Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Available evidence suggests that the M3 receptor on endothelial cells is responsible for acetylcholine (Ach)-dependent vasodilatation.
Data from human studies only provide indirect evidence for this, and results are more difficult to interpret.
WHAT THIS STUDY ADDS
This study used the new M3 receptor antagonist darifenacin as a pharmacological ‘tool’ to investigate the role of M3 receptor in the human forearm circulation.
It provides evidence for a major role for the M3 receptors in ACh-dependent vasodilatation in the forearm vascular bed.
AIMS
Acetylcholine (ACh) is a muscarinic agonist that causes receptor-mediated, endothelium-dependent vasodilatation in the forearm vasculature. Previous indirect evidence suggests this effect may be mediated by muscarinic M3 receptors. Darifenacin is a recently developed antimuscarinic drug with greater M3 selectivity, and our main objective was to investigate whether darifenacin affects dose-dependent vasodilatation to ACh in the forearm circulation.
METHODS
Healthy subjects were enrolled in two studies designed to assess the effects of atropine and darifenacin on the forearm blood flow (FBF) response to ACh.
RESULTS
In both studies ACh caused similar dose-dependent vasoditation in the forearm vasculature. In study I (5 subjects), the FBF response to ACh was largely attenuated by pretreatment with the nonselective muscarinic antagonist atropine. In study II (10 subjects), oral administration of darifenacin 15 mg for 1 week significantly reduced the FBF dose-dependent response to ACh 20 µg min−1 {mean difference from placebo 5.8 [95% confidence interval (CI) 3.1, 8.7] ml min−1 per 100 ml of forearm volume, P < 0.001} and to ACh 60 µg min−1[mean difference from placebo 5.9 (95% CI 3.1, 8.7) ml min−1 per 100 ml of forearm volume, P < 0.001]. After darifenacin, the AUC of change in FBF from baseline was reduced by almost 50% compared with placebo.
CONCLUSIONS
These results suggest that, in the forearm vasculature, muscarinic M3 receptors play a major role in ACh-induced endothelium-mediated vasodilatation.
Keywords: acetylcholine, atropine, darifenacin, forearm blood flow, muscarinic receptors
Introduction
Muscarinic receptors present on vascular endothelial cells are associated with acetylcholine (ACh)-dependent vasodilatation [1]. It is not clear which muscarinic (M) receptor subtype mediates the vasodilator effect of ACh, although previous studies have suggested that, in most vascular preparations from animals, the M3 receptor present on endothelial cells is responsible for this [2, 3]. With regard to human vessels, fewer data are available, sometimes with conflicting results, depending on the vascular bed investigated [4, 5].
Darifenacin is a recently introduced muscarinic antagonist with high affinity and selectivity for the M3 receptor [6] and is clinically used for the treatment of the symptoms of overactive bladder (OAB) [7]. We hypothesized that ACh acts primarily as an M3 agonist in the vascular endothelium of humans, and darifenacin would reduce or abolish ACh-mediated vasodilatation. The main aim of the study was to investigate whether darifenacin affects dose-dependent vasodilatation to ACh in the forearm circulation.
Methods
Subjects
Healthy, nonsmoking, men were recruited from the community and enrolled in the study. The study was undertaken with the approval of the local research ethics committee and in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject before entry into the study.
Measurements
Arterial vasodilatation in the forearm was measured as change in forearm blood flow (FBF) using the venous occlusion plethysmography technique, as previously described [8].
Drugs
Atropine, a nonselective muscarinic antagonist, was used in study I as an active control to confirm the muscarinic nature of the response to ACh. The M3 selective antagonist darifenacin was used in study II. Darifenacin (Emselex®) is clinically available as prolonged-release tablets at two doses, 7.5 and 15 mg. In our study the highest (15 mg) of the two recommended doses was used, and administered orally once daily. This dose was chosen on the basis of available evidence suggesting that improvement of OAB symptoms with darifenacin is dose-dependent [9]. This same dose has also been previously used in healthy volunteer studies to assess the pharmacodynamic effects of darifenacin, suggesting its M3 selectivity when compared with a selective M1/M2 antagonist [10]. There is no available evidence indicating that darifenacin has nonmuscarinic effects. Previous receptor-binding studies have analysed the affinity of darifenacin for the muscarinic receptors [antagonist binding affinity estimates (pKi values, nm): 7.8 for M1, 7.0 for M2, 8.8 for M3, 7.7 for M4 and 8.0 for M5]11] and also reported selectivity differences, showing that darifenacin is more selective for the M3 receptors, with a selectivity at M3 receptors that is 9.3 times that at M1 receptors, 59 times that at M2 or M4, and 12 times that at M5 receptors [12].
ACh was purchased from Clinalfa (Läufelfingen, Switzerland); atropine sulphate (Antigen Pharmaceuticals Ltd, Roscrea, Ireland) was purchased from the local hospital pharmacy. Both agents were used after dilution in saline (Baxter Healthcare Ltd, Thetford, UK). The doses of ACh and atropine used were selected on the basis of previous published data [13] and confirmed by pilot studies conducted in healthy volunteers (data not shown).
