Abstract
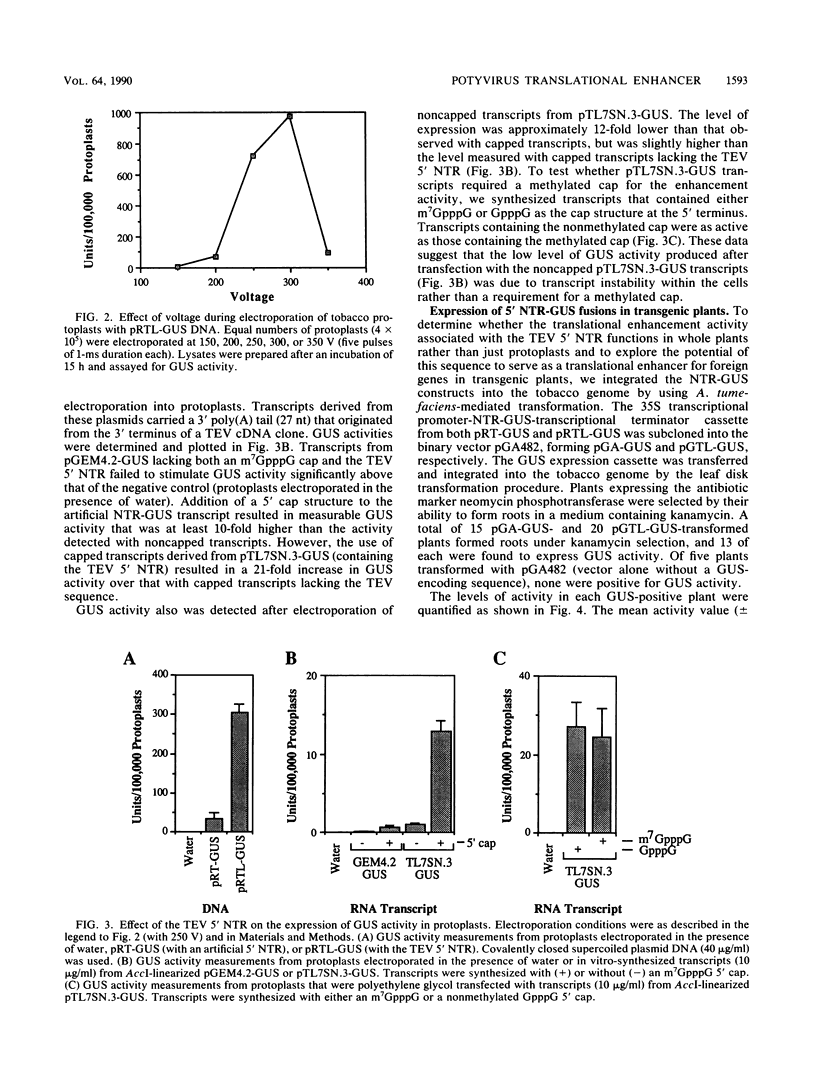
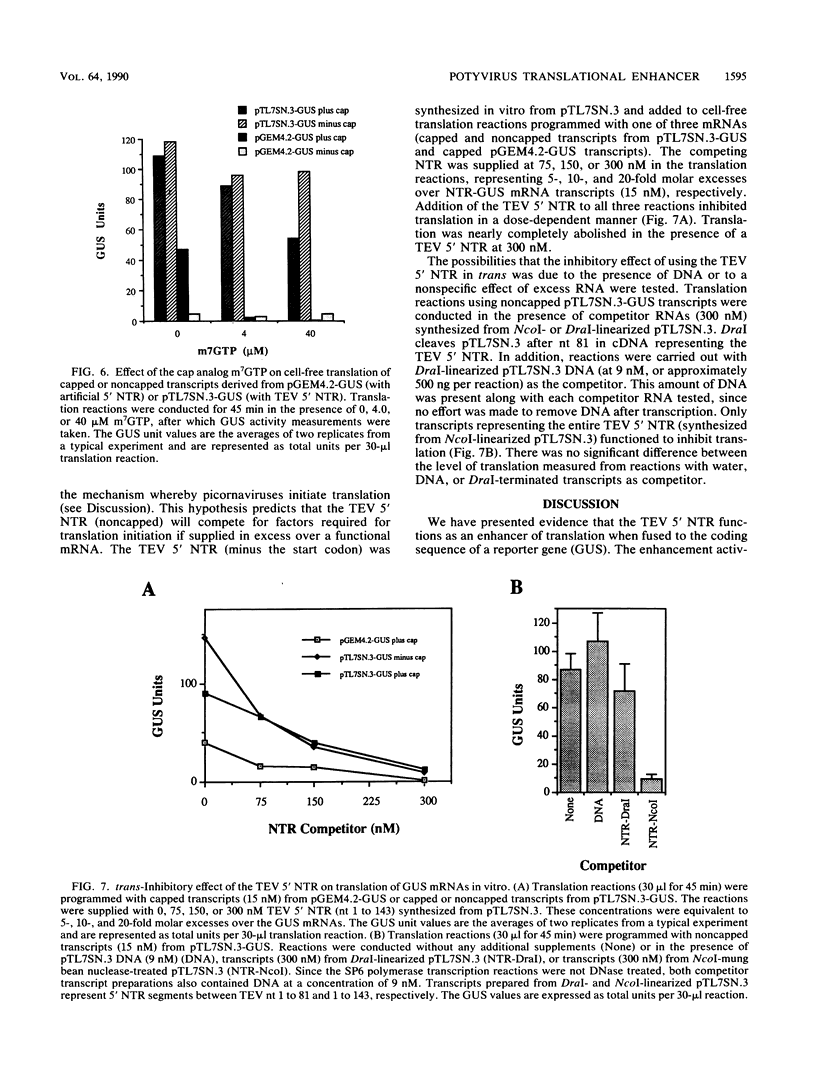
The RNA genome of tobacco etch virus (TEV), a plant potyvirus, functions as an mRNA for synthesis of a 346-kilodalton polyprotein that undergoes extensive proteolytic processing. The RNA lacks a normal 5' cap structure at its terminus, which suggests that the mechanism of translational initiation differs from that of a normal cellular mRNA. We have identified a translation-enhancing activity associated with the 144-nucleotide, 5' nontranslated region (NTR) of the TEV genome. When fused to a reporter gene encoding beta-glucuronidase (GUS), the 5' NTR results in an 8- to 21-fold enhancement over a synthetic 5' NTR in a transient-expression assay in protoplasts. A similar effect was observed when the 5' NTR-GUS fusions were expressed in transgenic plants. By using a cell-free translation system, the translation enhancement activity of the TEV 5' NTR was shown to be cap independent, whereas translation of GUS mRNA containing an artificial 5' NTR required the presence of a cap structure. Translation of GUS transcripts containing the TEV 5' NTR was relatively insensitive to the cap analog m7GTP, whereas translation of transcripts containing the artificial 5' NTR was highly sensitive. The 144-nucleotide TEV 5' NTR synthesized in vitro was shown to compete for factors that are required for protein synthesis in the cell-free translation reaction mix. Competition was not observed when a transcript representing the initial 81 nucleotides of the TEV 5' NTR was tested. These results support the hypothesis that the TEV 5' NTR promotes translation in a cap-independent manner that may involve the binding of proteins and/or ribosomes to internal sites within the NTR.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska-Szewczyk K., Ehrenfeld E. An internal 5'-noncoding region required for translation of poliovirus RNA in vitro. J Virol. 1988 Aug;62(8):3068–3072. doi: 10.1128/jvi.62.8.3068-3072.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning K. S., Lax S. R., Humphreys J., Ravel J. M., Jobling S. A., Gehrke L. Evidence that the 5'-untranslated leader of mRNA affects the requirement for wheat germ initiation factors 4A, 4F, and 4G. J Biol Chem. 1988 Jul 15;263(20):9630–9634. [PubMed] [Google Scholar]
