Abstract
Toll-like receptor 3 (TLR3) participates in the innate immune response by recognizing viral pathogens. In this study, human brain astrocytes were found to constitutively express TLR3, and this expression was increased by interferon-γ (IFN-γ) or double-stranded RNA (dsRNA). Treatment employing dsRNA in astrocytes induced IFN regulatory factor 3 (IRF3) phosphorylation, dimer formation and nuclear translocation followed by STAT1 activation. This treatment also activated nuclear factor-κB, p38 and c-Jun N-terminal kinase significantly, while activating extracellular signal-regulated kinase to a lesser extent. Treatment with anti-TLR3 antibody inhibited dsRNA-mediated interleukin-6 (IL-6) production. In the presence of mitogen-activated protein kinase inhibitors, astrocytes failed to secrete IL-6 in response to dsRNA treatment. Therefore, dsRNA-induced IL-6 production is dependent on mitogen-activated protein kinases and type I IFN production is dependent on IRF3 in brain astrocytes. These results suggest that brain inflammation, which produces inflammatory cytokines and type I IFNs, may enhance TLR3 expression in astrocytes. Additionally, upregulated TLR3 might modulate inflammatory processes by producing proinflammatory cytokines.
Keywords: astrocytes, cytokine, interferon-regulatory factor 3, mitogen-activated protein kinase, Toll-like receptor 3
Introduction
Toll-like receptors (TLR) play a key role in the innate immune response by recognizing pathogens. They regulate the production of inflammatory mediators and cytokines.1 Eleven members of the TLR family have been identified, each recognizing unique molecular patterns associated with different classes of pathogens.1,2 TLR3 mediates signalling in response to double-stranded RNA (dsRNA),3 suggesting that TLR3 may recognize viral infections. The other TLRs mainly appear to be responsible for recognizing bacterial ligands.
Recently, TLR expression and function have been reported in the central nervous system (CNS). Human astrocytes and oligodendrocytes express TLR2 and TLR3, whereas microglia express various TLRs.4,5 TLR4 is exclusively localized in microglia and mediates lipopolysaccharide (LPS)-induced oligodendrocyte injury.6 Innate immunity, especially TLR-mediated immune responses on glial cells, has been related both to protection against bacterial infections (Staphylococcus aureus)7 and CNS immunopathology.8
Astrocytes play a significant role during infectious and autoimmune CNS diseases. Astrocytes have privileged and strategic locations at the blood–brain barrier and within CNS tissue, so could be ideal sentinel cells, capable of sensing viral pathogens. This would induce a ‘state of alert’ in the CNS before viruses infect their target cells. Despite the importance of viral inflammation and innate immunity in neuropathies,9 molecular responses to dsRNA in the brain are poorly understood. Human cellular RNA was reported to be a potential TLR3 ligand,10 and it is well known that RNA is a constituent of the plaques found in patients with Alzheimer disease.11,12 Therefore, RNA released from necrotic cells could come in contact with microglia or astrocytes. Although studies report that TLR3 is expressed preferentially in astrocytes,5,13 the function of TLR3 in the brain is not completely understood. We investigated the role of TLR3 in human brain astrocytes. Primary human astrocytes were isolated, and TLR3 expression and activation in response to dsRNA and other signalling molecules were examined in these cells. Our results indicate that astrocytes have a distinct TLR3 cytoplasmic expression pattern and directly respond to dsRNA, leading to activation of interferon regulatory factor 3 (IRF3), mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) and to interleukin-6 (IL-6) secretion.
Materials and methods
Primary astrocyte preparation and maintenance
Five different astrocyte cultures were prepared from five 20- to 25-week-old human fetuses obtained from therapeutic abortions after obtaining the permission of the institutional review board (IRB) (IRB No. CR07014). Astrocytes were purified as previously described with slight modifications.14 The tissue was dissected using a syringe and was digested with trypsin–ethylenediaminetetraacetic acid (EDTA). The cells were cultured in 10% fetal calf serum (FCS; Gibco BRL, Grand Island, NY)–Dulbecco’s modified Eagle’s minimum essential medium (DMEM; Gibco BRL) containing 1% non-essential amino acids (Sigma, St Louis, MO), penicillin and streptomycin (Gibco BRL) at 37° in 5% CO2. After 2 weeks in culture, cells were shaken (200 r.p.m. for 2 hr) on a shaker (Vision Scientific Co., Bucheon, Korea) once a week for 2 weeks and the non-adherent cells were removed. Astrocyte cultures were routinely > 98% positive for glial fibrillary acidic protein, and < 1% of the cells were microglia based on human leucocyte antigen DR positive staining. Cell subcultures started at 2 × 104 cells/ml in a 75-cm2 tissue culture flask. The medium was changed on the third day and then twice a week. An astrocyte monolayer was usually observed on the 10th day. The astrocytes were maintained for up to 8 weeks after purification.
