Abstract
During systemic disease in mice, Salmonella enterica grows intracellularly within discrete foci of infection in the spleen and liver. In concomitant infections, foci containing different S. enterica strains are spatially separated. We have investigated whether functional interactions between bacterial populations within the same host can occur despite the known spatial separation of the foci and independence of growth of salmonellae residing in different foci. In this study we have demonstrated that bacterial numbers of virulent S. enterica serovar Typhimurium C5 strain in mouse tissues can be increased by the presence of the attenuated aroA S. Typhimurium SL3261 vaccine strain in the same tissue. Disease exacerbation does not require simultaneous coinjection of the attenuated bacteria. SL3261 can be administered up to 48 hr after or 24 hr before the administration of C5 and still determine higher tissue numbers of the virulent bacteria. This indicates that intravenous administration of a S. enterica vaccine strain could potentially exacerbate an established infection with wild-type bacteria. These data also suggest that the severity of an infection with a virulent S. enterica strain can be increased by the prior administration of a live attenuated vaccine strain if infection occurs within 48 hr of vaccination. Exacerbation of the growth of C5 requires Toll-like receptor 4-dependent interleukin-10 production with the involvement of both Toll/interleukin-1 receptor-domain-containing adaptor inducing interferon-β and myeloid differentiation factor 88.
Keywords: exacerbation, immunity, infection, interleukin-10, myeloid differentiation factor 88, Salmonella, Toll/interleukin-1 receptor-domain-containing adaptor inducing interferon-β, Toll-like receptor 4
Introduction
Salmonella enterica causes enteric fever, gastroenteritis and septicaemia in humans worldwide. The S. enterica serovar Typhi causes approximately 22 million cases of typhoid fever and over 200 000 deaths annually; serovar paratyphi causes about 5·5 million illnesses in humans.1 Other non-typhoidal Salmonella serotypes (e.g. Typhimurium, enteritidis) cause gastroenteritis in humans and animals and can spread via contaminated food, which is a major concern for the food industry. Non-typhoidal Salmonella are a common cause of bacteraemia and sepsis in immunocompromised individuals (e.g. patients with human immunodeficiency virus infection or malaria) and in children, especially in developing countries where they constitute a major cause of death.2–13 The control of S. enterica infections is challenging because of the emergence of multi-drug-resistant S. enterica strains, the insufficient efficacy of currently licensed vaccines and the lack of effective vaccines against non-typhoidal Salmonella infections.14 Single-dose live attenuated oral vaccines, such as the aroC ssaV Typhi strain, are currently being developed.15
Prevention and treatment of S. enterica infections will benefit from a clearer understanding of the location and dynamics of spread and distribution of individual bacterial populations within the host and from the knowledge of how individual bacteria interact with each other during infection.
In murine models of systemic infection S. enterica initially reside mainly within resident phagocytes of the spleen and liver16–20 and subsequently within inflammatory phagocytes that are recruited to multicellular foci of infection and are activated via the production of proinflammatory cytokines.21–33 Microscopic observation of individual bacteria in infected cells has revealed that the ability of S. enterica to disseminate within the tissues is an important trait of virulent bacteria in addition to their ability to grow intracellularly and resist killing by phagocytes.34,35 The in vivo growth of virulent S. enterica is associated with the continuous spread of bacteria in the tissues to form spatially independent new infection foci, each containing low bacterial numbers. The number of foci increases in parallel with the net bacterial growth rate of the infecting S. enterica strain.34 The integration of mathematical models and microscopic observations has generated testable predictions on the parameters that govern bacterial growth and spread with implications for pathogen dynamics, evolution and control. Current modelling frameworks indicate that spread of S. enterica within the liver is governed by density-independent necrotic lysis of infected phagocytes underlain by density-dependent intracellular bacterial replication.35
These approaches have also revealed striking spatial and functional independence between individual bacterial populations within an infected animal. Different populations or clones of S. enterica always segregate to individual foci of infection and do not mix.34 Functional independence between infection foci is illustrated by independent growth rates and infection kinetics of S. enterica strains when simultaneously inoculated into mice.36
The effects of host immune responses on different bacterial strains within the same host are also determined by events that occur at the level of individual infection foci. For example, in concurrent staggered infections, started 3 days apart with two S. enterica strains of similar virulence, the growth curves of the two bacterial strains in the tissues are comparable, but the suppression of bacterial growth occurs independently for each strain.36 The events leading to the control of one strain do not affect the development of the concurrent infection. These observations are intriguing given the known essential role of systemic mediators [e.g. interferon-γ (IFN-γ) and tumour necrosis factor-α] in the recruitment and activation of phagocytes at the foci of infection.21,22,28,32,37,38 In summary, different bacterial populations segregate spatially within the same host and local immunological events that occur at the level of each focus of infection affect differentially and independently each bacterial population despite the additional necessary contribution of the systemic immune response.
It is still unclear whether situations exist where simultaneous infections can affect one another via signals that are independently generated in spatially separated phagocytes or infection foci. It has previously been suggested that infected cells may be capable of globally conditioning uninfected phagocyte populations within the host. In fact, parenteral administration of dead Gram-negative bacteria or lipopolysaccharide (LPS) can been shown to exacerbate the growth of virulent S. enterica in mice39 and can cause the resurgence of typhoid fever in asymptomatic typhoid carriers,40 but the mechanisms underlying these phenomena are largely unknown. Testing the possibility that infected cells can affect cohorts of infected and/or non-infected cells within the same host, and understanding the mechanisms that regulate these interactions, have important implications for understanding how bacteria interact at the local level within the host tissues and for predicting the possible consequences of interactions between S. enterica vaccines and environmentally acquired wild-type virulent organisms.
