Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Domperidone is an effective treatment for some mothers with insufficient milk supply.
However, dose–effect data are not available, and the safety of domperidone use in both mother and infant has been questioned.
WHAT THIS STUDY ADDS
Domperidone only increases milk production in about two-thirds of preterm mothers with insufficient milk supply.
On average, the responders showed increasing levels of milk production with dose escalation from 30 mg to 60 mg daily.
The amount of domperidone that transferred into breast milk was very low, and the risk to the breastfed infant is minimal.
AIMS
To investigate the possibility of a dose–response relationship for the use of domperidone in treating insufficient milk supply in mothers of preterm infants, and to quantify the exposure of the breastfed infant to domperidone.
METHODS
Six preterm mothers received domperidone (30 mg daily or 60 mg daily) in a double-blind, randomized, crossover trial. Milk production and serum prolactin were measured before and during the trial, and domperidone concentration in milk was measured during drug treatment.
RESULTS
For milk production, two of the mothers were ‘nonresponders’, whereas the other four were ‘responders’ and showed a significant increase in milk production from 8.7 ± 3.1 g h−1 in the run-in phase (mean ± SEM), 23.6 ± 3.9 g h−1 for the 30-mg dose (P = 0.0217) and 29.4 ± 6.6 g h−1 for the 60-mg dose (P = 0.0047). In all participants, serum prolactin was significantly increased for both doses, but the response was not dose dependent. Median (interquartile range) domperidone concentrations in milk over a dose interval at steady-state were 0.28 µg l−1 (0.24–0.43) and 0.49 µg l−1 (0.33–0.72) for the 30-mg and 60-mg doses, respectively. The mean relative infant dose was 0.012% at 30 mg daily and 0.009% at 60 mg daily.
CONCLUSION
In one-third of mothers, domperidone did not increase milk production. In the remainder, milk production increased at both domperidone doses, and there was a trend for a dose–response relationship. The amount of domperidone that transfers into milk was extremely low, and infant exposure via breastfeeding was not considered to be significant.
Keywords: breastfeeding, domperidone, infant dose, insufficient milk supply, lactation, preterm infants
Introduction
Human milk is considered to be the ideal food for infants in the first few months of life as it provides appropriate nutrition [1] as well as unique advantages such as reduced morbidity [2], increased immunity and protection against infections [3], improved retinal function [4], enhanced cognitive development [5, 6], lowered prevalence of diabetes [7] and possibly with a reduction in some cardiorespiratory risk factors [8] in later life. Most new mothers delivering full-term infants are able to produce a sufficient supply of breast milk provided they physiologically stimulate lactation within the first week post partum [4]. However, mothers who deliver preterm infants often have difficulty in achieving adequate milk production [9, 10]. Since human milk is equally, if not more important for the preterm infant [11], every effort needs to be made to assist mothers to establish lactation. When standard physiological measures (e.g. correct infant attachment and feeding technique combined with regular pumping) are only partially effective, treatment with galactagogues such as domperidone and metoclopramide is sometimes used [12].
Domperidone, a potent dopamine D2 receptor antagonist, was developed by Janssen Pharmaceutica in 1974 as a prokinetic and antiemetic agent [13]. Currently, domperidone is approved only for the treatment of gastroparesis, nausea and vomiting in most countries, with the exception of the USA, where it is not approved [14]. By blocking dopamine D2receptors in the anterior pituitary [13], domperidone stimulates the release of prolactin, which is essential for the initiation and establishment of lactation [15]. The potential of domperidone to be a stimulant of milk production was recognized early in its development [16, 17], with targeted investigations showing a marked increase in serum prolactin in both sexes [18, 19]. Subsequently, oral domperidone (30 mg daily) has been shown to increase serum prolactin significantly [20–23] and milk production [22–24] in mothers with insufficient milk supply (IMS). However, it is important to note that domperidone is only registered as a prokinetic and antiemetic and that there is no approved indication for its use in lactation. Previous studies have reported mean concentrations in milk following a 30-mg daily dose regimen as means of 2.6 µg l−1 (1.75–3 h after last dose; n = 2) [20] and 1.2 µg l−1 (random sampling; n = 6) following a 30-mg daily dosage regimen [22].
The aims of our study were to investigate if increasing the dose of domperidone from 30 mg daily to 60 mg daily could further increase milk production in mothers of preterm infants, and to provide additional data on the concentration of domperidone in milk and hence enable a comprehensive assessment of its safety for the breastfed, preterm infant.