Reagents
The following reagents were used at the indicated concentrations and times: polyriboinosinic polyribocytidylic acid [poly(I:C); Amersham Pharmacia Biotech, Milwaukee, WI], polyribocytidylic acid [poly(C); Amersham], polydeoxyriboinosinic polydeoxyribocytidylic acid [poly(dI:dC); Amersham], interferon-γ (IFN-γ; Genzyme, Cambridge, MA), LPS (Escherichia coli 026:B6; Sigma), U0126 (Calbiochem, La Jolla, CA), SB203580 (Calbiochem), c-Jun N-terminal kinase (JNK) inhibitor II (Calbiochem).
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated using an RNeasy kit (Qiagen, Santa Claris, CA). Total RNA (2 μg) was used to synthesize complementary DNA with 0·1 optical density (O.D.) random hexamer (Pharmacia, Uppsala, Sweden) and 200 U Moloney-murine leukaemia virus reverse transcriptase (Gibco BRL). The TLR3 primers used were: forward, 5′-GATCTGTCTCATAATGGCTTG-3′; reverse, 5′-GACAGATTCCGAATGCTTGTG-3′. Conditions for the PCR were as follows: denaturing at 94° for 30 seconds, annealing at 52° for 30 seconds and extension at 72° for 1 min. The PCR buffer contained 10 mm Tris–HCl (pH 10), 2·0 mm MgCl2, 50 mm KCl and 1·25 U Taq polymerase (Takara, Tokyo, Japan). After 28 cycles, an additional 10 min extension at 72° was added. β-Actin was used as an internal control and the RT-PCR assays were performed three times and representative results are shown.
Flow cytometry
Astrocytes (5 × 106) were incubated with goat anti-human TLR3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min at 4° in phosphate-buffered saline (PBS) with 1% bovine serum albumin. After the cells were washed twice, fluorescein isothiocyanate-labelled rabbit anti-goat immunoglobulin G (IgG; Jackson ImmunoResearch, Baltimore, MD) was added and incubated for 30 min at 4°. For intracellular staining, cells were fixed with 4% paraformaldehyde for 20 min at room temperature and permeabilized with 1% bovine serum albumin-PBS containing 0·1% Triton X-100 for 5 min at room temperature. After washing, the cells were incubated with anti-TLR3 antibody for 30 min at 4°. Cells were then washed twice and incubated with fluorescein isothiocyanate-labelled secondary antibody at 4° for 30 min. Cell fluorescence was determined using FACSCaliber (BD Biosciences, San Diego, CA) and analysed using CellQuest 3.3 (BD Biosciences).
Electrophoretic mobility shift assay (EMSA)
The EMSA was performed using 6 μg of nuclear extract. Double-stranded NF-κB synthetic oligonucleotides (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were purchased from Promega (Madison, WI) and end-labelled with [γ-32P]ATP (Amersham) and T4 polynucleotide kinase (NEB, Beverly, MA). The nuclear extract was incubated with 0·0175 pmol (>104 counts/min) radiolabelled NF-κB probe and 1 μg poly(dI:dC)–poly(dI:dC) (Amersham) for 30 min in binding buffer [10 mm Tris–HCl (pH 7·6), 20 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 5% glycerol]. Binding reactions were performed on ice in a 20 μl total volume. Samples were loaded on a non-denaturing 6% polyacrylamide gel and electrophoresis was performed for 3 hr at 150 V. The gel was dried and exposed to high-performance autoradiography film. One-hundred-fold excesses of unlabelled NF-κB probe and mutant NF-κB probe (Santa Cruz Biotechnology) were used as specific competitors, and an unrelated oligonucleotide AP-1 probe was used as a non-specific competitor. The supershift assays were performed by preincubating nuclear extracts with 2 μg polyclonal p65 or p50 antibodies (Santa Cruz Biotechnology) on ice for 30 min in the aforementioned reaction conditions.
Enzyme-linked immunosorbent assay (ELISA)
Astrocytes were cultured in a 96-well plate, with 2 × 104 cells per well. The culture medium was collected to measure IL-6 production. The IL-6 in the supernatant was quantified using commercial ELISA kits (BD Biosciences) according to the manufacturer’s instructions. The experiments were performed in triplicate. The anti-TLR3 antibody was purchased from R&D Systems (Minneapolis, MN).
