Abstract
CD80 and CD86 play a critical role in the initiation of T-cell responses. However, their role in the in vivo effector CD4+ T-cell responses has been less extensively investigated. The current studies have examined the functional relevance of CD80 and CD86 in the effector CD4+ T-cell responses inducing antigen-induced arthritis. Arthritis was induced in C57BL/6 mice by sensitization to methylated bovine serum albumin (mBSA) on day 0, booster immunization (day 7) and intra-articular injection of mBSA (day 21). Control or anti-CD80 and/or anti-CD86 monoclonal antibodies were administered from day 21 to day 28. Arthritis severity and immune responses were assessed on day 28. The development of arthritis was significantly suppressed by inhibition of CD80 or CD86. Blockade of both CD80 and CD86 caused a trend towards reduced disease severity compared to control antibody-treated mice. Neutralization of CD80 attenuated accumulation of CD4+ T cells in joints and enhanced splenocyte production and circulating levels of interleukin-4. Inhibition of CD86 or both CD80 and CD86 reduced T-cell accumulation in joints without affecting T helper type 1/type 2 (Th1/Th2) differentiation or antibody levels. Blockade of CD86, and not CD80, significantly suppressed splenocyte interleukin-17 (IL-17) production. These results provide further in vivo evidence that CD80 and CD86 play important pathogenic roles in effector T-cell responses. CD80 exacerbates arthritis by downregulating systemic levels of IL-4 and increasing T-cell accumulation in joints without affecting IL-17 production. CD86 enhances disease severity by upregulating IL-17 production and increasing the accumulation of effector T cells in joints without affecting Th1/Th2 development.
Keywords: antigen-induced arthritis, CD80, CD86, costimulation, effector T cell, interleukin-17
Introduction
Rheumatoid arthritis (RA) is a common chronic autoimmune disease characterized by joint inflammation and destruction. The inflammatory infiltrates in the rheumatoid synovium consist of many cell types including T cells, B cells, dendritic cells, macrophages and neutrophils.1–5 Although the present understanding of disease pathogenesis is still incomplete and the specific antigen(s) eliciting the autoimmune response is unknown, a large body of evidence suggests that acute and chronic rheumatoid inflammation is driven by activated CD4+ T cells. First, RA is associated with particular major histocompatibility complex class II allelles such as human leucocyte antigen DR4.6 Second, activated CD4+ T cells are present in inflammatory infiltrates of rheumatoid synovium,4,7 third, various T-cell-directed therapies have clearly conferred clinical benefit in RA,8,9 and, finally, early RA is characterized by a distinct T-cell-related cytokine profile.10 Activated T cells in rheumatoid joints are predominantly interferon-γ (IFN-γ)-secreting T helper type 1 (Th1) effectors11,12 and several treatment modalities currently employed in RA exert their protective effects in part by shifting the Th1/Th2 balance towards Th2.13,14 However, the recent discovery of a new subset of Th cells characterized by interleukin-17 (IL-17) production (termed Th17) has produced new interest in the role of T cells in RA and challenged previous views of Th1-driven rheumatoid inflammation.15,16 The IL-17 is upregulated in synovial fluid of patients with RA17 and may contribute to the pathogenesis of RA by promoting joint inflammation as well as cartilage and bone destruction.18 Evidence from animal models including collagen-induced arthritis (CIA) and adjuvant arthritis has also emerged suggesting that autoimmune inflammation in RA is Th17-mediated.19–21 Furthermore, chronic rheumatoid inflammation is thought to be regulated not only by injurious effector T cells, but also by CD4+ CD25+ T regulatory (Treg) cells with diminished suppressive activity, which are present in the synovial fluid of patients with RA.16,22,23 In addition to T cells, activated macrophages in joints are thought to significantly contribute to joint injury by producing proinflammatory mediators such as tumour necrosis factor-α (TNF-α) and IL-1, as well as matrix metalloproteinases (MMPs).1,24