Materials and methods
Materials
Domperidone and t-butyl methyl ether were purchased from Sigma Chemical Co. (St Louis, MO, USA). Citalopram hydrobromide was a gift from Lundbeck Australia Pty Ltd (Baulkham Hills, Australia). Acetonitrile and n-hexane [high-performance liquid chromatography (HPLC) grade] were purchased from LAB-SCAN Analytical Sciences (Bangkok, Thailand), and hydrochloric acid (HPLC grade) from Merck Pty Ltd (Kilsyth, Australia).
Participants and study protocol
The recruitment site was the King Edward Memorial Hospital (KEMH) (Subiaco, Western Australia), and mothers were assessed for their suitability for the trial by a lactation consultant. Preterm mothers were considered as having IMS if their milk production was <300 ml day−1, despite the usual remedial physiological interventions over a period of 2–3 weeks. The study was approved by the Ethics Committee of the King Edward Memorial and Princess Margaret Hospitals (no. 951/EW) and subsequently was also registered with the Australian Government Department of Health and Ageing Therapeutic Goods Administration under the Clinical Trial Notification Scheme (2004/360). Using a within-subjects repeated measures design, milk production and the transfer of domperidone into milk were assessed at two different domperidone dose levels. All participants were tested in three phases: run-in, phase 1 and phase 2. The run-in phase was the no-drug control phase (1–3 days), and phases 1 and 2 defined the dose administrations of 30 mg or 60 mg domperidone daily (as 10 mg or 20 mg every 8 h). The duration of phases 1 and 2 was between 1 and 2 weeks. Following the run-in period, mothers were randomized to either the 30-mg or 60-mg daily dose in phase 1 and to the alternate dose in phase 2. Participant allocation was carried out by the staff in the Pharmacy Department at KEMH according to a predetermined randomization schedule. Domperidone tablets were pre-packed into opaque capsules so that the mothers could not identify the dose they were receiving at any time. The investigators were also blinded to the dose being administered. During the trial, the mothers continued with the regular pumping schedule (every 2–4 h) recommended by their lactation consultant.
Milk production was measured [25] after the participants had been on the current dose for 1 week and could therefore be expected to be at steady state (half-life of domperidone is approximately 7–9 h [26]). The amount available by pumping both breasts simultaneously (Medela Symphony® pump; Medela AG, Baar, Switzerland) was measured over 15-min sessions carried out on arrival at the clinic when the morning dose of domperidone was given, and 1, 2 and 3 h after this dose. The amounts (g) produced in each of 1-, 2- and 3-h sessions were statistically similar (ANOVA, data not shown) and were therefore averaged and expressed as g h−1 for the run-in, 30-mg and 60-mg dose trial assessments. For each participant, preliminary trials established pumping conditions that were effective and comfortable, and these were utilized throughout the trial. Milk samples (1 ml each; two to six per participant) for assay of domperidone were also collected by the mothers, usually on the day (or day after) they attended for the pumping sessions for both the 30-mg and 60-mg dose trials.
Analysis of domperidone by HLPC
Following the addition of citalopram (40 ng) as the internal standard, 1-ml aliquots of milk were buffered to pH 9.2 with 0.15 ml 2% w/v borax and extracted into 10 ml t-butyl methyl ether by shaking vigorously for 5 min. After centrifugation (2500g for 5 min), 8 ml of the supernatant was transferred to a clean tube and back-extracted into 0.15 ml 0.05 m HCl by shaking vigorously for 1 min. After further centrifugation as above, the supernatant was aspirated to waste, and the remaining HCl layer was transferred to a 1.5 ml tube, where it was washed by vortexing with 0.15 ml hexane for 45 s. After centrifugation at 7800 gfor 2 min, a 0.1 ml aliquot of the HCl phase was injected onto the HPLC. The HPLC system consisted of a Hewlett Packard Series 1100 isocratic pump, autosampler and variable wavelength UV detector (Agilent Technology, Waldbronn, Germany). A Lichrospher RP Select B™ C8 column (5 µm, 250 × 4 mm i.d.; E. Merck GmbH, Darmstadt, Germany) was used with a mobile phase of 28% v/v CH3CN in 45 mm KH2PO4 (pH 3.4) pumped at 1.3 ml min−1, and with detection of analytes at 210 nm. Data were analysed using Chemstation Software Ver. 9 (Agilent Technology, Waldbronn, Germany). Relative standard deviations for the assay at 0.25, 0.5, 1, 2.5 and 5 µg l−1 of domperidone in milk ranged from 2.7 to 8.7% intraday and 3.3 to 9.5% interday. The limit of quantification was 0.15 µg l−1. A quality control was run with each batch of samples and was considered acceptable if the calculated value was within 10% of the reference value.