Western blotting
Cells were lysed by sonicating three times for 10 seconds. Lysates were centrifuged for 10 min at 10 000 g, and the soluble supernatant was used for Western blot analysis. The total protein was resolved on 10% or 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membrane. The blots were blocked in PBS with 0·1% Tween-20 (PBST) containing 5% non-fat milk, and were incubated with the appropriate primary antibody at a 1 : 1000 dilution. After three washes in PBST, the blots were incubated in peroxidase-conjugated secondary antibody at 1 : 5000. They were then washed three times in PBST and developed with an ECL system (Amersham). The polyclonal anti-TLR3 antibody was purchased from IMGENEX (San Diego, CA) (IMG-315A). Anti-extracellular signal-regulated kinase (ERK), anti-phospho-ERK, anti-p38 MAPK, anti-phospho-p38 MAPK, anti-JNK, anti-phospho-JNK, anti-phospho-signal transducer and activator of transcription 1 (STAT1), anti-STAT1, anti-phospho-IκBα and anti-IκBα were all purchased from Cell Signaling Technology (Beverly, MA). Goat anti-mouse IgG (BD Biosciences), goat anti-rabbit IgG (Jackson ImmunoResearch) and rabbit anti-goat IgG (Jackson ImmunoResearch) secondary antibodies were used.
Native PAGE
Astrocytes were collected and lysed in native lysis buffer [50 mm Tris–HCL, pH 7·5, 150 mm NaCl, 1 mm EDTA, pH 8·0, 1 mm Na3VO4, 1 mmβ-glycerophosphate, 1 mm phenylmethylsulphonyl fluoride in ethanol, protease inhibitor cocktail and 1% Nonidet P-40]. An SDS-free 7·5% polyacrylamide gel was prerun with 25 mm Tris–HCl and 192 mm glycine with 1% deoxycholate in the cathode chamber only for 30 min at 40 mA. Samples (10 μg) were combined 1 : 1 with native sample buffer (Biorad, Hercules, CA), loaded and electrophoresed for 60 min at 35 mA. Proteins were transferred to membranes and probed for IRF3 dimerization with rabbit anti-IRF3 (Novus Biologicals, Inc., Littleton, CO).
Immunofluorescence staining and analysis by confocal microscopy
Astrocytes were grown in eight-well Nunc Lab-Tec II chambered coverglasses (Nalge Nunc, Naperville, IL) with 10% FCS–DMEM (Gibco BRL) containing 1% non-essential amino acids (Sigma), penicillin and streptomycin (Gibco BRL) at 37° in 5% CO2. Cells were fixed, permeabilized and stained with rabbit anti-IRF3 polyclonal antibody (Novus Biologicals, Inc.) for 45 min at room temperature. Cells were washed three times and further incubated with 2·5 μg/ml Alexa Fluor-conjugated secondary antibodies (Alexa-594, Molecular Probes, Carlsbad, CA) for 1 hr at room temperature and analysed by confocal microscopy. The confocal microscopy studies were performed with a Zeiss Axiovert 100-M inverted microscope equipped with an LSM 510 (Carl Zeiss, Jena, Germany) laser scanning unit and a 1·4 NA × 63 Plan-Apochromat oil-immersion objective.
Luciferase reporter gene assay
Luciferase reporter gene assay was performed with the pIL6-κB-Luc (NF-κB promoter) plasmid. Fetal astrocytes were grown in 12-well plates with 10% FCS–DMEM (Gibco BRL) containing 1% non-essential amino acids (Sigma), penicillin and streptomycin (Gibco BRL) at 37° in 5% CO2. Cells were cotransfected with 0·05 μg/ml of pIL6-κB-Luc plasmid and pCMV-β-galactosidase vector (Promega). After 24 hr, cells were stimulated with poly(I:C) for 6 hr, and thereafter, cell extracts were prepared. The luciferase activity was measured by a Luciferase Reporter Assay System (Promega). β-Galactosidase activity was measured with O-nitrophenyl-β-d-galactopyranoside as substrate. The luciferase activity reported in the figures is normalized for transfection efficiency with the β-galactosidase activity.
Statistical analysis
Data were statistically evaluated by one-way analysis of variance (anova) followed by the Fisher’s protected least significant difference test for multiple comparisons. A P-value < 0·05 was considered to be statistically significant.