Antigen-induced arthritis (AIA) is a widely used animal model of RA with similar histopathological features. It is induced by sensitization of animals to methylated bovine serum albumin (mBSA) in complete Freund’s adjuvant (CFA), followed by an injection of mBSA into the joint cavity. The development of joint injury in AIA is dependent on CD4+ T cells,25–27 while CD8+ T cells and B cells are not involved.25 The development of AIA is affected by various Th subsets. Earlier data suggested that disease severity in AIA correlates with the development of Th1 responses.27,28 However, these observations have been challenged by more recent studies using IFN-γ-deficient mice that have shown that IFN-γ has a protective role in AIA.29,30 In contrast, studies using neutralizing anti-IL-17 monoclonal antibodies (mAb) have shown that joint inflammation and destruction in AIA are promoted by IL-17,29,31 whereas Th2 cytokines such as IL-4 play a protective role in AIA.28 Depletion and adoptive transfer studies have also shown that CD4+ CD25+ Treg cells play a suppressive role at the onset of AIA.32 In addition to T cells, activated macrophages are present in AIA joints where they are thought to contribute to tissue injury.33–35
Optimal activation of naïve CD4+ T cells requires engagement of the T-cell receptor as well as a non-antigen-specific, costimulatory signal. The best characterized costimulatory molecules are CD80 and CD86. They are expressed by antigen-presenting cells, such as dendritic cells, macrophages and B cells, but can also be upregulated on other cell types such as endothelial cells36 by proinflammatory stimuli. The interaction of CD80 or CD86 with the T-cell receptor, CD28, promotes T-cell responses by decreasing apoptosis and enhancing the expansion of antigen-specific T cells.37,38 CD80 and CD86 have also been shown to regulate Th1/Th2 differentiation39–41 and are critical for antibody production and isotype switching.39,42 Studies using mice deficient in both CD80 and CD86 have shown that these molecules also play an important role in the generation of Th17 and CD4+ CD25+ Treg cells,43,44 although the role of CD80 or CD86 in the effector Th17 responses is not known. Following T-cell activation, cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), a homologue of CD28, is upregulated on the surface of activated T cells.36 It binds CD80 and CD86 with a much higher affinity than CD28, thus preventing engagement of CD28 and resulting in suppression of ongoing T-cell responses.36,45
CD80 and CD86 are well known for the critical role they play during the initiation of adaptive immune responses and a variety of immune-mediated diseases.39,41,46 However, the contribution of these CD28 ligands to the in vivo effector immune responses has not been given much focus. Activation and function of memory and effector CD4+ T cells has been generally considered to be CD28-independent. More recently, however, it has been demonstrated that CD80 and CD86 can play an important local role during the effector phase of inflammatory disease models such as crescentic glomerulonephritis and experimental autoimmune encephalomyelitis.46,47
The CD28/B7 pathway plays an important role in the initiation of experimental arthritis. CD28−/− mice are resistant to CIA,48 and inhibition of both CD80 and CD86 during the induction phase of CIA prevents the development of disease.49–51 However, the role of CD80 and CD86 in the effector phase of RA is less well defined. Expression of CD80 and, to a larger extent CD86,52,53 has been demonstrated in the joints of patients with RA suggesting that blocking these costimulatory molecules has therapeutic potential. Indeed, treatment with the CTLA-4–immunoglobulin fusion protein, which blocks both CD80 and CD86, has significantly improved the signs and symptoms of RA in clinical trials54 and CTLA-4–immunoglobulin treatment of established CIA suppressed the course of disease.49,55 Given the increasing importance of Th17 effector cells in the pathogenesis of RA and the potential of CD80 and CD86 to regulate pathogenic effector T-cell responses and Th17 development, the current studies have examined the functional role of CD80 and CD86 in the effector phase of Th17-mediated AIA. They demonstrate that CD80 and CD86 both contribute to joint injury during the effector phase of AIA, but do so by different mechanisms. CD80 is pathogenic in AIA by attenuating systemic levels of IL-4, as well as enhancing CD4+ T-cell accumulation in joints, without affecting IL-17 production. In contrast, CD86 contributes to joint injury by upregulating IL-17 production and increasing effector T-cell accumulation in joints, without an effect on Th1/Th2 development. These results, together with the increasing importance of IL-17 in rheumatoid inflammation and greater expression of CD86 in the joints of patients with RA, suggest that CD86 may be a more appealing target than CD80 for the treatment of RA. Furthermore, the current study provides new in vivo evidence that CD86, and not CD80, plays an important role in the production of IL-17 by effector/memory T cells.