Serum prolactin measurements
These were performed using Chemiluminescent Microparticle Immunoassay on an Architect® analyser (Abbott Diagnostics, Sydney, Australia) according to the manufacturer's specifications.
Data analysis
Absolute infant dose (µg kg−1 day−1) was calculated as the product of the concentration of domperidone in milk from each mother and an estimated infant milk intake of 0.15 l kg−1 day−1[27]. Relative infant dose was calculated as absolute infant dose/maternal dose (µg kg−1 day−1) and expressed as a percentage [27]. Data have been summarized as median [interquartile range (IQR)] or mean [95% confidence interval (CI) or range], as appropriate. Statistical analyses were performed using SigmaStat Ver. 3.5 (SPSS Inc., Chicago, IL, USA).
Monitoring of side-effects and compliance
Participants were asked to record any side-effects experienced on a daily chart of the main side-effects listed in the manufacturer's Product Information [26] and to rank the severity of these on a Likert scale of none, mild, moderate, severe and extreme. Domperidone dose times were also recorded daily on the same chart, and adherence was also assessed by tablet count on the days that milk production was assessed.
Results
Ten preterm mothers with IMS were identified for recruitment, but only seven agreed to participate. One 28-year-old woman (71 kg) withdrew early during phase 1 of the trial (60-mg dose) because of severe abdominal cramping. The remaining six participants had a mean (range) age of 29 years (24–37) and a mean weight of 76 kg (61–108). With the exception of one normal vaginal delivery, their infants (two sets of twins and four singletons) were delivered by caesarean section. Their mean gestational age was 26.5 weeks (24–29.4). For two women this was their first pregnancy, and for the remainder their second. The mean time of recruitment was 53 days (16–117) postpartum.
Side-effects reported by the mothers during the trial are summarized in Table 1. Dry mouth, headache and abdominal cramping (usually mild to moderate) were reported at both domperidone doses, and were more prevalent at the 60-mg dose. However, one woman who started on the 60-mg dose in phase 1 withdrew after 3 days of treatment because of severe abdominal cramping. Adherence to prescribed therapy was very good in the six participants who completed both dose phases of the trial.
Table 1.
Side-effects reported by the participants during the trial
| Number of participants reporting during domperidone treatment | ||
|---|---|---|
| Side-effect | 30 mg daily | 60 mg daily |
| Abdominal cramping | 1 | 2 |
| Constipation | 0 | 1 |
| Dry mouth | 3 | 5 |
| Depressed mood | 0 | 1 |
| Headache | 1 | 3 |
Milk production data are summarized in Figure 1. Four of the six mothers (PA, PB, PD, PG) showed increased milk production during treatment with both doses of domperidone and were classified as responders. Two mothers (PC, PF) who had no response to treatment at either dose were classified as nonresponders. In the responders, the mean (± SEM) amount of milk produced was 8.7 ± 3.1 g h−1 in the run-in phase, 23.6 ± 3.9 g h−1 for the 30-mg dose and 29.4 ± 6.6 g h−1 for the 60-mg dose. The mean amounts following both the 30-mg (P = 0.0217) and 60-mg (P = 0.0047) doses were significantly greater (215% and 367%, respectively) than those recorded in run-in period (repeated-measuresanova with Holm–Sidak test). However, despite three of four mothers showing a clear increase in production between the 30-mg and 60-mg doses, the number of observations was too small to support a significant overall increase.
Figure 1.