In the present paper we show that an attenuated S. enterica strain can induce an increase in the splenic and hepatic numbers of a virulent strain via early production of interleukin-10 (IL-10). Serum levels of IL-10 were transient but exerted a lasting conditioning effect on the global population of phagocytes in the spleen and liver resulting in exacerbation of the growth of virulent bacteria. The increase in the numbers of the virulent strain seen in double infections did not require simultaneous coinjection of the attenuated bacteria because the effect was still apparent when the two infections were staggered. Toll-like receptor 4 (TLR4) signalling was required for early IL-10 production. Both Toll/IL-1 receptor (TIR)-domain-containing adaptor inducing IFN-β (TRIF) and myeloid differentiation factor 88 (MyD88) could mediate TLR4-dependent IL-10 production with optimal levels of IL-10 production requiring the presence of both signalling molecules.
Materials and methods
Animals
C57BL/6 and BALB/c mice (both Sc11a1s) were purchased from Harlan Olac Ltd (Bicester, UK). Mice that were homozygous for mutations in TLR4 (TLR4−/−)41 and TRIF (TRIF−/−)42 (backcrossed at least six times onto the C57BL/6 background) were bred and housed in pathogen-free conditions at Harlan Ltd, UK or at the Department of Veterinary Medicine, Cambridge. Mice that were homozygous for mutations in MyD88 (MyD88−/−)42,43 on a C57BL/6 background were bred and housed at the University of Edinburgh. Mice with homozygous mutations in IL-10 (IL-10−/−)44 were obtained from B&K Universal (Hull, UK).
Bacteria
Salmonella Typhimurium C5 is a highly virulent bacterial strain with a lethal dose for 50% (LD50) by the intravenous route of infection of < 10 colony-forming units (CFU) in BALB/c mice.45 The S.Typhimurium SL3261 strain is an aroA attenuated derivative of S.Typhimurium SL1344 with an intravenous LD50 for BALB/c mice of approximately 107 CFU.46 Rifampicin-resistant SL3261 and nalidixic-acid-resistant C5 were generated by selection on media containing antibiotics. SL3261 were heat killed by boiling for 10 min.
Inoculation of mice and enumeration of bacteria within organ homogenates
Bacterial suspensions for injection were prepared from single-colony cultures of 15 ml Luria–Bertani (LB) broth incubated without shaking overnight at 37°. Cultures were diluted as appropriate in phosphate-buffered saline and injected into a lateral tail vein. Inocula were enumerated by pour-plating with LB agar supplemented with rifampicin or nalidixic acid (Sigma, Poole, UK). The C5 strain was injected at 103 CFU per animal and SL3261 was injected at 106 CFU per animal. Mice were killed by cervical dislocation and spleens and livers were aseptically removed. Organs were placed into stomacher bags and homogenized in 10 ml sterile water in a Colworth Stomacher 80. The resulting homogenate was 10-fold serially diluted in phosphate-buffered saline and pour-plated with LB agar supplemented with rifampicin (100 μg/ml) or nalidixic acid (15 μg/ml) as appropriate.
Assay of serum IL-10
Blood was collected from a lateral tail vein, incubated for 90 min at room temperature and centrifuged at 12 000 g for 10 min. The serum supernatant was split into aliquots and stored at −80°. Interleukin-10 was measured by Biotrack enzyme-linked immunosorbent assay (ELISA; GE Healthcare UK Ltd, Buckinghamshire, UK) according to the manufacturer’s instructions.
Macrophage culture and stimulation
Mouse femurs were flushed with RPMI-1640 and the resultant cells were grown at 37° in a 5% CO2 atmosphere on bacteriological Petri dishes in 80% RPMI-1640 [supplemented with 10% heat-inactivated fetal calf serum, 2 mm l-glutamine, 0·05 mm 2-mercaptomethanol, 1 mm sodium pyruvate, 5% horse serum (all reagents from Sigma-Aldrich, UK)] with 20% medium supernatant from L929 cells. After 7 days of culture, cells were detached by scraping and replated in fresh medium at a density of 3 × 106 per plate for a further 7 days. For stimulation, cells were scraped and plated at a density of 105 per well in 96-well plates and incubated overnight. Culture medium was removed and replaced with fresh medium containing 1 μg/ml S.Minnesota LPS (Invivogen, Toulouse, France) or heat-killed SL3261 (multiplicity of infection 10) and the cells were incubated at 37° in 5% CO2. Supernatants were removed at the appropriate times and frozen at −80°. Interleukin-10 was measured by DuoSet sandwich ELISA (R&D Systems Europe Ltd, Abingdon, UK).
Statistics
To assess the difference in the growth rates between the C5 single infection and the C5 and SL3261 double infections over time (Fig. 1a), a multiple linear regression model was used. The data for the spleen and liver counts were analysed separately, and when regressing log10(CFU) against time the best fitting model was the one that had a common intercept but different slopes (i.e. statistically significantly different growth rates) for the alternative infection types.
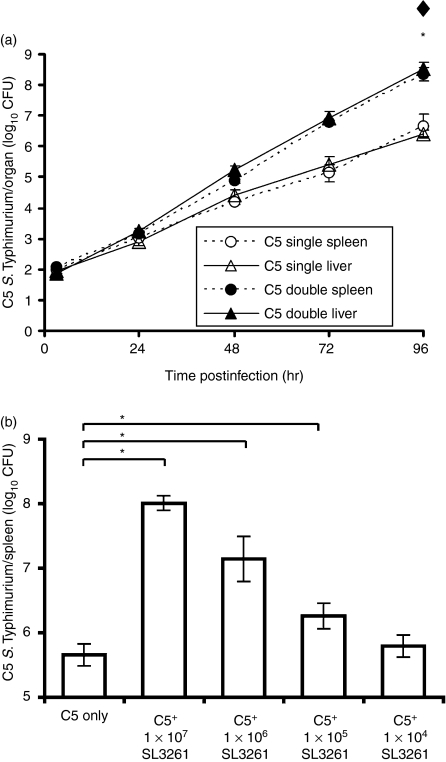
Figure 1.