Materials and methods
Experimental design
Eight- to ten-week-old male C57BL/6 mice obtained from Monash University Animal Services (Melbourne, Australia) were immunized on day 0 with 200 μg mBSA (Sigma Aldrich, St Louis, MO) emulsified in 0·2 ml CFA and injected subcutaneously into flank skin. On day 7, the mice were given 100 μg mBSA in 0·1 ml CFA by intradermal injection at the base of the tail. Arthritis was induced on day 21 by intra-articular injection of 30 μg mBSA in 10 μl sterile saline into the left knee, the right knee being injected with sterile saline alone. The mice received intraperitoneal injections of 100 μg protein G purified control control antibodies [both hamster and rat immunoglobulin G (IgG); n = 7] or hamster anti-mouse CD80 (clone 16-10A1; n = 6), rat anti-mouse CD86 (clone GL1; n = 7) or both anti-CD80 and anti-CD86 mAb (n = 7). The 16-10A1 and GL1 hybridomas were provided by Dr David Tarlinton (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). Treatment was started on day 21 and repeated every second day until the end of the experiments. Arthritis severity and immune responses were analysed on day 28.
Assessment of arthritis severity
Knee joints were dissected and fixed in 10% buffered formalin for 7 days. Fixed tissues were decalcified for 3 weeks in 15% ethylenediaminetetraacetic acid, dehydrated, and embedded in paraffin. Sagittal sections (5 μm) of the knee joint were stained with Safranin-O and counterstained with fast green/iron haematoxylin. Histological sections were scored semiquantitatively in a blinded fashion for each of the four parameters of joint injury as follows: Synovitis, defined as hypercellularity of the synovium, was scored using a scale of 0–3, where 0 = normal, 1 = mild synovial infiltrate, 2 = moderate synovial infiltrate, and 3 = extensive hypercellular pannus; Joint space exudate, defined as leucocytes (known to be mainly neutrophils56) in the joint space, was scored using a scale of 0–3, where 0 = no cells in joint space, 1 = a few scattered cells in joint space, 2 = occasional clusters of cells in joint space, and 3 = numerous clusters and rafts of cells in joint space; Cartilage degradation, defined on the basis of the loss of Safranin-O staining, was scored using a scale of 0–3, where 0 = no loss of staining and 3 = 100% loss of staining; and Bone damage, defined as the extent and depth of subchondral bone invasion by pannus, was scored using a scale of 0–3, where 0 = no invasion, 1 = mild invasion by pannus, 2 = moderate invasion by pannus, and 3 = extensive invasion by pannus. A combined arthritis score was generated from the sum of scores for the above four parameters (maximum possible score 12).
Accumulation of CD4+ T cells and macrophages in the synovium
CD4+ T cells and macrophages were demonstrated in joints by three-layer immunoperoxidase staining of formalin-fixed paraffin-embedded 5 μm-thick joint sections. Sections were first dewaxed by immersion in Histosol for 10 min, then passed through 100% followed by 70% ethanol and washed in water. After blocking with 15% normal swine serum in 5% BSA/phosphate-buffered saline (PBS), primary mAb were added and incubated overnight at 4°. GK1.5 (rat IgG2b anti-mouse CD4; American Type Culture Collection, Manassas, VA) was used to detect CD4+ T cells, whereas M1/70 (rat IgG2b anti-mouse Mac-1; American Type Culture Collection) was used for macrophages. Isotype-matched control immunoglobulin (rat IgG2b; Pharmingen, San Diego, CA) served as a negative control. Sections were washed and endogenous peroxidase was blocked with 1% hydrogen peroxide in methanol. After washing, secondary antibody (rabbit anti-rat immunoglobulin; DAKO, Carpinteria, CA) was applied for 1 hr at room temperature. Sections were washed and horseradish peroxidase (HRP)-conjugated swine anti-rabbit immunoglobulin (DAKO) was added and incubated for 1 hr at room temperature. After washing, slides were developed with diaminobenzidine (brown), counterstained with haematoxylin and coverslips were placed on them. Five high-power fields (HPFs) were assessed for each animal to semiquantitatively determine the percentage of CD4+ T cells and macrophages per HPF, where percentage refers to the percentage of the HPF area occupied by positive staining.
Splenocyte production of IFN-γ, IL-4 and IL-17, as well as circulating levels of IL-4
To assess splenocyte cytokine production, spleens were removed from mice and single cell suspensions were obtained. Splenocytes (4 × 106 cells/well) were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum in the presence of 10 μg/ml mBSA for 72 hr. Concentrations of IFN-γ and IL-4 in splenocyte supernatants were measured by enzyme-linked immunosorbent assay (ELISA), as previously described,57 while IL-17 was assessed by a mouse IL-17 DuoSet ELISA kit according to manufacturer’s instructions (R&D Systems, Minneapolis, MN). Circulating levels of IL-4 were assessed by ELISA on serum collected at the end of the experiments, as previously described,57 with recombinant mouse IL-4 standards and serum samples (1/2 dilution) diluted in 2% BSA/PBS.