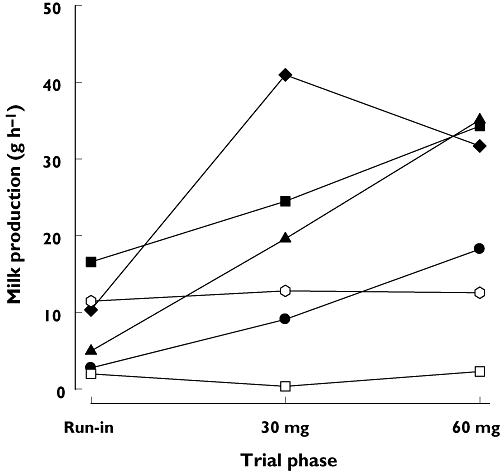
Average milk production for the individual mothers (PA, PB, PC, PD, PF and PG) for the run-in, 30 mg domperidone and 60 mg domperidone phases of the trial. PA, (•); PB, (▴); PC, (□); PD, (♦); PF, ( ); PG, (▪)
); PG, (▪)
Serum prolactin concentration profiles were similar in both responders and nonresponders, and data for all six women are therefore summarized in Figure 2. In the run-in phase, there was a trend (P = 0.05) for increased prolactin between pre-pumping and 45 min post pumping, whereas during treatment with domperidone the mean 45-min post-pumping concentrations were slightly but not significantly lower than in the corresponding pre-pumping trial. Compared with the run-in values, the mean pre-pumping prolactin concentrations were significantly increased (433%; anova) at both the 30-mg (P < 0.007) and 60-mg (405%; P < 0.01) domperidone doses. However, during domperidone dosing the mean 45-min post-pumping prolactin concentrations were similar to those in the run-in phase.
Figure 2.
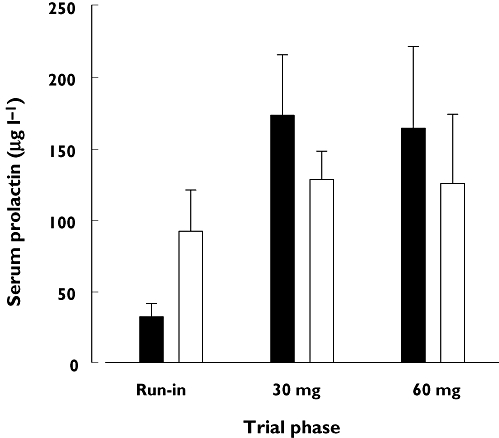
Serum prolactin concentrations (mean ± SEM) before pumping, and 45 min after initiating pumping for the run-in, 30 mg domperidone and 60 mg domperidone phases of the trial
The measured concentrations of domperidone in milk at steady state were very low (Figure 3). The median (IQR) concentration for the 30-mg dose [0.28 µg l−1 (0.24, 0.43); n = 30 samples from six mothers] was significantly lower (Mann–Whitney U = 256, P = 0.007) than that for the 60-mg dose [0.49 µg l−1 (0.33, 0.72); n = 28 samples from five mothers]. Maternal and infant (via milk) dose data are summarized in Table 2. The median absolute and relative infant doses ranged from 0.04 to 0.07 µg kg−1 day−1 and 0.012 to 0.009%, respectively, at 30 mg and 60 mg.
Table 2.
Maternal and estimated infant doses for domperidone
| Parameter estimate during dosing with | ||
|---|---|---|
| Parameter | 30 mg daily | 60 mg daily |
| Maternal dose (µg kg−1 day−1)* | 410 (326, 494) | 820 (652, 988) |
| Domperidone in milk (µg l−1)† | 0.28 (0.24–0.43) | 0.49 (0.33–0.72) |
| Absolute infant dose (µg kg−1 day−1)‡ | 0.04 (0.03–0.07) | 0.07 (0.05–0.11) |
| Relative infant dose (%)‡ | 0.012 (0.009–0.014) | 0.009 (0.006–0.012) |
Mean (95% CI).
Median (IQR) for n = 30 samples from six mothers at 30 mg and n = 28 samples from five mothers at 60 mg.
Median (IQR).
Figure 3.
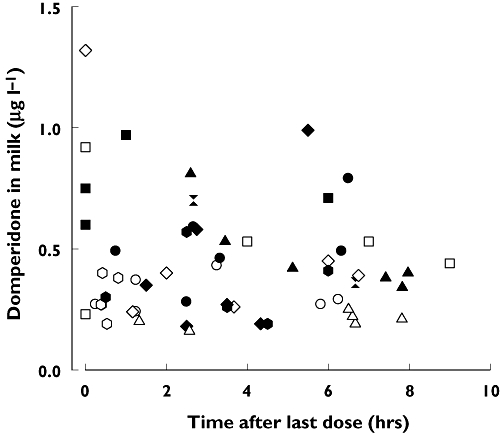
Concentration of domperidone in milk at steady-state. Individual participants shown with different symbols, with open symbols for the 30-mg dose, and closed symbols for the 60-mg dose
Discussion
Domperidone and metoclopramide are two prokinetic drugs that have been used as galactagogues for mothers with IMS. Domperidone is preferred as it does not readily cross the blood–brain barrier [28] and therefore rarely causes extrapyramidal adverse reactions. The anterior pituitary is outside the blood–brain barrier, and hence domperidone is able to alter prolactin synthesis. The side-effects of note in our study were dry mouth, abdominal cramping and headache. Prevalence was as expected from previous data [13] and mostly of mild to moderate intensity. However, severe abdominal cramping was the cause of the only withdrawal from the trial.