Results
TLR3 expression in astrocytes and upregulation in response to poly(I:C) or IFN-γ
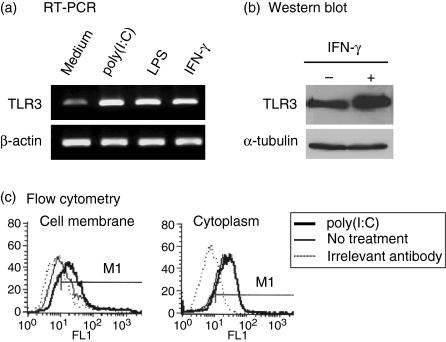
To evaluate TLR3 expression in astrocytes, RT-PCR, Western blotting and flow cytometric analysis were performed. TLR3 messenger RNA was detected in cultured astrocytes and upregulated by inflammatory stimulation, such as dsRNA, LPS or IFN-γ treatment (Fig. 1a). In response to IFN-γ, TLR3 protein levels increased significantly (Fig. 1b). Flow cytometric analysis revealed that TLR3 was localized more in the cytoplasm than on the cell surface but after stimulation with dsRNA, the cell surface localization of TLR3 increased (Fig. 1c). These results show constitutive TLR3 expression in astrocytes, which increased robustly with inflammatory stimuli.
Figure 1.
Expression and upregulation of Toll-like receptor 3 (TLR3) in astrocytes. (a) Cells were treated with 25 μg/ml poly(I:C), 100 ng/ml lipopolysaccharide (LPS), 1000 U/ml interferon-γ (IFN-γ) for 5 hr or left untreated. Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) for TLR3 expression was performed. β-Actin was used as an internal control. (b) Cells were incubated with 1000 U/ml IFN-γ for 24 hr, and Western blots were performed. β-Tubulin was used as an internal control. (c) Cells were stimulated with 25 μg/ml poly(I:C) for 24 hr. Results shown are representative of three independent samples.
NF-κB activation following poly(I:C) treatment in brain astrocytes
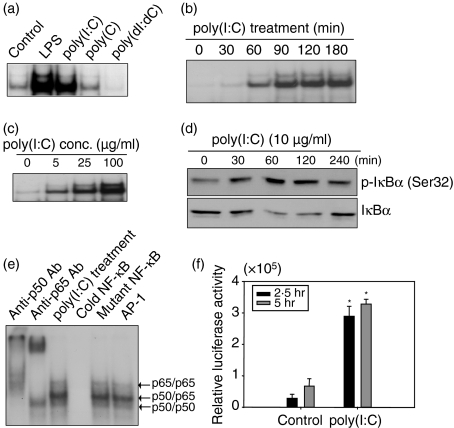
To examine whether dsRNA activates NF-κB in astrocytes EMSA was performed. Poly(I:C) efficiently activated NF-κB (Fig. 2a) in a manner similar to the positive control (LPS stimulation). In contrast, poly(C) or poly(dI:dC) did not activate NF-κB efficiently (Fig. 2a). The activation of NF-κB occurred 30 min after poly(I:C) treatment and reached a maximum level at 180 min (Fig. 2b). The activation of NF-κB increased with increased poly(I:C) concentration (Fig. 2c); treatment with 100 μg/ml poly(I:C) induced significantly more NF-κB activation than a 5 μg/ml poly(I:C) treatment. The IκBα phosphorylation occurred efficiently after poly(I:C) treatment (Fig. 2d). In a competition experiment, a 100-fold excess of unlabelled NF-κB probe was incubated with either a mutant NF-κB probe or an unrelated oligonucleotide probe. A supershift assay using anti-p50 antibody or anti-p65 antibody identified NF-κB components (Fig. 2e). A luciferase reporter gene assay in primary astrocytes also showed NF-κB activation induced by poly(I:C) (Fig. 2f).
Figure 2.
Nuclear factor-κB (NF-κB) activation with poly(I:C) stimulation in astrocytes. (a) Cells were treated with 100 ng/ml lipopolysaccharide (LPS), 25 μg/ml poly(I:C), 25 μg/ml poly(C) or 25 μg/ml poly(dI:dC) for 1 hr or left untreated. The NF-κB activation was analysed by electrophoretic mobility shift assay. (b) NF-κB activation increased in a time-dependent manner. Cells were stimulated with 25 μg/ml poly(I:C) for the indicated times. (c) NF-κB activation increased in a dose-dependent manner. Cells were treated with the indicated doses of poly(I:C) for 1 hr. (d) Cells were incubated with 10 μg/ml poly(I:C). After stimulation, cells were lysed by sonication and the lysates were analysed by Western blot using antibodies to p-IκBα or IκBα. (e) Antibody supershift assays were performed by preincubating poly(I:C)-treated nuclear extracts from astrocytes with the indicated antibodies. A 100-fold excess of unlabelled oligonucleotide (cold NF-κB), mutant oligonucleotide (mutant NF-κB) or non-specific oligonucleotide (AP-1) was used for competition assays. The data are representative of results obtained from three different experiments. (f) Luciferase reporter gene assay was performed. Fetal astrocytes were cotransfected with 0·05 μg/ml of pIL6-κB-Luc plasmid and pCMV-β-galactosidase vector. After 24 hr, cells were stimulated with poly(I:C). The luciferase activity was measured by a Luciferase Reporter Assay System. Luciferase activity is normalized for transfection efficiency with the β-galactosidase activity. *P< 0·05, significantly different from controls at the same treated time.