Circulating antibody levels
Serum levels of antigen-specific IgG, as well as of IgG subclasses, IgG1 and IgG2a, were assessed by ELISA on serum collected at the end of the experiments. Microtitre plates (Maxisorp plates, NUNC, Roskilde, Denmark) were coated with 100 μl/well of 10 μg/ml mBSA in carbonate/bicarbonate buffer (pH = 9·6) and incubated overnight at 4°. Plates were washed with 0·05% Tween/PBS and blocked with 2% casein/PBS for 2 hr at room temperature. Serum samples (total IgG: 1/100 to 1/12500; IgG1: 1/1000; IgG2a: 1/1000 dilution) were added to wells and incubated overnight at 4°. After washing, total IgG and IgG1 were detected with HRP-conjugated sheep anti-mouse IgG (1/2000; Amersham, Little Chalfont, UK) and HRP-conjugated goat anti-mouse IgG1 (1/4000; Southern Biotechnology Assoc., Birmingham, AL), respectively. IgG2a was detected using biotinylated rat anti-mouse IgG2a mAb (Pharmingen) and streptavidin-HRP (Chemicon, Tenecula, CA). Tetramethylbenzidine (Sigma) was added to plates and the reaction was stopped with 0·5 m sulphuric acid, after which the absorbance was read at 450 nm using a microplate reader. Sera from normal C57BL/6 mice provided baseline antibody levels.
Statistical analysis
Results are expressed as the mean ± SEM. One-way analysis of varianve (anova) followed by Dunnett’s multiple comparison test was used for statistical analysis of parametric data. The Kruskal–Wallis test followed by Dunn’s multiple comparison test was used for statistical analysis of non-parametric data. All statistical analyses were performed using graphpad prism (GraphPad Software Inc., San Diego, CA). Differences were considered to be statistically significant if P< 0·05.
Results
The development of arthritis and leucocyte accumulation in the joints of mice receiving control antibodies
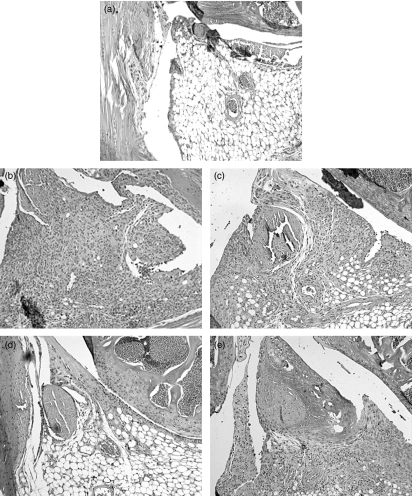
Mice receiving control antibodies (hamster and rat IgG) developed severe arthritis 7 days after intra-articular injection of mBSA. They had inflammation in the joints as assessed by synovitis and exudation of leucocytes (known to be mainly neutrophils56) in the joint space. The mice also showed signs of severe joint damage as indicated by cartilage degradation and bone loss (Figs 1 and 2b). Joints injected with saline only did not exhibit any signs of inflammation or joint damage (Fig. 2a). Control antibody-treated mice also had significant accumulation of CD4+ T cells (Fig. 3a) and macrophages (Fig. 3b) in the synovium of the diseased joints.
Figure 1.
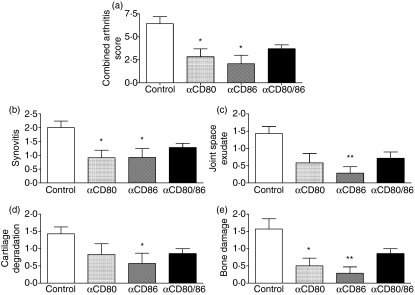
Inhibition of CD80 and/or CD86 during the effector phase of antigen-induced arthritis attenuates the severity of arthritis. The extent of joint injury was assessed by Safranin-O staining of formalin-fixed paraffin-embedded sections using four parameters: (b) synovitis, hypercellularity of the synovium; (c) joint space exudate, as leucocytes in the joint space; (d) cartilage degradation, as loss of Safranin-O staining (0 = fully stained cartilage, 3 = completely unstained cartilage); and (e) bone damage, as extent and depth of subchondral bone invasion by pannus. (a) The combined arthritis score was generated from the sum of scores for the above four parameters. *P< 0·05 versus control, **P< 0·01 versus control.