The milk production data clearly showed that one-third of our participants were nonresponders at either dose, and suggest that in those who show no response at 30 mg, there is little prospect of a higher dose being effective. In the remaining four participants, both 30-mg and 60-mg doses resulted in significant increases (215% and 367%, respectively) in milk production. However, our study was not sufficiently powered to discriminate between the two doses. Poor adherence to treatment was not an explanation for the nonresponse in two participants. Previous studies using 30 mg vs. placebo in parallel groups have shown percent increases of 175% [22] and 79% [23] in daily milk production. Our data suggest that dose escalation from 30 mg to 60 mg may further increase a partial response obtained at the lower dose level. However, the higher dose may also result in increased side-effects. A previous study has demonstrated that milk production as assessed in our study is a reliable indicator of total daily production [25]. Thus, the milk production achieved in the responders confirms the efficacy of domperidone as a treatment for IMS in preterm mothers.
The serum prolactin data were interesting, in that the overall pattern of response was similar in responders and nonresponders. In the run-in phase, pumping significantly increased the mean prolactin concentration to 92 µg l−1 from a baseline of 33 µg l−1, which is about a half to a third of the concentrations reported in normal lactation [29, 30]. Both doses of domperidone significantly increased serum prolactin to a similar extent (405% at 30 mg and 433% at 60 mg). A previous study using 30 mg has reported a 533% increase in serum prolactin vs. a parallel control group [22]. The high pre-pumping prolactin concentrations in our study were not maintained or increased following the pumping stimulus, and mean concentrations at 45 min actually decreased slightly. The latter finding suggests that there is a ceiling effect of domperidone dose on prolactin synthesis and that the pumping stimulus somehow uncouples the normal synthesis/release mechanisms. Similar effects have been noted for metoclopramide in preterm mothers with IMS [31], and also following prolonged domperidone treatment in healthy volunteers [32]. The fact that dose escalation further increased milk production in some participants without a parallel increase in serum prolactin suggests that mediators and/or mechanisms other than prolactin must be involved in the increased milk production. Although serum prolactin is permissive for lactation, Cox et al. have shown that it does not directly regulate milk synthesis [30]. However, milk prolactin was directly correlated to fullness of the breast, suggesting a local role in autocrine control of milk synthesis.
During the course of our study we were able to collect a substantial body of data on domperidone transfer into milk, showing a significant milk concentration–dose relationship. Moreover, the profile was low across the dose interval. Two previous studies at 30 mg have reported means of 2.6 µg l−1 in two patients (1.75–3 h after last dose) [20] and 1.2 µg l−1 in six patients (unspecified random sampling) [22]. The median absolute infant dose in our study (0.04 µg kg−1 day−1 at 30 mg and 0.07 µg kg−1 day−1 at 60 mg) is very much lower than the 100–300 µg kg−1 four to six times daily dose recommended for the treatment of gastrointestinal stasis in infants [33]. Similarly, the median relative infant dose for domperidone at both 30-mg and 60-mg doses ranged from 0.014% to 0.008% of the weight-adjusted maternal dose, which again is well below the 10% notional level of concern [27]. Given that the oral bioavailability of domperidone is 13–17% in adults [26], an additional safety margin could also be anticipated during oral absorption in infants, as a result of first-pass metabolism in the gut wall and liver. Finally, two studies have reported no domperidone-related adverse effects in exposed breastfed neonates [22, 23]. We conclude that maternal domperidone use in the context of IMS is not a significant risk for the breastfed infant.