Exacerbation of Salmonella enterica serovar Typhimurium C5 viable counts caused by coadministration of S. enterica serovar Typhimurium SL3261. (a) Wild-type (WT) BALB/c mice were infected intravenously (i.v.) with 103 colony-forming units (CFU) of virulent C5 (single infections, ○ and Δ). Another group of wild-type BALB/c mice was infected i.v. with 103 CFU virulent C5 together with 1 × 106 CFU S. enterica serovar Typhimurium SL3261 (double infections, • and ▴). Spleen (circles, dotted lines) and liver (triangles, solid lines) counts of viable bacteria were obtained at times thereafter by plating organ homogenates on selective media (see Materials and methods). The results are expressed as mean log10 CFU ± standard deviation from groups of three mice per point. Rates of C5 growth in C5 single and C5 double infections were compared by linear regression analysis. *Significance at P < 0·05 for difference in rates of C5 growth in the spleen. ♦Significance at P < 0·05 for differences in rates of C5 growth in the liver. (b) Groups of five wild-type C57BL/6 mice were infected i.v. with 103 CFU of virulent C5 (C5 only), or with the same dose of C5 together with 107, 106, 105 or 104 CFU of SL3261. Three days later, viable bacterial counts in livers and spleens were assayed. For brevity, viable counts for spleen are shown but equivalent results were observed in the liver. The results are expressed as mean log10 CFU ± standard deviation. *P < 0·05 when comparing the double infected groups to the single C5-only group.
Multiple linear regression was also employed for all other analyses in the paper. This allowed us to make inferences about differences in the response variable (C5 CFU or IL-10 serum production) across all groups within one modelling framework, negating the need to use any multiple adjustment procedures that arise from traditional null hypothesis significance testing (such as pairwise t-tests). Analysis of variance (anova) was used to compare the fits of two models, the first with genotype and presence/absence of SL3261 as the main effects, and the second with an additional interaction term between these two factors. A statistically significant improvement in the fit of the second model suggested that the effect of double infection with SL3261 on the response variable in the wild-type control mice was different when compared to the corresponding effect in the gene-targeted animals. The net result was that we could compare the difference in rates of increase/decrease between single and double infections in different genotypes by studying the corresponding parameter estimate from the model (information that we cannot obtain using null hypothesis testing procedures).
Multiple repeats were also analysed and as a cautionary measure we also reassessed the significance of the parameter estimates using non-parametric (usually bootstrapping) techniques before reporting the results. This was to ascertain possible non-normality of the data. Reported significant results are those that both analyses (standard and non-parametric) return as significant at the 5% level. All data analysis was undertaken using the R statistical language (R Development core team, Vienna, Austria. http://www.R-project.org), using the ‘boot’ library.47
Results
The administration of the attenuated S. enterica SL3261 strain can increase the net growth rate of the virulent S. enterica C5 strain within the same host
BALB/c mice were injected intravenously with combinations of a low dose of virulent nalidixic-acid-resistant S. enterica C5 bacteria with a larger dose of a rifampicin-resistant S. enterica SL3261 attenuated strain. Parallel groups of mice received either the C5 strain alone or the SL3261 strain alone. The net bacterial growth rates of both strains were followed in the spleens and livers of different groups of mice for a 96-hr period. In all cases, the numbers of viable S. enterica SL3261 remained between 105 and 106 throughout the observation period fluctuating only minimally (not shown). In mice receiving C5 alone, a net increase in numbers of bacteria of about 10-fold per day was observed (Fig. 1a). Linear regression analysis was used to evaluate the differences in growth rate of the C5 strain between single and double infections. This showed that there were statistically significant differences in the rates of C5 growth between the single and double infections in both the spleen (P < 0·0001) and liver (P < 0·0001). Thus, coadministration of S. enterica SL3261 with C5 resulted in higher bacterial loads in the later stages of the infection (Fig. 1a).
The higher bacterial loads of C5 were dependent on the dosage of strain SL3261 (Fig. 1b). The strongest exacerbation of the C5 infection was observed when it was simultaneously administered with 107 CFU of SL3261, while negligible exacerbation was seen for the 104 dosage in C57BL/6 mice. It is noteworthy that the intermediate dosages of 105 and 106 CFU, which are routinely used for parenteral vaccination of mice when testing the immunogenicity of live attenuated vaccine strains, induced a statistically significant increase in numbers of C5 (Fig. 1b).
Administration of S. enterica SL3261 can increase the severity of the infection with the virulent S. enterica C5 strain in staggered infections
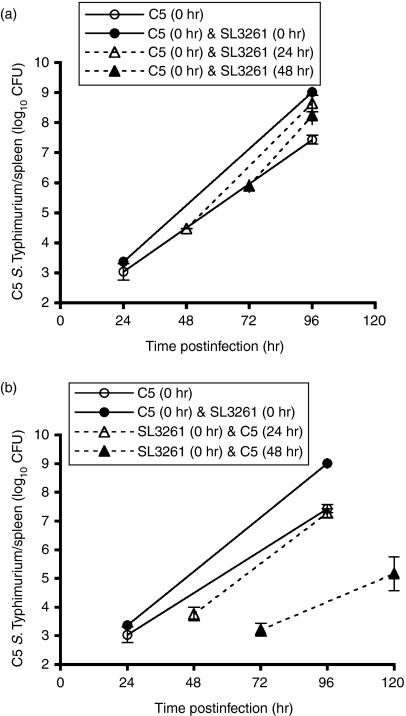
To investigate whether the higher bacterial numbers of C5 seen in double infections were dependent on the simultaneous administration of SL3261, the two strains were administered to the same mice but the infections were staggered by 24 or 48 hr. Figure 2(a) suggests that administration of SL3261 to mice carrying an already established C5 infection resulted in an increase in the growth of C5, leading to higher bacterial numbers in the tissues at the 96-hr time point [this result was highly significant when tested using multiple regression but was borderline (P = 0·08) when using Mann–Whitney (Holm-adjusted) pairwise comparisons – see Materials and methods]. Injection of C5 24 hr after the administration of SL3261 also resulted in increased growth of the virulent bacteria (Fig. 2b). Interestingly, the presence of a pre-existing SL3261 infection for 48 hr resulted in a lower bacterial growth rate of the C5 strain compared to the single C5 infection. The data suggest that the exacerbation of the C5 infection does not require simultaneous administration of SL3261.