Figure 2.
Representative photomicrographs of joint injury in control antibody-treated mice and mice receiving anti-CD80 and/or anti-CD86 monoclonal antibody during the effector phase of antigen-induced arthritis. Knee joints were fixed in formalin, decalcified and embedded in paraffin, after which 5-μm thick sagittal sections were stained with Safranin-O and counterstained with fast green/iron haematoxylin. Control antibody-treated mice developed severe arthritis 7 days after the intra-articular injection of methylated bovine serum albumin (b), while no joint inflammation or injury was observed in joints injected with saline only (a). Inhibition of CD80 (c), CD86 (d), or CD80 and CD86 (e) reduced arthritis severity compared to control antibody-treated mice (b). Original magnification: × 400.
Figure 3.
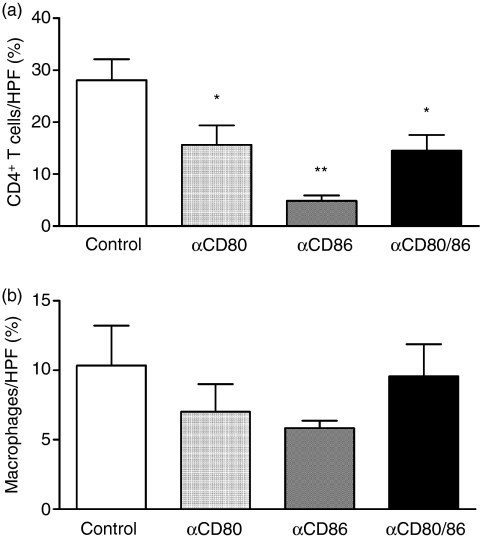
Accumulation of CD4+ T cells and macrophages in joints of control antibody-treated mice and in mice receiving anti-CD80 and/or anti-CD86 monoclonal antibodies (mAb) during the effector phase of antigen-induced arthritis. T cells and macrophages were demonstrated by immunoperoxidase staining of formalin-fixed paraffin-embedded joint sections. All mAb treatments significantly reduced T-cell accumulation in the synovium (a), while no statistically significant change in macrophage infiltration was observed (b). HPF, high-power field; % refers to the percentage of the HPF area occupied by positive staining. *P< 0·05 versus control, **P< 0·01 versus control and P< 0·05 versus anti-CD80.
The effect of anti-CD80 and/or anti-CD86 mAb treatment on the development of arthritis
To assess the role of CD80 and CD86 in the efferent phase of AIA, mice developing arthritis were treated with anti-CD80 and/or anti-CD86 mAb only during the effector phase of the arthritogenic immune response. Blockade of CD80 attenuated the development of arthritis compared to control antibody-treated mice as assessed by the combined arthritis score (Fig. 1a), synovitis (Figs 1b and 2c) and bone damage (Figs 1e and 2c). Exudation of leucocytes in the joint space (Figs 1c and 2c) and cartilage degradation (Figs 1d and 2c) were also diminished in mice receiving anti-CD80 antibody compared to control antibody-treated animals; however, these differences did not reach statistical significance. Similarly, inhibition of CD86 decreased arthritis severity as indicated by reduced joint inflammation (synovitis and joint space exudate) and joint damage (cartilage degradation and bone loss) (Figs 1 and 2d). Neutralization of both CD80 and CD86 caused a trend towards decreased arthritis severity compared to control antibody-treated mice (Figs 1 and 2e). However, the combined blockade resulted in a similar degree of protection from joint injury compared to the individual mAb treatments (Fig. 1) and no statistically significant differences were observed in any of the parameteres between any of the treatment groups (anti-CD80 versus anti-CD86, anti-CD80 versus anti-CD80/86, anti-CD86 versus anti-CD80/86).