- Carrington J. C., Dougherty W. G. Small nuclear inclusion protein encoded by a plant potyvirus genome is a protease. J Virol. 1987 Aug;61(8):2540–2548. doi: 10.1128/jvi.61.8.2540-2548.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Shahabuddin M., Hellmann G. M., Overmeyer J. H., Hiremath S. T., Siaw M. F., Lomonossoff G. P., Shaw J. G., Rhoads R. E. The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res. 1986 Jul 11;14(13):5417–5430. doi: 10.1093/nar/14.13.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. A comparison of eukaryotic viral 5'-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res. 1987 Nov 11;15(21):8693–8711. doi: 10.1093/nar/15.21.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. The 5'-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Walbot V., Hershey J. W. The ribosomal fraction mediates the translational enhancement associated with the 5'-leader of tobacco mosaic virus. Nucleic Acids Res. 1988 Sep 12;16(17):8675–8694. doi: 10.1093/nar/16.17.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S. A., Cuthbert C. M., Rogers S. G., Fraley R. T., Gehrke L. In vitro transcription and translational efficiency of chimeric SP6 messenger RNAs devoid of 5' vector nucleotides. Nucleic Acids Res. 1988 May 25;16(10):4483–4498. doi: 10.1093/nar/16.10.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Inability of circular mRNA to attach to eukaryotic ribosomes. Nature. 1979 Jul 5;280(5717):82–85. doi: 10.1038/280082a0. [DOI] [PubMed] [Google Scholar]