Figure 2.
Salmonella Typhimurium SL3261 can cause acceleration in the C5 growth rate even if not administered simultaneously. (a) Wild-type (WT) C57BL/6 mice were infected intravenously (i.v.) with 103 colony-forming units (CFU) of virulent C5 at time 0 (○). Three further groups of wild-type C57BL/6 mice were all given 103 CFU of virulent C5 at time 0 and then given 106 CFU SL3261 at 0 hr (•), 24 hr (▴) or 48 hr (▴). Viable bacterial counts were obtained from spleens 24 hr after SL3261 infection and then at 96 hr. Mice in the C5-only group were assayed for viable bacterial counts at 24 and 96 hr postinfection. The results are expressed as mean log10 CFU ± standard deviation for four mice per point. (b) Wild-type C57BL/6 mice were infected i.v. with 103 CFU of virulent C5 at time 0 (○). Three further groups of wild-type C57BL/6 mice were given 106 CFU of SL3261 at time 0 and then given 103 CFU of C5 at 0 hr (•), 24 hr (▴) or 48 hr (▴). Viable bacterial counts were obtained from the spleen at 24 hr after C5 infection and then at 96 or 120 hr. The C5-only group was assayed for viable bacterial counts at 24 and 96 hr postinfection. The results are expressed as mean log10 CFU ± standard deviation of four mice per point. For brevity, viable counts for spleen are shown but equivalent results were observed in the liver.
IL-10 contributes to the exacerbation of the growth of S. enterica C5 induced by coadministration of S. enterica SL3261
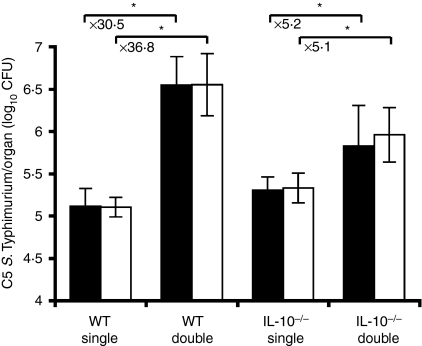
In search of a systemic mechanism that might be responsible for the suppression of macrophage functions we investigated the possibility that immunosuppressant soluble factors could account for a global downregulation of phagocyte functions that would result in exacerbated infection with the virulent C5 strain. Both IL-10−/− mice and congenic C57BL/6 control mice were infected either with C5 alone (single infections) or with a combination of C5 and SL3261 (double infections). An increase in tissue numbers of C5 as a result of double infections was seen in both the spleens and livers of C57BL/6 and IL-10−/− mice at day 3 postinfection (Fig. 3). There was no evidence to suggest that the C5 viable counts differed significantly between C57BL/6 and IL-10−/− mice in single infections. We also found that there was a significant interaction effect between mouse genotype and the presence or absence of the SL3261 strain. Moreover this effect was negative. This suggests that although coinfection with C5 and SL3261 resulted in increased growth of C5 bacteria in both C57BL/6 and IL-10−/− mice, the rate of increase was reduced when IL-10 was absent. The net result of this was that the mean increase in log10 C5 viable counts seen in the IL-10−/− mice as a result of double infection was much reduced (× 5·2 in livers, × 5·1 in spleens) as compared to the increase seen in wild-type C57BL/6 mice (× 36·8 in livers, × 30·5 in spleens). These data suggest that IL-10 is an important mediator of the higher tissue numbers of C5 seen after coadministration of SL3261.
Figure 3.
Growth of C5 in wild-type (WT) and interleukin-10 deficient (IL-10−/−) mice. Both IL-10−/− mice and wild-type C57BL/6 mice were infected with 103 colony-forming units (CFU) of C5 (single infections) or double infections with 103 CFU of C5 combined with 106 CFU of SL3261. Spleen (solid bars) and liver (open bars) counts of C5 were determined at 3 days postinfection. The results are expressed as mean log10 CFU ± standard deviation of four mice per data point. *P < 0·05 when comparing the double infected group to the single C5-only group for each mouse genotype.
In vivo production of IL-10 is transient and TLR4 dependent
To determine the kinetics of IL-10 release, groups of five C57BL/6 mice undergoing single and double infections were bled 1 hr after administration of the bacteria and IL-10 was measured in the sera by ELISA. The IL-10 was detected in the sera of mice simultaneously infected with both the C5 and the SL3261 strains (982 ± 405 pg/ml), but not in mice injected with the C5 strain only (limit of detection 188 pg/ml). Levels of IL-10 were highest at 1 hr postinfection, IL-10 was still detectable at lower levels in some animals at 5 hr but was absent from the sera of all mice by 24 hr postinfection. Levels of IL-10 in mice injected with SL3261 alone were similar to those seen in the sera of mice undergoing double infections (unpublished data). Thus, an early transient peak of IL-10 was seen in double infections in C57BL/6 mice.
To examine the mechanism of early IL-10 serum release in vivo, groups of five C57BL/6 and TLR4−/− mice were injected with C5 alone or with C5 and SL3261 together. No IL-10 was detected in the sera of TLR4−/− mice (limit of detection 188 pg/ml). Similarly, TLR4−/− mice infected with SL3261 alone did not produce detectable amounts of IL-10. Early IL-10 production was entirely dependent upon TLR4 signalling.