The effect of CD80 and/or CD86 blockade on leucocyte accumulation in the joints
The development of AIA is dependent on joint-infiltrating CD4+ T cells and macrophages. Accumulation of T cells and macrophages at various sites of inflammation has been previously shown to be dependent on CD80 and CD86. Thus, infiltration of T cells and macrophages in the joints was assessed. All mAb treatments significantly decreased the accumulation of CD4+ T cells in the synovium compared to control antibody-treated mice (Fig. 3a). However, inhibition of CD86 alone had a greater effect than the anti-CD80 mAb treatment in reducing CD4+ T-cell infiltration in joints (Fig. 3a). Furthermore, none of the mAb treatments resulted in statistically significant changes in joint macrophage accumulation compared to control antibody-treated mice (Fig. 3b).
The effect of anti-CD80 and/or anti-CD86 mAb treatment on splenocyte cytokine production
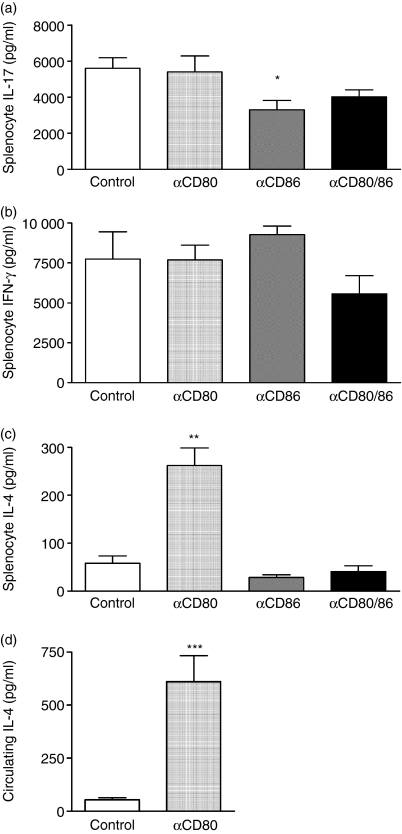
CD80 and CD86 have been shown to affect the development of Th1, Th2 and Th17 cells, and, in turn, Th1, Th2 and Th17-associated cytokines including IFN-γ, IL-4 and IL-17, respectively, can impact on disease severity in AIA. Therefore, levels of those cytokines were measured in supernatants obtained from ex vivo cultured antigen-restimulated splenocytes. Inhibition of CD80 alone did not affect IL-17 (Fig. 4a) or IFN-γ production (Fig. 4b), but it significantly increased splenocyte production of IL-4 (Fig. 4c). The enhanced splenocyte production of IL-4 correlated with increased circulating levels of IL-4 in mice treated with anti-CD80 mAb (Fig. 4d). In contrast, blockade of CD86 alone or both CD80 and CD86 did not significantly affect splenocyte production of IFN-γ (Fig. 4b) or IL-4 (Fig. 4c). However, neutralization of CD86 alone significantly suppressed IL-17 secretion by antigen-restimulated splenocytes (41% reduction; Fig. 4a). The combined CD80/86 blockade caused a trend towards reduced IL-17 production when compared to control antibody-treated mice (28% reduction; Fig. 4a).
Figure 4.
The effect of CD80 and/or CD86 inhibition on splenocyte cytokine production and serum levels of interleukin-4 (IL-4). Splenocyte IL-17 (a), interferon-γ (IFN-γ) (b) and IL-4 (c) production was measured by enzyme-linked immunosorbent assay (ELISA) on supernatants collected from splenocytes cultured in the presence of antigen (methylated bovine serum albumin) for 72 hr. Circulating levels of IL-4 (d) were measured by ELISA on serum collected at the end of experiments. Inhibition of CD80 did not affect splenocyte IL-17 or IFN-γ production, but it enhanced splenocyte production and serum levels of IL-4. Blockade of CD86 or both CD80 and CD86 did not affect IFN-γ or IL-4 production by cultured splenocytes. However, inhibition of CD86 alone significantly suppressed splenocyte IL-17 production. *P< 0·05 versus control, **P< 0·01 versus control, ***P< 0·001 versus control.