IL-6 production following poly(I:C) treatment in fetal brain astrocytes
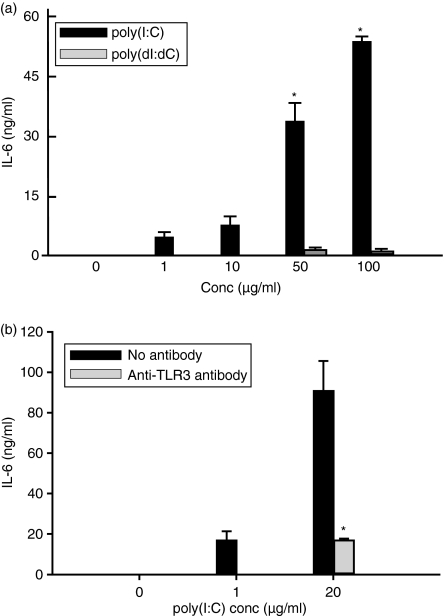
To investigate whether dsRNA induces IL-6 in astrocytes, cells were incubated with poly(I:C) or poly(dI:dC) and the culture supernatant was analysed using ELISA. Poly(I:C) induced IL-6 release in a dose-dependent manner (P< 0·001) (Fig. 3a) but poly(dI:dC) did not induce IL-6 production (Fig. 3a). To examine whether dsRNA-induced IL-6 production in astrocytes is TLR3 dependent, astrocytes were preincubated with anti-TLR3 antibody for 1 hr and cultured with poly(I:C) for 18 hr. Pretreating astrocytes with the anti-TLR3 antibody suppressed poly(I:C)-induced IL-6 release (Fig. 3b). This result indicates that TLR3 expressed in astrocytes specifically recognizes dsRNA and triggers downstream signals leading to IL-6 production.
Figure 3.
Double-stranded RNA induces interleukin-6 (IL-6) production in astrocytes through Toll-like receptor 3 (TLR3). (a) Cells were cultured with poly(I:C) and poly(dI:dC) at the indicated concentrations for 24 hr. IL-6 in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA). Data are representative of results obtained from three separate experiments. (b) Cells were pretreated with 10 μg/ml anti-TLR3 antibody for 1 hr. After incubating with 2 or 20 μg/ml poly(I:C) for 18 hr, the medium was collected and analysed by ELISA for IL-6 production. Pretreatment with goat serum was similar to no antibody treatment. *P< 0·05, significantly different from control or poly(dI:dC)-treated group (a) and also significantly different from the group not treated with anti-TLR3 antibody (b).
MAPK are involved in poly(I:C)-induced IL-6 production
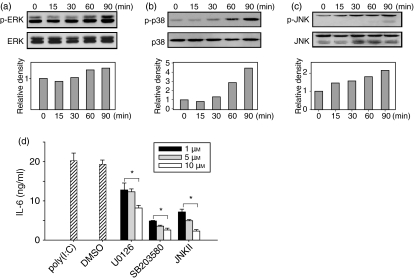
To address whether dsRNA induces MAPK to stimulate IL-6 production in astrocytes, cells were treated with poly(I:C) and activation of ERK, p38 and JNK were assessed by Western blotting. The dsRNA induced ERK, p38 and JNK phosphorylation (Fig. 4); MAPK phosphorylation was detected 30 min after poly(I:C) treatment and increased until 90 min. At 90 min, p38 was phosphorylated fourfold (Fig. 4b) and JNK was phosphorylated twofold compared with controls (Fig. 4c). Phosphorylation of ERK only increased slightly (1·3-fold) (Fig. 4a).
Figure 4.