The higher tissue numbers of the virulent C5 strain seen after coadministration of the SL3261 strain depend upon LPS responsiveness
Administration of a large bolus of dead bacteria (108 CFU/animal) or purified LPS can accelerate the growth rate of virulent S. enterica in mice, the effect depending on LPS responsiveness.39 So far we have shown that IL-10 greatly contributes to the higher tissue numbers of the C5 strain seen after coadministration of SL3261 and that IL-10 production is dependent on the presence of functional TLR4. To assess whether TLR4 was involved in the SL3261-dependent disease exacerbation, we performed single and double infections in TLR4−/− mice and monitored the bacterial burden in spleens and livers at 24-hr intervals for 3 days.
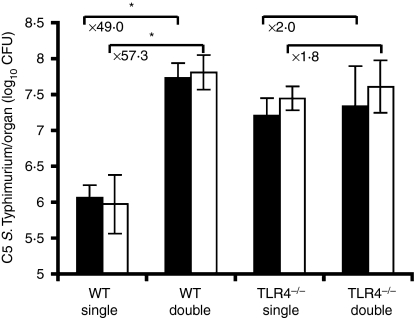
Figure 4 shows the viable C5 counts on day 3 postinfection in C57BL/6 and TLR4−/− mice in the liver and spleen. The results indicate that there was a significant difference (P < 0·05) in the log10 CFU count between C57BL/6 and TLR4−/− mice in single infections of S. enterica C5. Moreover, this effect was positive, suggesting that the presence of TLR4 inhibited growth of C5 single infections. There was evidence that double infections of C5 with SL3261 exacerbated growth of C5 in C57BL/6 mice but the effect of the double infection in TLR4−/− mice was negligible. TLR4 and presumably LPS signalling are therefore required for the exacerbation of the growth of C5 in C57BL/6 mice. The numbers of viable SL3261 were similar in C57BL/6 and TLR4−/− mice up to day 3 postinfection (unpublished data).
Figure 4.
Toll-like receptor 4 (TLR4) plays an important role in exacerbating the growth of C5 in the double infections. Both TLR4−/− and wild-type (WT) C57BL/6 mice were infected with 103 colony-forming units (CFU) of C5 (single infections) or double infections of 103 CFU of C5 combined with 106 CFU of SL3261. Spleen (solid bars) and liver counts (open bars) were determined at day 3 postinfection. The results are expressed as mean log10 CFU ± standard deviation of five mice per group. *P < 0·05 when comparing the double infected group to the single C5-only group for each mouse genotype.
TLR4-dependent and TLR4-independent signalling pathways involving both MyD88 and TRIF contribute to IL-10 production from bone marrow derived macrophages
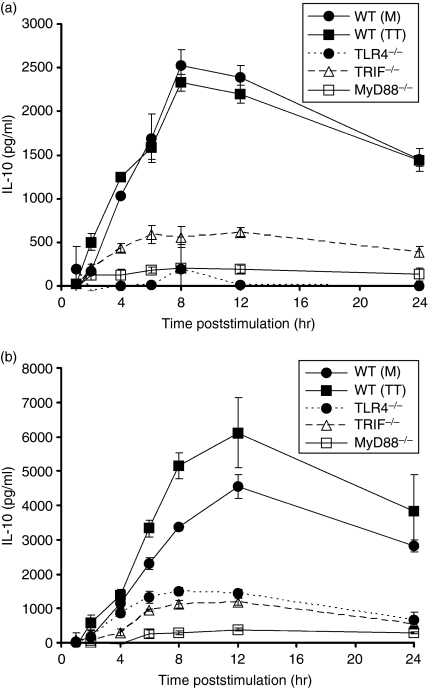
To dissect the signalling pathways that lead to IL-10 production in response to S. enterica LPS and to whole S. enterica bacterial cells, we used in vitro cultures of bone-marrow-derived macrophages (BMDM) from C57BL/6 mice, from TLR4−/− mice and from mice deficient in the adaptor proteins TRIF and MyD88, which could potentially be involved in TLR4-dependent IL-10 production.48 TRIF and MyD88 form two separate signalling pathways originating from TLR4. Recruitment of MyD88 to TLR4 leads to the activation of nuclear factor-κB and the mitogen-activated protein kinases p38 and JNK resulting in proinflammatory cytokine production. The TRIF pathway also results in late nuclear factor-κB activation but additionally activates interferon response factor-3 resulting in the production of type I interferon-responsive genes.49
Macrophage cultures were exposed to LPS or whole bacterial cells and IL-10 release was measured in the supernatants at various times thereafter. The BMDM from wild-type C57BL/6 mice, but not from TLR4−/− mice, produced IL-10 in response to LPS with peak levels reached after 8–12 hr. The BMDM from TRIF−/− and MyD88−/− mice produced detectable levels of IL-10, indicating that both of these signalling molecules can mediate the LPS-induced release of IL-10. However, IL-10 release from TRIF−/− and MyD88−/− BMDM was low compared to that seen in cells from wild-type C57BL/6, indicating that both TRIF and MyD88 are needed for optimal IL-10 production (Fig. 5a). Similar results were obtained when stimulating BMDM with heat-killed S. enterica bacterial cells except for the detectable level of IL-10 in the supernatants of TLR4−/− BMDM (Fig. 5b), indicating that receptors other than TLR4 also contribute to IL-10 release from BMDM in response to S. enterica bacterial cells. IL-10 production in response to S. enterica was mediated by LPS recognition via TLR4 but may also involve other signalling receptors. Both TRIF and MyD88 were sufficient for IL-10 production by cells in culture in response to S. enterica with optimal responses requiring the simultaneous presence of both of these signalling molecules.
Figure 5.
Interleukin-10 (IL-10) production induced by lipopolysaccharide (LPS) and heat-killed Salmonella enterica is MyD88 and Toll/IL-1 receptor-domain-containing adaptor inducing interferon-β (TRIF)-dependent. Bone-marrow-derived macrophages cultured from wild-type (WT) C57BL/6, MyD88−/−, Toll-like receptor 4 deficient (TLR4−/−) and TRIF−/− mice were stimulated with either 1 μg/ml LPS (a) or heat-killed SL3261 (multiplicity of infection 10) (b) for 24 hr. Samples were removed at various time-points for IL-10 detection by enzyme-linked immunosorbent assay. The graphs are assimilations of two experiments, MyD88−/− with WT control [WT(M)] and a second experiment of TLR4−/−, TRIF−/− and WT control [WT(TT)]. Data points represent the mean IL-10 production ± standard deviation from three wells per time point, minus the mean IL-10 production from the untreated wells.