Study I
This study was undertaken in order to confirm the effect of the nonselective muscarinic receptor antagonist atropine on ACh-mediated vasodilatation in five healthy subjects. On the study day, after cannulation of the brachial artery and baseline FBF measurements, FBF responses to two doses of ACh (6 and 20 µg min−1) were assessed before and after atropine infusion (0.2 mg).
Study II
The effect of a 1-week period of treatment with darifenacin was investigated in a two-way, double-blind, randomized, placebo-controlled, crossover FBF study. Ten subjects were randomized to receive either placebo or darifenacin 15 mg orally once daily for 1 week. At day 8 of each arm of the study, separated by at least 1 week, subjects received a 6-min intra-arterial infusion of ACh at five consecutive and incremental doses (0.6, 2, 6, 20 and 60 µg min−1).
Statistical analysis
Results are expressed as mean ± SEM. FBF data are presented as changes in absolute blood flow (ml min−1 per 100 ml of forearm volume) in the infused arm [8]. In study II, responses to ACh after darifenacin and placebo were also quantified as area under the curve (AUC) of change in absolute FBF from baseline, expressed in arbitrary units. Comparisons were made with repeated measures anova and two-tailed Student's t-test, as appropriate. Statistical analysis was performed with Graph-Pad Prism (GraphPad Software Inc., San Diego, CA, USA). Significance was accepted at the 5% level in all cases.
Results
Table 1 shows characteristics of the study participants. The only side-effect reported during treatment with darifenacin was dry mouth, which occurred in two of the ten subjects enrolled in study II. Blood pressure and heart rate did not change during any study day comparing baseline and post-treatment values.
Table 1.
Clinical characteristics of the study subjects
| Study I | Study II | |
|---|---|---|
| Number of subjects | 5 | 10 |
| Age, years | 28 (4) | 37 (4) |
| Systolic BP before/after treatment (II), mmHg | 115 (3) | 117 (2)/113 (2) |
| Diastolic BP before/after treatment (II), mmHg | 72 (3) | 69 (3)/66 (4) |
| BMI, kg m−2 | 22 (1) | 24 (1) |
Data are mean (SEM). BMI, body mass index; BP, blood pressure.
Study I
ACh dose-dependently increased FBF in the infused arm (P < 0.001, anova). Pretreatment with atropine significantly decreased the vasodilator response to both doses of ACh (P < 0.001, anova) (Figure 1a).
Figure 1.
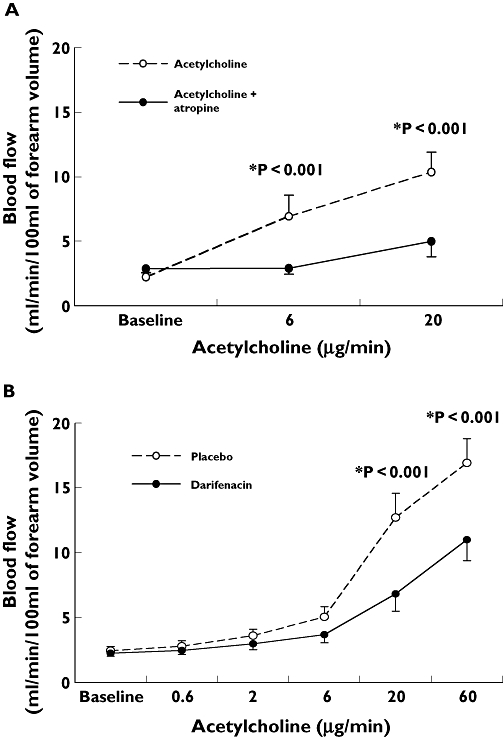
(a) Changes in absolute blood flow (ml min−1 per 100 ml of forearm volume) in the infused arm in response to acetylcholine (Ach) infusion (6 and 20 µg min−1) before and after atropine. Data are mean ± SEM. P < 0.001 ACh vs. ACh plus atropine. (b) Changes in absolute blood flow (ml min−1 per 100 ml of forearm volume) in the infused arm in response to ACh infusion (0.6, 2, 6, 20 and 60 µg min−1) after a 1-week period of treatment with darifenacin compared with placebo. Data are mean ± SEM. P < 0.001 darifenacin vs. placebo
Study II
ACh dose-dependently increased FBF in the infused arm (P < 0.0001, anova). Pretreatment with darifenacin significantly decreased the vasodilator response to ACh 20 µg min−1 {mean difference from placebo 5.8 [95% confidence interval (CI) 3.1, 8.7] ml min−1 per 100 ml of forearm volume, P < 0.001} and to ACh 60 µg min−1[mean difference from placebo 5.9 (95% CI 3.1, 8.7) ml min−1 per 100 ml of forearm volume, P < 0.001] (Figure 1b). After darifenacin, the AUC of change in FBF from baseline was reduced by almost 50% [mean difference in AUC from placebo 291.6 (95% CI 121.7, 461.5) arbitrary units, P = 0.003].