Mitogen-activated protein kinase (MAPK) activation by double-stranded RNA in brain astrocytes. (a–c) Cells were incubated with 25 μg/ml poly(I:C) for the indicated times or left untreated. After the stimulation, cells were lysed by sonication and the lysates were analysed by Western blot using antibodies to phosphorylated extracellular signal-regulated kinase (p-ERK), ERK, p-p38, p38, phosphorylated c-Jun N-terminal kinase (p-JNK) and JNK. The fold induction was calculated as the ratio of phosphorylated MAPK to unphosphorylated MAPK. The data are representative of three separate experiments (a–c). (d) Astrocytes were preincubated with the indicated concentrations of U0126 (ERK inhibitor), SB203580 (p38 inhibitor), JNK inhibitor II (JNK inhibitor) or dimethyl sulphoxide (DMSO; control) for 30 min. After treatment, cells were cultured with 100 μg/ml poly(I:C) for 18 hr. The supernatant was collected and analysed by enzyme-linked immunosorbent assay for interleukin-6 (IL-6) production. *P< 0·05, significantly different from poly(I:C) control at varying concentrations.
This result prompted the investigation of MAPK involvement in dsRNA-induced IL-6 production. To confirm specific inhibition, cells were treated with MAPK inhibitors at different concentrations. The astrocytes were pretreated with MAPK inhibitors for 30 min and cultured with poly(I:C) for 18 hr. Then the inhibition of IL-6 production by MAPK inhibitors was measured by ELISA. The MAPK inhibitors suppressed IL-6 production in a dose-dependent manner. Treatment with U0126 (an ERK inhibitor) moderately decreased poly(I:C)-induced IL-6 production (Fig. 4d) while treatment with either SB203580 (a p38 inhibitor) or JNK inhibitor II (a JNK inhibitor) led to a marked suppression of IL-6 production in poly(I:C)-stimulated astrocytes. All the inhibitors reduced MAPK activity in a dose-dependent manner (Fig. 4d).
IRF3, STAT1 and IκBα are activated by poly(I:C) treatment
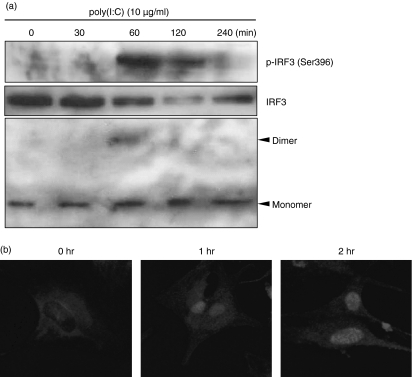
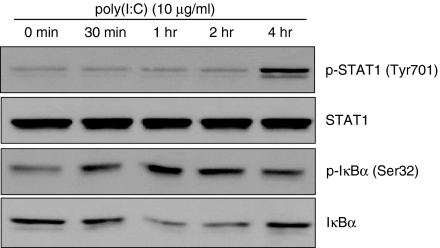
IRF3 phosphorylation, dimer formation and nuclear translocation were assessed as measures of IRF3 activation in response to dsRNA. The dsRNA induced IRF3 phosphorylation at 60 min, but this disappeared at 240 min after poly(I:C) treatment (Fig. 5a). Formation of IRF3 dimers was also detected at 60 min after poly(I:C) treatment (Fig. 5a). Nuclear translocation of IRF3 was observed at 1 hr after poly(I:C) treatment and persisted until 2 hr after stimulation (Fig. 5b). The STAT1 activation began 240 min after poly(I:C) treatment (Fig. 6). Phosphorylation of IκBα occurred 30 min after poly(I:C) treatment (Fig. 6), which suggests the activation of NF-κB.
Figure 5.
Poly(I:C) induced interferon regulatory factor 3 (IRF3) activation in astrocytes. (a) Cells were incubated with 10 μg/ml poly(I:C). After stimulation, cell lysates were separated by native polyacrylamide gel electrophoresis (PAGE). Both monomeric and dimeric IRF3 were detected by rabbit anti-IRF3 antibody. (b) Cells were stimulated with 10 μg/ml poly(I:C) for the indicated time, fixed, permeabilized and stained with rabbit anti-IRF3 polyclonal antibody for 45 min at room temperature. Then cells were washed three times and incubated with 2·5 μg/ml Alexa Fluor-conjugated secondary antibodies (Alexa-594) for 1 hr at room temperature. Cells were analysed by confocal microscopy at × 40 magnification.
Figure 6.
Poly(I:C) induced signal transducer and activator of transcription 1 (STAT1) and nuclear factor-κB (NF-κB) activation in astrocytes. Cells were stimulated with 10 μg/ml poly(I:C) for the indicated time. Western blotting was performed with anti-phospho-STAT1, anti-STAT1, anti-phospho-IκBα or anti-IκBα antibodies. Goat anti-rabbit immunoglobulin G was used as secondary antibody.