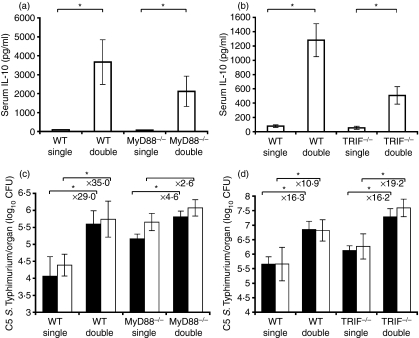
Both MyD88 and TRIF contribute to IL-10 production in vivo
Both TRIF and MyD88 were involved in IL-10 production from BMDM upon exposure to S. enterica so we proceeded to study the relative contribution of these adaptor molecules to the early in vivo release of IL-10 seen in the sera of mice undergoing double infections. As expected, IL-10 was detectable in the sera of C57BL/6 mice injected with both S. enterica strain SL3261 and strain C5 (Fig. 6a,b). Detectable levels of IL-10 were also seen in the sera of both TRIF−/− and MyD88−/− mice undergoing double infections, although the levels were significantly reduced compared to those found in C57BL/6 mice. In single C5 infections neither the mutant mice nor the C57BL/6 mice produced detectable levels of IL-10 (Fig. 6a,b). Either signalling via TRIF or via MyD88 was sufficient for in vivo IL-10 production, but the high levels of IL-10 observed in C57BL/6 mice required both adaptor molecules to be present.
Figure 6.
Signalling via TRIF or MyD88 adaptor molecules is sufficient for early in vivo interleukin-10 (IL-10) release and results in an increased C5 growth rate in the host. Wild-type (WT) C57BL/6, MyD88−/− and TRIF−/− mice were infected with single C5-only and double (C5 and SL3261) combined infections and bled at 1 hr postinfection and serum was analysed by enzyme-linked immunosorbent assay for the presence of IL-10 (a) and (b). The results are expressed as the mean IL-10 ± standard deviations from five mice per point. *P < 0·05 when comparing the double infected group to the single C5-only group for each mouse genotype. (c) MyD88−/− and WT C57BL/6 mice were infected with single C5-only and double (C5 and SL3261). Spleen (solid bars) and liver counts (open bars) were determined at day 2 postinfection from three to four mice per point. (d) TRIF−/− and WT C57BL/6 mice were infected with single C5-only and double (C5 and SL3261). Spleen (solid bars) and liver counts (open bars) were determined at day 3 postinfection from four to five mice per point. The results are expressed as mean log10 viable count ± standard deviation. *P < 0·05 when comparing the double infected group to the single C5-only group for each mouse genotype.
Relative contribution of TRIF and MyD88 to the increased net growth rate of virulent C5
We proceeded to assess the role of these adaptor molecules in causing the higher tissue numbers of C5 observed in double infections. As with TLR4−/− mice, lack of MyD88 resulted in higher bacterial numbers in single C5 infections (Fig. 6c) and although double infections with SL3261 exacerbated C5 growth in both C57BL/6 and MyD88−/− mice, the relative increase was significantly less in the latter case (shown by a significant negative interaction term between genotype and type of infection). Statistically significantly increased tissue numbers of C5 were seen in the spleens and livers of TRIF−/− mice in double infections. However, this time there was no evidence to suggest that the degree of exacerbation of C5 growth in TRIF−/− mice as a result of coinfection with SL3261 was different compared to C57BL/6 control mice (Fig. 6d). The ability of TRIF and MyD88 to mediate the higher tissue numbers of C5 seen in double infections was therefore consistent with the fact that either TRIF-dependent or MyD88-dependent signalling pathways can mediate production of IL-10 in response to S. enterica in vitro and in vivo.
Discussion
The work presented in this paper shows that one bacterial strain can influence the growth of another when these strains are known to be living in spatially separate foci of infection34 within the host via the release of systemic mediators. The administration of an attenuated S. enterica strain with limited ability to grow in the mouse tissues produced a dramatic increase in tissue numbers of a virulent S. enterica strain. The disease acceleration was dependent upon the release of the immunosuppressive cytokine IL-10. The study illustrates that IL-10 production in response to S. enterica requires TLR4 and occurs via signalling pathways involving both TRIF and MyD88 adaptor molecules.
