study groups Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 8.Tibotec Pharmaceuticals Ltd. PREZISTA (darunavir): summary of product characteristics (EMEA) Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/prezista/prezista.htm (last accessed: 23 March 2007.
- 9.Cupp-Vickery JR, Garcia C, Hofacre A, McGee-Estrada K. Ketoconazole-induced conformational changes in the active site of cytochrome P450eryF. J Mol Biol. 2001;311:101–10. doi: 10.1006/jmbi.2001.4803. [DOI] [PubMed] [Google Scholar]
- 10.Grub S, Bryson H, Goggin T, Ludin E, Jorga K. The interaction of saquinavir (soft gelatin capsule) with ketoconazole, erythromycin and rifampicin: comparison of the effect in healthy volunteers and in HIV-infected patients. Eur J Clin Pharmacol. 2001;57:115–21. doi: 10.1007/s002280100277. [DOI] [PubMed] [Google Scholar]
- 11.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:481–5. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 12.Khaliq Y, Gallicano K, Venance S, Kravchik S, Cameron DW. Effect of ketoconazole on ritonavir and saquinavir concentrations in plasma and cerebrospinal fluid from patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2000;68:637–46. doi: 10.1067/mcp.2000.112363. [DOI] [PubMed] [Google Scholar]
- 13.Abbott Laboratories. Kaletra (lopinavir/ritonavir) US Prescribing Information. Available at http://www.kaletra.com/ (last accessed: 21 February 2007.
- 14.Tibotec BVBA. Mechelen, Belgium: Tibotec BVBA; Data on file. [Google Scholar]
- 15.Sekar V, Kestens D, Spinosa-Guzman S, De Pauw M, De Paepe E, Vangeneugden T, Lefebvre E, Hoetelmans R. The effect of different meal types on the pharmacokinetics of darunavir (TMC114)/ritonavir in HIV-negative healthy volunteers. J Clin Pharmacol. 2007;47:479–84. doi: 10.1177/0091270006298603. [DOI] [PubMed] [Google Scholar]
- 16.Sekar V, Guzman S, Stevens T, De Paepe E, Lefebvre E, Hoetelmans R. Absolute bioavailability of TMC114, administered in the absence and presence of low-dose ritonavir. 7th International Workshop on Clinical Pharmacology of HIV Therapy, Lisbon, Portugal, 20–22 April 2006. Abstract P86.
- 17.Bouche MP, Michielsen L, Piot M, Timmerman P. Swift and simultaneous determination of darunavir (TMC114) and ritonavir in human plasma using LC-MS/MS. Abstract. TuP-042. Seventeenth Int. Mass Spectrom. Conf., Prague, Czech Republic, 27 August to 1 September 2006.
- 18.Abbott Laboratories. Norvir (ritonavir). US Prescribing Information. Available at http://www.norvir.com/ (last accessed: 21 February 2007.
- 19.GlaxoSmithKline and Vertex. Lexiva (fosamprenavir) US prescribing information. Available at http://www.lexiva.com/ (last accessed: 21 February 2007.
- 20.Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984–96. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- 21.Merck. Crixivan (indinavir) US Prescribing Information. Available at http://www.crixivan.com/ (last accessed: 21 February 2007.
- 22.Cardiello PG, Samor T, Burger D, Hoetelmans R, Mahanontharit A, Ruxrungtham K, Lange JM, Cooper DA, Phanuphak P. Pharmacokinetics of lower doses of saquinavir soft-gel caps (800 and 1200 mg twice daily) boosted with itraconazole in HIV-1-positive patients. Antivir Ther. 2003;8:245–9. [PubMed] [Google Scholar]
- 23.Koks CH, Crommentuyn KM, Hoetelmans RM, Burger DM, Koopmans PP, Mathôt RA, Mulder JW, Meenhorst PL, Beijnen JH. The effect of fluconazole on ritonavir and saquinavir pharmacokinetics in HIV-1-infected individuals. Br J Clin Pharmacol. 2001;51:631–5. doi: 10.1046/j.0306-5251.2001.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristol Myers Squibb Company. Reyataz (atazanavir) US Prescribing Information. Available at http://www.reyataz.com/ (last accessed: 21 February 2007.
- 25.Owen A, Hoggard P, Khoo SH. The role of drug transporters in HIV treatment failure. HIV/AIDS Curr Trends. 2002;8:11–12. [Google Scholar]