Discussion
In the present study, flow cytometry was performed to assess the TLR3 expression pattern in cultured brain astrocytes. A previous study found TLR3 exclusively on the surface of cultured astrocytes, and within intracellular vesicles in cultured microglia.4 Here, we detected TLR3 protein through cell surface and intracellular staining, both of which were upregulated by dsRNA stimulation. Localization of TLR3 varies between different cell types: TLR3 was detected on the cell surface in human fibroblasts,15 while it is intracellular in dendritic cells and retinal pigment epithelial cells.16
Double-stranded RNA recognition by TLR3 has been shown to be dependent on TLR3 localization. In this study, we have shown that TLR3 is expressed on the cell surface of astrocytes, although the expression is higher in the cytoplasm. Recognition of dsRNA by extracellular TLR3 is sufficient to induce cytokine production in brain astrocytes. In our study, astrocytes released IL-6 in response to dsRNA treatment, but not in response to single-stranded RNA or dsDNA. Adding an anti-TLR3 antibody inhibited dsRNA-induced IL-6 secretion. Even in cells with TLR3 localized in the endosomes, the inhibitory function of anti-TLR3 antibodies is still controversial. Inhibiting endosomal maturation, however, also inhibited dsRNA-induced cytokine production in immature dendritic cells with TLR3 localized to intracellular vesicles.17 Inhibition of dsRNA-induced cytokine production with an anti-TLR3 antibody was shown in retinal pigment epithelial cells as well.16
Recent studies have demonstrated that TLR3 signals through a MyD88-independent pathway, utilizing a Toll/IL-1 receptor (TIR)-containing adaptor molecule (TICAM-1)18 and a TIR domain-containing adaptor-inducing IFN-β (TRIF)19 to NF-κB and IFN-β promoters. In primary astrocytes, we assessed NF-κB activation after dsRNA treatment. Activation of NF-κB was detected at 30 min and sustained for up to 180 min. It has been suggested that MAPK are critical signalling molecules for TLR-mediated cell activation.3 When HeLa cells were pretreated with ERK1/2 and p38 MAPK inhibitors, IL-6 production in response to dsRNA treatment was suppressed.20 Cyclooxygenase-2 expression was found to be dependent on MAPK activation21 and preincubation with MAPK inhibitors decreased IL-6 production in a dose-dependent manner. Therefore, MAPK phosphorylation plays a central role in IL-6 production. Astrocytes respond to TLR3 ligation by producing IL-6, CXCL-10 and IFN-β, implicating these cells as contributors to proinflammatory responses.22,23 A recent paper showed that TLR3 uses different signalling mechanisms. Inhibitors of NF-κB, JNK and ERK reduce IL-8 and IFN-inducible protein 10 (IP-10) expression in astrocytes, while phosphoinositide 3 kinase inhibition suppressed IP-10 expression, but upregulated IL-8 expression.24
Our data show that poly(I:C) induces IRF3 phosphorylation and dimer formation in brain astrocytes. The activated IRF3 was translocated to astrocyte nuclei 1 hr after poly(I:C) treatment. Similar to other TLR3-expressing cells,25 astrocytes efficiently produce type I IFNs in response to dsRNA, or participate in provoking anti-viral immunity. TLR3 enhanced the production of anti-inflammatory cytokines, including IL-9, IL-10 and IL-11, but not of proinflammatory cytokines or type I IFNs, and it downregulated the p40 subunit of IL-12 and IL-23.26 This suggests an immunomodulatory function of TLR3 signalling.
The protective role played by TLR3 against viruses in astrocytes is still controversial. Transcription factors and chemokine genes are activated through the TLR3 pathway, but TLR3-mediated activation did not affect the level of replication of Theiler’s murine encephalomyelitis virus.27,28 The fact that poly(I:C) induced viperin/cig5 to inhibit human immunodeficiency virus replication in astrocytes also suggests a protective role.29 Two other papers show that microglia recognize dsRNA through TLR3 in mice,30 and that human neurons express TLR3 as intrinsic machinery to trigger antiviral responses.31
In addition to protecting against infection, other immunological roles of TLR3 in the brain remain to be described. There may be endogenous ligands for TLRs, so it would be interesting to clarify the function of TLR3 and its molecular mechanisms in the brain. Some host-derived TLR ligands have been identified. These include heat-shock proteins32 and extracellular matrix breakdown products.33,34 These molecules can activate TLRs, modulating the immune response. Despite the ubiquitous RNAse distribution, pathophysiological conditions might be associated with the appearance of extracellular RNA. For example, RNA released from degenerative or necrotic cells could contact microglia or astrocytes. Such RNAs may be recognized by TLR3 or RIG-1, promoting inflammation.10 This extracellular RNA could conceivably stimulate TLR3 signalling, inducing an immune response in the brain. A report suggested that increased tau protein phosphorylation at the PHF-1 site (Ser396/404), and JNK and p38 MAPK activation were observed in poly(I:C)-treated cells.35
In summary, we demonstrated that TLR3 is abundantly expressed in cultured brain astrocytes, and its activation by dsRNA results in MAPK-dependent IL-6 secretion. Therefore, TLR3 in astrocytes could sense immune responses during viral infection or some immunological brain diseases, and play an important role in modulating immune responses in the brain.