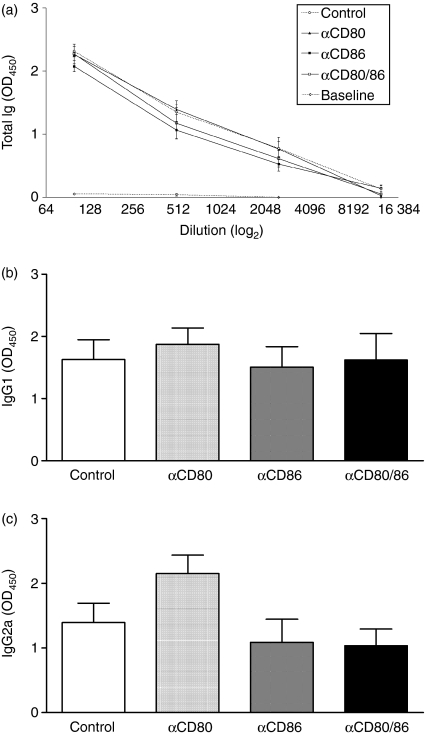
The effect of anti-CD80 and/or anti-CD86 mAb treatment on humoral immunity
CD80 and CD86 have also been shown to play a critical role in the development of humoral immune responses to various antigens. Thus, antigen-specific antibody levels were measured in serum collected at the end of experiments. However, none of the mAb treatments significantly affected circulating levels of mBSA-specific total IgG (Fig. 5a), or IgG subclasses, IgG1 (Fig. 5b) and IgG2a (Fig. 5c).
Figure 5.
Circulating antigen-specific antibody levels in control antibody-treated mice and mice receiving anti-CD80 and/or anti-CD86 monoclonal antibody (mAb) during the effector phase of antigen-induced arthritis. Serum levels of total immunoglobulin G (IgG) (a), IgG1 (b) and IgG2a (c) were measured by enzyme-linked immunosorbent assay on serum collected at the end of experiments. None of the mAb treatments significantly affected circulating antibody levels. Baseline represents values from sera of non-immunized mice.
Discussion
The role of CD80 and CD86 costimulatory molecules in the in vivo effector T-cell responses has not been extensively investigated. The current studies have examined the functional role of CD80 and CD86 in the effector phase of a widely used, CD4+ T-cell-dependent animal model of RA. To investigate the role of these costimulatory molecules in the effector arthritogenic T-cell responses, administration of inhibitory anti-CD80 and/or anti-CD86 mAb commenced at disease onset, on the day of intra-articular injection of mBSA, after the establishment of the immune response to the immunizing antigen.
Inhibition of CD80 or CD86 during the effector phase of AIA markedly attenuated signs of joint inflammation (synovitis and exudation of leucocytes in the joint space) and damage (cartilage degradation and bone loss). These results indicate that the presence of both molecules during the effector arthritogenic immune responses is required for the full development of disease. No further additive reduction in arthritis severity was observed when both CD80 and CD86 were blocked, suggesting that inhibition of either costimulatory molecule is adequate to obtain maximal disease amelioration. In addition, no statistically significant differences were observed in any of the parameters of joint injury measured between any of the treatment groups, indicating that all mAb treatments were similarly effective at diminishing arthritis severity.
Studies using adoptive transfer,26 CD4 depletion27 and CD4 deficiency25 have demonstrated that AIA is critically dependent on CD4+ T cells, while experiments using β2-microglobulin−/− and μ-chain−/− mice have shown that this model of RA is independent of CD8+ T cells and B cells,25 respectively. Macrophages are also implicated in the pathogenesis of both RA and experimental arthritis through the production of injurious proinflammatory cytokines such as TNF-α, IL-1 and IL-6, tissue-degrading enzymes such as MMPs and reactive oxygen species.1,24,33,35 In the current studies, accumulation of CD4+ T cells in joints was significantly decreased by all mAb treatments, correlating with reduced disease severity. These results suggest that one of the mechanisms by which CD80 and CD86 may contribute to joint injury in AIA is by locally augmenting CD4+ T-cell accumulation in diseased joints. Inhibition of CD86 had a greater effect in attenuating T-cell accumulation in joints than anti-CD80 mAb treatment, indicating that CD86 plays a more important role in local recruitment of pathogenic effector T cells than CD80. Blockade of CD80 and/or CD86 did not result in statistically significant reduction in local macrophage accumulation, indicating that decreased disease severity in any of the treatment groups cannot be attributed to alterations in macrophage recruitment. However, because CD4+ T cells are known to activate macrophages, the possibility that inadequate macrophage activation as a result of the lack of T cells in joints is contributing to the observed reduction in injury is not excluded.