Discussion
The main aim of this study was to investigate the effects of darifenacin on the dose–response curve of ACh-mediated vasodilatation in the forearm vasculature. Our results show that pretreatment with darifenacin substantially reduced the response to ACh, indicating the importance of the M3 receptors in mediating ACh-induced vasodilatation. However, darifenacin did not completely abolish vasodilatation to ACh, and one possible explanation is that other muscarinic receptor subtypes located in the vessel wall may contribute to this response. This is supported by the results of study I, in which atropine almost abolished the vasodilator response to intra-arterial infusion of ACh. It is also possible that nonmuscarinic receptors are involved in the residual ACh-dependent vasodilatation and might contribute to explaining our findings.
From a clinical point of view, there are also possible practical implications: indeed, our results suggest that darifenacin may interfere with endothelial function testing, and in people taking darifenacin, assessment of endothelial function with ACh may therefore be confounded by the use of this drug.
In conclusion, results from this study indicate that, in the human forearm vascular bed, the muscarinic M3 receptors play a major role in ACh-mediated vasodilatation.
Study limitations
Although our study provides evidence for a major role for M3 receptor in ACh-mediated vasodilatation, we are aware of some limitations that need to be addressed in future studies. First, plasma darifenacin levels were not measured in our subjects, and selectivity of the drug at the dose used for M3 receptors on the endothelium is not known. It is therefore possible that concentrations required to block the M3 receptors may also result in blockade of other muscarinic receptors. However, in Phase III studies conducted in patients [9] and healthy volunteers [10] darifenacin 15 mg showed a selective M3 profile when compared with a M1/M2 antagonist.
Competing interests
None to declare.
REFERENCES
- 1.Eglen RM, Whiting RL. Heterogeneity of vascular muscarinic receptors. J Auton Pharmacol. 1990;10:233–45. doi: 10.1111/j.1474-8673.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 2.Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–24. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal N, Lambrecht G, Mutschler E, Tacke R, Malik KU. Pharmacological characterization of the vascular muscarinic receptors mediating relaxation and contraction in rabbit aorta. J Pharmacol Exp Ther. 1991;258:842–50. [PubMed] [Google Scholar]
- 4.Bruning TA, Hendriks MG, Chang PC, Kuypers EA, van Zwieten PA. In vivo characterization of vasodilating muscarinic-receptor subtypes in humans. Circ Res. 1994;74:912–9. doi: 10.1161/01.res.74.5.912. [DOI] [PubMed] [Google Scholar]
- 5.Norel X, Walch L, Costantino M, Labat C, Gorenne I, Dulmet E, Rossi F, Brink C. M1 and M3 muscarinic receptors in human pulmonary arteries. Br J Pharmacol. 1996;119:149–57. doi: 10.1111/j.1476-5381.1996.tb15688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada S, Maruyama S, Takagi Y, Uchida S, Oki T. In vivo demonstration of M3 muscarinic receptor subtype selectivity of darifenacin in mice. Life Sci. 2006;80:127–32. doi: 10.1016/j.lfs.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Haab F, Corcos J, Siami P, Glavind K, Dwyer P, Steel M, Kawakami F, Lheritier K, Steers WD. Long-term treatment with darifenacin for overactive bladder: results of a 2-year, open-label extension study. BJU Int. 2006;98:1025–32. doi: 10.1111/j.1464-410X.2006.06439.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–46. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapple C, Steers W, Norton P, Millard R, Kralidis G, Glavind K, Abrams P. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int. 2005;95:993–1001. doi: 10.1111/j.1464-410X.2005.05454.x. [DOI] [PubMed] [Google Scholar]
- 10.Kay GG, Wesnes KA. Pharmacodynamic effects of darifenacin, a muscarinic M selective receptor antagonist for the treatment of overactive bladder, in healthy volunteers. BJU Int. 2005;96:1055–62. doi: 10.1111/j.1464-410X.2005.05745.x. [DOI] [PubMed] [Google Scholar]
- 11.Eglen RM, Choppin A, Watson N. Therapeutic opportunities from muscarinic receptor research. Trends Pharmacol Sci. 2001;22:409–14. doi: 10.1016/s0165-6147(00)01737-5. [DOI] [PubMed] [Google Scholar]
- 12.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–78. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawes M, Chowienczyk PJ, Ritter JM. Quantitative aspects of the inhibition by N(G)-monomethyl-L-arginine of responses to endothelium-dependent vasodilators in human forearm vasculature. Br J Pharmacol. 2001;134:939–44. doi: 10.1038/sj.bjp.0704338. [DOI] [PMC free article] [PubMed] [Google Scholar]