A key feature of S. enterica infections is the spatial segregation of different bacterial populations within the same host. Individual bacterial clones grow in the animal tissues and spread to form spatially independent new infection foci each containing low bacterial numbers.34 The individual foci appear to be functionally independent as shown by the fact that multiple S. enterica strains of different virulence retain their individual net growth profiles even when administered to the same host in simultaneous infections.36
We observed that the administration of an attenuated strain, at an intermediate dose routinely used for parenteral vaccination of mice, can induce a significant acceleration of disease progression with wild-type S. enterica within the same host. These results indicate that there are circumstances in which a set of infected cells (i.e. the phagocyte recipients of the attenuated strain) can globally influence the host immune system, resulting in the suppression of the antibacterial functions of phagocytes at distant sites. Upon parenteral infection with attenuated SL3261, resident macrophages release a systemic factor that acts upon uninfected macrophages to condition them to become less able to control the net growth rate of the C5 bacteria. In fact, the accelerated growth of C5 coincides with continuous spread from infected to non-infected cells, with spatial segregation from the sites of persistence of SL3261 (unpublished data) and with a large excess of uninfected cells in the tissues throughout the infection.50,51
In search of a systemic mechanism that might be responsible for the suppression of macrophage functions we considered the possibility that the injection of the attenuated SL3261 strain might induce an early endotoxin-dependent release of inflammatory cytokines followed by a state of endotoxin tolerance.52 This would prevent the host producing those key cytokines (e.g. tumour necrosis factor-α and IFN-γ) that are needed for phagocyte recruitment and activation and for the control of S. enterica in the tissues.21,28,37 We did not observe a reduction in cytokine production nor in cytokine messenger RNA expression in the animals undergoing double infections (unpublished data). We investigated the possibility that the release of immunosuppressant soluble factors could be responsible for the global downregulation of phagocyte functions that resulted in the exacerbated growth of the virulent C5 strain. We identified IL-10 as one of the important mediators of the increase in net growth rate of C5 induced by coadministration of intermediate doses of SL3261. These findings are consistent with the known cellular effects of IL-10, which include reduced antibacterial functions of phagocytes (e.g. impaired reactive oxygen intermediates, reactive nitrogen intermediates, proinflammatory cytokine production and phagocytosis).53–56 Neutralization of IL-10 also reduces the net bacterial growth rate of Salmonella in vivo.57
The precise intracellular antibacterial functions that are suppressed by IL-10 require further clarification. After the initial blood clearance phase, bacterial killing by phagocytes is negligible in the first few days of growth of S. enterica systemic infections in mice58,59 and therefore it is likely that IL-10 acts by inhibiting the bacteriostatic functions of infected phagocytes, resulting in acceleration of the division of the virulent S. enterica strain.
In this study we have revealed for the first time the role of MyD88 and TRIF in the early in vivo production of IL-10 in response to live Salmonella infections.
The initial in vivo IL-10 production in response to Salmonella is entirely dependent upon TLR4, which further demonstrates the predominant role of TLR4 during Salmonella infections.60,61 Also, no exacerbation of the infection was seen in TLR4−/− mice, indicating the requirement for this receptor in the IL-10-dependent increase in disease severity that we observed in double infections. In single infections, the numbers of the C5 strain in the tissues of TLR4−/− mice were higher compared to those in control animals. This might raise the concern that bacterial growth in TLR4 mice has already reached the ‘theoretical’ maximum of the system, making it difficult to appreciate any further exacerbation in a double infection. We are confident that this is not the case given the fact that we routinely observe a much faster progression of the disease in other experimental conditions (e.g. in gp91−/−phox mice). Also, we were able to detect a disease exacerbation in double infections in MyD88−/− mice where single infections were already more severe than in control animals.
The general picture that is emerging from several studies indicates that several mechanisms and adaptor molecules contribute to optimal production of IL-10 in response to LPS stimulation. In fact, our results indicate that S. enterica-induced IL-10 production requires functional MyD88 and TRIF with maximal cytokine production requiring the function of both adaptor molecules. A recent publication by Boonstra et al.48 demonstrated that both MyD88 and TRIF adaptors were required to regulate IL-10 production in response to LPS by BMDM in vitro. Recently it has also been discovered that TRIF-mediated, LPS-induced IL-10 production may be secondarily upregulated through LPS-induced IFN-β production.62 Interferon-β then binds to the type I IFN receptor (IFNAR) to activate the JAK–STAT pathway (janus kinase–signal transducer and activator of transcription), which in turn leads to IL-10 production through as yet unknown transcription factors. Each adaptor can lead to IL-10 production independently of the other but optimal induction in response to LPS stimulation is only possible when there is interaction between the two signalling arms. The relative contribution of IFN-β to in vivo production of IL-10 will be the object of further studies.
The work described here provides proof of principle that attenuated Salmonella strains can exacerbate the growth of virulent strains acquired concurrently, although this has yet to be proved using the oral route of infection. This research may help inform procedures and safety guidelines when using live vaccine strains in areas of the world where typhoid fever is endemic and there is a strong likelihood that individuals could become infected with environmentally acquired strains during vaccination programmes.
Acknowledgments
This work was funded by The Wellcome Trust in a PhD training studentship awarded to Gemma Foster.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Bachou H, Tylleskar T, Kaddu-Mulindwa DH, Tumwine JK. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6:160. doi: 10.1186/1471-2334-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enwere G, Biney E, Cheung YB, et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J. 2006;25:700–5. doi: 10.1097/01.inf.0000226839.30925.a5. [DOI] [PubMed] [Google Scholar]
- 4.Gilks CF, Brindle RJ, Otieno LS, et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336:545–9. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MA, Banda HT, Gondwe M, et al. Non-typhoidal Salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. Aids. 2002;16:1633–41. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Graham SM, Hart CA, Molyneux EM, Walsh AL, Molyneux ME. Malaria and Salmonella infections: cause or coincidence? Trans R Soc Trop Med Hyg. 2000;94:227. doi: 10.1016/s0035-9203(00)90286-4. [DOI] [PubMed] [Google Scholar]
- 7.Graham SM, Molyneux EM, Walsh AL, Cheesbrough JS, Molyneux ME, Hart CA. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–96. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–4. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 9.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–9. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 10.Kankwatira AM, Mwafulirwa GA, Gordon MA. Non-typhoidal Salmonella bacteraemia – an under-recognized feature of AIDS in African adults. Trop Doct. 2004;34:198–200. doi: 10.1177/004947550403400404. [DOI] [PubMed] [Google Scholar]