During the conduct of our study, the US Food and Drug Administration (FDA) raised concerns about the uncontrolled marketing of domperidone in the USA, citing the lack of an approved indication for IMS and the possibility of adverse effects in both the mother and her breastfed infant [34]. Their main concern in the mother was ‘long-QT syndrome’, as cardiac deaths were seen following intravenous domperidone use in cancer chemotherapy, and also because of a report of abnormal cardiac repolarization in an in vitrostudy [35]. Detailed commentaries on such adverse effects for domperidone use in lactation [12] and gastroparesis [14] suggests that the FDA concern is grossly overstated, except in patients who already have a prolonged QTc interval, where the drug may be contraindicated. As an added maternal-safety precaution prior to domperidone use in lactation, QTc interval could be checked by doing an ECG. In terms of safety for the breastfed infant, the very low concentrations of domperidone reported in the present and previous studies [21, 22], and the miniscule absolute and relative infant doses that we calculated clearly show that safety in the breastfed infant is also not an issue.
Acknowledgments
We are most grateful to Sandra Cummings, Liz Ashton, Diana Langton, Debra Oosterbaan, Mary Wallbank and Judith Kristensen for assistance with patient identification and recruitment, and to Dr John Beilby for measurements of serum prolactin.
REFERENCES
- 1.American Academy of Pediatrics Section on Breastfeeding. Policy statement. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. [Google Scholar]
- 2.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr. 1995;126:696–702. doi: 10.1016/s0022-3476(95)70395-0. [DOI] [PubMed] [Google Scholar]
- 3.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ. 1990;300:11–6. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uauy RD, Birch DG, Birch EE, Tyson JE, Hoffman DR. Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr Res. 1990;28:485–92. doi: 10.1203/00006450-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Arch Dis Child Fetal Neonatal Ed. 2001;84:F23, 7. doi: 10.1136/fn.84.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddy WH, Kendall GE, Blair E, de Klerk NH, Stanley FJ, Landau LI, Silburn S, Zubrick S. Breast feeding and cognitive development in childhood: a prospective birth cohort study. Paediatr Perinat Epidemiol. 2003;17:81–90. doi: 10.1046/j.1365-3016.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus – a nationwide population-based case–control study in pre-school children. Diabetes Metab Res Rev. 2007;24:211–22. doi: 10.1002/dmrr.791. [DOI] [PubMed] [Google Scholar]
- 8.Rudnicka AR, Owen CG, Strachan DP. The effect of breastfeeding on cardiorespiratory risk factors in adult life. Pediatrics. 2007;119:e1107–15. doi: 10.1542/peds.2006-2149. [DOI] [PubMed] [Google Scholar]
- 9.Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81:870–7. doi: 10.1034/j.1600-0412.2002.810913.x. [DOI] [PubMed] [Google Scholar]
- 10.Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: the first 6 weeks after birth. J Hum Lact. 2005;21:22–30. doi: 10.1177/0890334404272407. [DOI] [PubMed] [Google Scholar]
- 11.Wight NE, Morton JA. Human milk, breastfeeding and the pre-term infant. In: Hale TW, Hartmann PE, editors. Hale & Hartmann's Textbook of Human Lactation. 1st. Amarillo, TX: Hale Publishing L.P; 2007. pp. 215–53. [Google Scholar]
- 12.Hale TW. Medications that alter milk production. In: Hale TW, Hartmann PE, editors. Hale & Hartmann's Textbook of Human Lactation. 1st. Amarillo, TX: Hale Publishing L.P; 2007. pp. 479–89. [Google Scholar]
- 13.Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33:429–40. doi: 10.1345/aph.18003. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad N, Keith-Ferris J, Gooden E, Abell T. Making a case for domperidone in the treatment of gastrointestinal motility disorders. Curr Opin Pharmacol. 2006;6:571–6. doi: 10.1016/j.coph.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Czank C. Hormonal control of the lactation cycle. In: Hale TW, Hartmann PE, editors. Hale & Hartmann's Textbook of Human Lactation. 1st. Amarillo, TX: Hale Publishing L.P; 2007. pp. 89–111. [Google Scholar]