- Maiss E., Timpe U., Brisske A., Jelkmann W., Casper R., Himmler G., Mattanovich D., Katinger H. W. The complete nucleotide sequence of plum pox virus RNA. J Gen Virol. 1989 Mar;70(Pt 3):513–524. doi: 10.1099/0022-1317-70-3-513. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal binding of eucaryotic ribosomes on poliovirus RNA: translation in HeLa cell extracts. J Virol. 1989 Jan;63(1):441–444. doi: 10.1128/jvi.63.1.441-444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends Biochem Sci. 1988 Feb;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Robaglia C., Durand-Tardif M., Tronchet M., Boudazin G., Astier-Manifacier S., Casse-Delbart F. Nucleotide sequence of potato virus Y (N Strain) genomic RNA. J Gen Virol. 1989 Apr;70(Pt 4):935–947. doi: 10.1099/0022-1317-70-4-935. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Park I. W., Evans C. L., Jaynes J. M., Palmenberg A. C. Effects of cDNA hybridization on translation of encephalomyocarditis virus RNA. J Virol. 1987 Jun;61(6):2033–2037. doi: 10.1128/jvi.61.6.2033-2037.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D. E., Hull R., Turner P. C., Wilson T. M. Studies on the mechanism of translational enhancement by the 5'-leader sequence of tobacco mosaic virus RNA. Eur J Biochem. 1988 Jul 15;175(1):75–86. doi: 10.1111/j.1432-1033.1988.tb14168.x. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Lee K. A. Capped mRNAs with reduced secondary structure can function in extracts from poliovirus-infected cells. Mol Cell Biol. 1982 Dec;2(12):1633–1638. doi: 10.1128/mcb.2.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D., Andino R., Baltimore D. An RNA sequence of hundreds of nucleotides at the 5' end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988 Jul;62(7):2291–2299. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D., Pelletier J., Sonenberg N., Baltimore D. Translation in mammalian cells of a gene linked to the poliovirus 5' noncoding region. Science. 1988 Jul 22;241(4864):445–448. doi: 10.1126/science.2839901. [DOI] [PubMed] [Google Scholar]
- Töpfer R., Matzeit V., Gronenborn B., Schell J., Steinbiss H. H. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987 Jul 24;15(14):5890–5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]