- 11.Mastroeni P, Ugrinovic S, Chandra A, MacLennan C, Doffinger R, Kumararatne D. Resistance and susceptibility to Salmonella infections: lessons from mice and patients with immunodeficiencies. Rev Med Microbiol. 2003;14:53–62. [Google Scholar]
- 12.Mulholland EK, Adegbola RA. Bacterial infections – a major cause of death among children in Africa. N Engl J Med. 2005;352:75–7. doi: 10.1056/NEJMe048306. [DOI] [PubMed] [Google Scholar]
- 13.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J. 2000;19:312–8. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989;11(Suppl 3):S552–67. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick BD, McKenzie R, O’Neill JP, et al. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine. 2006;24:116–23. doi: 10.1016/j.vaccine.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Biozzi G, Howard JG, Halpern BN, Stiffel C, Mouton D. The kinetics of blood clearance of isotopically labelled Salmonella enteritidis by the reticulo-endothelial system in mice. Immunology. 1960;3:74–89. [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlap NE, Benjamin WH, McCall RD, Tilden AB, Briles DE. A ‘safe-site’ for Salmonella typhimurium is within splenic cells during the early phase of infection in mice. Microb Pathog. 1991;10:297–310. doi: 10.1016/0882-4010(91)90013-z. [DOI] [PubMed] [Google Scholar]
- 18.Salcedo SP, Noursadeghi M, Cohen J, Holden DW. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–97. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 19.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yrlid U, Svensson M, Hakansson A, Chambers BJ, Ljunggren HG, Wick MJ. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2001;69:5726–35. doi: 10.1128/IAI.69.9.5726-5735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everest P, Roberts M, Dougan G. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect Immun. 1998;66:3355–64. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–6. [PubMed] [Google Scholar]
- 23.Hirose K, Nishimura H, Matsuguchi T, Yoshikai Y. Endogenous IL-15 might be responsible for early protection by natural killer cells against infection with an avirulent strain of Salmonella choleraesuis in mice. J Leukoc Biol. 1999;66:382–90. doi: 10.1002/jlb.66.3.382. [DOI] [PubMed] [Google Scholar]
- 24.Kincy-Cain T, Clements JD, Bost KL. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–40. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastroeni P, Clare S, Khan S, Harrison JA, Hormaeche CE, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–83. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastroeni P, Harrison JA, Chabalgoity JA, Hormaeche CE. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–96. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroeni P, Harrison JA, Robinson JH, Clare S, Khan S, Maskell DJ, Dougan G, Hormaeche CE. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–76. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastroeni P, Skepper JN, Hormaeche CE. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect Immun. 1995;63:3674–82. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastroeni P, Villarreal B, Demarco de Hormaeche R, Hormaeche CE. Serum TNF alpha inhibitor in mouse typhoid. Microb Pathog. 1992;12:343–9. doi: 10.1016/0882-4010(92)90097-8. [DOI] [PubMed] [Google Scholar]
- 30.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Effect of late administration of anti-TNF alpha antibodies on a Salmonella infection in the mouse model. Microb Pathog. 1993;14:473–80. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- 31.Muotiala A, Makela PH. Role of gamma interferon in late stages of murine salmonellosis. Infect Immun. 1993;61:4248–53. doi: 10.1128/iai.61.10.4248-4253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–4. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tite JP, Dougan G, Chatfield SN. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991;147:3161–4. [PubMed] [Google Scholar]
- 34.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 35.Brown SP, Cornell SJ, Sheppard M, Grant AJ, Maskell DJ, Grenfell BT, Mastroeni P. Intracellular demography and the dynamics of Salmonella enterica infections. PLoS Biol. 2006;4:e349. doi: 10.1371/journal.pbio.0040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maskell DJ, Hormaeche CE, Harrington KA, Joysey HS, Liew FY. The initial suppression of bacterial growth in a Salmonella infection is mediated by a localized rather than a systemic response. Microb Pathog. 1987;2:295–305. doi: 10.1016/0882-4010(87)90127-6. [DOI] [PubMed] [Google Scholar]
- 37.Mastroeni P, Arena A, Costa GB, Liberto MC, Bonina L, Hormaeche CE. Serum TNF alpha in mouse typhoid and enhancement of a Salmonella infection by anti-TNF alpha antibodies. Microb Pathog. 1991;11:33–8. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- 38.Muotiala A, Makela PH. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–41. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 39.Hormaeche CE. Dead salmonellae or their endotoxin accelerate the early course of a Salmonella infection in mice. Microb Pathog. 1990;9:213–8. doi: 10.1016/0882-4010(90)90023-j. [DOI] [PubMed] [Google Scholar]
- 40.Wilson GS. The Hazards of Immunization. London: Athlone press; 1967. pp. 265–80. [Google Scholar]
- 41.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 42.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 43.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 45.Hormaeche CE. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979;37:311–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 47.Canty A. Bootstrap R (S- Plus) Functions. Vienna: R Development core team; 2007. R package version 1.2-28. (R port, Ripley B. [Google Scholar]
- 48.Boonstra A, Rajsbaum R, Holman M, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol. 2006;177:7551–8. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 49.Dunne A, O’Neill LA. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 2005;579:3330–5. doi: 10.1016/j.febslet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 50.Anthony Jones E, Summerfield JA. The Liver: Pathobiology. 2nd edn. New York: Raven Press; 1988. pp. 683–704. [Google Scholar]
- 51.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–83. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonnella PA, Starr S, Rodrick ML, Wilmore DW. Induced hyporesponsiveness in rat Kupffer cells is not specific for lipopolysaccharide. Immunology. 1994;81:402–6. [PMC free article] [PubMed] [Google Scholar]
- 53.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 54.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 55.Laichalk LL, Danforth JM, Standiford TJ. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med Microbiol. 1996;15:181–7. doi: 10.1111/j.1574-695X.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 56.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arai T, Hiromatsu K, Nishimura H, Kimura Y, Kobayashi N, Ishida H, Nimura Y, Yoshikai Y. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology. 1995;85:381–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamin WH, Hall P, Jr, Roberts SJ, Briles DE. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J Immunol. 1990;144:3143–51. [PubMed] [Google Scholar]
- 59.Hormaeche CE. The in vivo division and death rates of Salmonella typhimurium in the spleens of naturally resistant and susceptible mice measured by the superinfecting phage technique of Meynell. Immunology. 1980;41:973–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Lembo A, Kalis C, Kirschning CJ, Mitolo V, Jirillo E, Wagner H, Galanos C, Freudenberg MA. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in mice. Infect Immun. 2003;71:6058–62. doi: 10.1128/IAI.71.10.6058-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 62.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–9. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]