- 16.Massara F, Camanni F, Amoroso A, Molinatti GM, Muller EE. Increased thyrotrophin and prolactin secretion induced by domperidone in hypothyroid subjects. Acta Endocrinol (Copenh) 1981;97:48–53. doi: 10.1530/acta.0.0970048. [DOI] [PubMed] [Google Scholar]
- 17.Camanni F, Ghigo E, Ciccarelli E, Massara F, Campagnoli C, Molinatti G, Muller EE. Defective regulation of prolactin secretion after successful removal of prolactinomas. J Clin Endocrinol Metab. 1983;57:1270–6. doi: 10.1210/jcem-57-6-1270. [DOI] [PubMed] [Google Scholar]
- 18.Camanni F, Genazzani AR, Massara F, La RR, Cocchi D, Muller EE. Prolactin-releasing effect of domperidone in normoprolactinemic and hyperprolactinemic subjects. Neuroendocrinology. 1980;30:2–6. doi: 10.1159/000122965. [DOI] [PubMed] [Google Scholar]
- 19.Uberti EC, Trasforini GC, Margutti AR, Sammaroli SR. Effect of domperidone on prolactin secretion in healthy subjects. Panminerva Med. 1980;22:13–5. [PubMed] [Google Scholar]
- 20.Hofmeyr GJ, Van Iddekinge B. Domperidone and lactation. Lancet. 1983;1:647. doi: 10.1016/s0140-6736(83)91818-4. [DOI] [PubMed] [Google Scholar]
- 21.Hofmeyr GJ, Van Iddekinge B, Blott JA. Domperidone: secretion in breast milk and effect on puerperal prolactin levels. Br J Obstet Gynaecol. 1985;92:141–4. doi: 10.1111/j.1471-0528.1985.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 22.da Silva OP, Knoppert DC, Angelini MM, Forret PA. Effect of domperidone on milk production in mothers of premature newborns: a randomized, double-blind, placebo-controlled trial. Can Med Assoc J. 2001;164:17–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Petraglia F, De LV, Sardelli S, Pieroni ML, D'Antona N, Genazzani AR. Domperidone in defective and insufficient lactation. Eur J Obstet Gynecol Reprod Biol. 1985;19:281–7. doi: 10.1016/0028-2243(85)90042-5. [DOI] [PubMed] [Google Scholar]
- 24.Blank C, Eaton V, Bennett J, James SL. A double blind RCT of domperidone and metoclopramide as pro-lactational agents in mothers of preterm infants. p. 73. Perinatal Society of Australia and New Zealand 5th Annual Conference, Canberra, Australia.
- 25.Lai CT, Hale TW, Kent JC, Simmer K, Hartmann PE. Hourly rate of milk synthesis in women. p. 48. 12th International Society for Research in Human Milk and Lactation International Conference, Queens' College, Cambridge, UK.
- 26.Anonymous. Motilium, MIMS Abbreviated Prescribing Information. St Leonards, Australia: MediMedia Australia Pty Ltd; 2008. Version 5.01.0089 Edition. [Google Scholar]
- 27.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and Human Lactation. 2nd. Amsterdam: Elsevier; 1996. pp. 67–74. [Google Scholar]
- 28.Laduron PM, Leysen JE. Domperidone, a specific in vitro dopamine antagonist, devoid of in vivo central dopaminergic activity. Biochem Pharmacol. 1979;28:2161–5. doi: 10.1016/0006-2952(79)90198-9. [DOI] [PubMed] [Google Scholar]
- 29.Hennart P, Delogne-Desnoeck J, Vis H, Robyn C. Serum levels of prolactin and milk production in women during a lactation period of thirty months. Clin Endocrinol (Oxf) 1981;14:349–53. doi: 10.1111/j.1365-2265.1981.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 30.Cox DB, Owens RA, Hartmann PE. Blood and milk prolactin and the rate of milk synthesis in women. Exp Physiol. 1996;81:1007–20. doi: 10.1113/expphysiol.1996.sp003985. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenkranz RA, Ackerman BA. Metoclopramide effect on faltering milk production by mothers of premature infants. Pediatrics. 1986;78:614–20. [PubMed] [Google Scholar]
- 32.Brouwers JR, Assies J, Wiersinga WM, Huizing G, Tytgat GN. Plasma prolactin levels after acute and subchronic oral administration of domperidone and of metoclopramide: a cross-over study in healthy volunteers. Clin Endocrinol (Oxf) 1980;12:435–40. doi: 10.1111/j.1365-2265.1980.tb02733.x. [DOI] [PubMed] [Google Scholar]
- 33.Anonymous. BNF for Children. London: BMJ Publishing Group; 2006. [Google Scholar]
- 34.Anonymous. FDA warns against women using unapproved drug, domperidone, to increase milk production. Available at http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01292.html (last accessed 31 January 2008.
- 35.Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation. 2000;102:1883–5. doi: 10.1161/01.cir.102.16.1883. [DOI] [PubMed] [Google Scholar]