Acknowledgments
This work was supported by KOSEF/MOST grant (R01-2002-000-00210-0) and KOSEF/the National Core Research Center for Nanomedical Technology Grant (R15-2004-024-00000-0).
Abbreviations
- CNS
central nervous system
- dsRNA
double-stranded RNA
- DMEM
Dulbecco’s modified Eagle’s minimum essential medium
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- IFN
interferon
- IgG
immunoglobulin G
- IL-6
interleukin-6
- IRF3
interferon regulatory factor 3
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAL
MyD88-adaptor-like
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PBST
PBS–Tween-20
- RT-PCR
reverse transcription–polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- STAT1
signal transducer and activator of transcription 1
- TICAM-1
Toll/interleukin-1 receptor-containing adaptor molecule-1
- TIRAP
Toll/interleukin-1 receptor domain-containing adaptor protein
- TLR
Toll-like receptor
- TRIF
Toll/interleukin-1 receptor domain-containing adaptor-inducing interferon-β
References
- 1.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–9. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 5.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–9. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–76. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalpke AH, Schäfer MKH, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–63. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–9. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg SD, Galvin JE, Chiu T-S, Lee VM, Masliah E, Trojanowski JQ. RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol (Berl) 1998;96:487–94. doi: 10.1007/s004010050923. [DOI] [PubMed] [Google Scholar]
- 12.Marcinkiewicz M. BetaAPP and furin mRNA concentrates in immature senile plaques in the brain of Alzheimer patients. J Neuropathol Exp Neurol. 2002;61:815–29. doi: 10.1093/jnen/61.9.915. [DOI] [PubMed] [Google Scholar]
- 13.Krasowska-Zoladek A, Banaszewska M, Kraszpulski M, Konat GW. Kinetics of inflammatory response of astrocytes induced by TLR3 and TLR4 ligation. J Neurosci Res. 2007;85:205–12. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- 14.Norris JG, Benveniste EN. Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. J Neuroimmunol. 1993;45:137–45. doi: 10.1016/0165-5728(93)90174-w. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364–9. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 16.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–62. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 18.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the toll-like receptor signaling. J Immunol. 2002;169:6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 20.Harcourt JL, Offermann MK. Multiple signaling cascades are differentially involved in gene induction by double stranded RNA in interferon-alpha-primed cells. Eur J Biochem. 2001;268:1373–81. doi: 10.1046/j.1432-1327.2001.02003.x. [DOI] [PubMed] [Google Scholar]
- 21.Steer SA, Moran JM, Christmann BS, Maggi LB, Corbett JA. Role of MAPK in the regulation of double-stranded RNA- and encephalomyocarditis virus-induced cyclooxygenase-2 expression by macrophages. J Immunol. 2006;177:3413–20. doi: 10.4049/jimmunol.177.5.3413. [DOI] [PubMed] [Google Scholar]
- 22.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–30. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 23.Scumpia PO, Kelly KM, Reeves WH, Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52:153–62. doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- 24.Park C, Lee S, Cho IH, et al. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–56. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 25.Uematsu S, Akira S. Toll-like receptors and type I interferons. J Biol Chem. 2007;282:15319–23. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 26.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–95. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 27.So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the toll-like receptor 3. Glia. 2006;53:858–67. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- 28.Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–52. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- 29.Rivieccio MA, Suh HS, Zhao Y, Zhao ML, Chin KC, Lee SC, Brosnan CF. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J Immunol. 2006;177:4735–41. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- 30.Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–12. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- 31.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–94. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 32.Beg AA. Endogenous ligands of toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–12. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 33.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by toll-like receptor 4. J Immunol. 2002;168:5233–9. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 34.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nessa BN, Tanaka T, Kamino K, et al. Toll-like receptor 3 mediated hyperphosphorylation of tau in hum an SH-SY5Y neuroblastoma cells. Psychiatry Clin Neurosci. 2006;60:S27–33. [Google Scholar]