Previous studies have demonstrated that CD80 and CD86 can play a role in the effector T-cell responses that cause tissue injury. For example, inhibition of CD80 and CD86 during the effector phase of crescentic glomerulonephritis significantly decreased glomerular accumulation of CD4+ T cells and macrophages and attenuated renal injury.47 Similarly, adoptive transfer of sensitized encephalitogenic T cells into CD80/86−/− mice markedly reduced clinical signs of experimental autoimmune encephalomyelitis which was associated with reduced leucocyte infiltration into the target tissue.46
It is currently not known what cell types express CD80 and/or CD86 in AIA joints or how these molecules may locally enhance T-cell accumulation in the tissue, but several possibilities exist. CD80 and CD86 are known to be expressed by professional antigen-presenting cells, but can also be induced on other cell types such as endothelial cells by proinflammatory cytokines.36 Therefore, CD80 and/or CD86 might be expressed by professional antigen-presenting cells including macrophages, B cells and dendritic cells that are present in diseased joints5 or by resident non-immune cells such as synovial and endothelial cells. Costimulation provided by CD80 and CD86 might enhance the initial infiltration of T cells into joints by upregulating local expression of adhesion molecules such as E-selectin, P-selectin and intercellular adhesion molecule 1 which are present in AIA joints.47,58,59 CD80/86 costimulation may alter the expression of chemokines, such as monocyte chemoattractant protein-1, interferon-γ-inducible protein-10 and macrophage inflammatory protein-1α, in the joints and/or chemokine receptors including CCR5 and CXCR3 on effector T cells that might be important for these cells to migrate into diseased tissue and mediate disease.60,61
The severity of joint inflammation and damage in AIA can be affected by various cytokines including Th1-associated IFN-γ, Th2-related IL-4 and Th17-associated IL-17. For example, inhibition of IL-4 during the initiation of AIA exacerbates disease.28 In addition, earlier experiments suggested that arthritis severity in AIA correlates with the development of Th1 responses and IFN-γ production.27,28 However, two recent studies using IFN-γ−/− mice have shown that IFN-γ plays a protective role in AIA,29,30 thus challenging previous concepts of Th1-mediated AIA. On the other hand, recent studies using neutralizing anti-IL-17 antibody have demonstrated that IL-17 plays a critical pathogenic role in the progression of joint inflammation and damage in AIA.29,31 Numerous studies have demonstrated that CD80 and CD86 can alter the Th1/Th2 balance and affect disease outcome.39,41,46 In addition, a recent study using mice that were deficient in both CD80 and CD86 has shown that these costimulatory molecules play an important role in the generation of Th17 cells.15 Therefore, IFN-γ, IL-4 and IL-17 release by antigen-restimulated splenocytes was measured to determine if reduced disease as the result of the anti-CD80 and/or anti-CD86 mAb treatments correlated with changes in cytokine production by effector/memory T cells. Reduced joint injury in mice treated with anti-CD80 mAb was associated with increased splenocyte production and circulating levels of IL-4. This suggests that another potential mechanism by which CD80 might exacerbate joint injury during the effector phase of AIA is by attenuating IL-4 production by effector T cells. Previous evidence has shown that CD80 can affect IL-4 production by recently activated CD4+ T cells.62 It is possible that increased IL-4 production is the result of interference with the interaction between CD80 and CTLA-4 because CD80 preferentially binds CTLA-4 and signalling through this receptor has been shown to downregulate Th2 responses.36,63 Inhibition of IL-4 has previously exacerbated the development of AIA28 and local or systemic delivery of IL-4 has resulted in the suppression of joint inflammation and destruction,64 demonstrating a protective role of IL-4. The IL-4 may exert its protective anti-inflammatory effects in the effector phase of AIA through a number of mechanisms, including (1) downregulation of macrophage activation and function,65 (2) inhibition of IL-1β, IL-6 and TNF-α production,66 (3) attenuation of MMP production,67 and (4) suppression of neutrophil influx into joints.68 Furthermore, in the current study, the potentially protective effect of IL-4 cannot be attributed to changes in Th1 or Th17 development because IFN-γ and IL-17 production by cultured splenocytes was not affected in mice receiving anti-CD80 mAb.
In contrast to anti-CD80 mAb treatment, the inhibition of CD86 alone or of both CD80 and CD86 did not alter the production of IFN-γ or IL-4 by antigen-challenged splenocytes, indicating that reduced disease severity in mice receiving anti-CD86 or anti-CD80/86 mAb was not the result of changes in Th1 or Th2 responses. However, blockade of CD86 alone or both CD80 and CD86 either significantly reduced or caused a trend towards decreased IL-17 production, respectively. This suggests that another mechanism by which CD86 may contribute to disease pathogenesis in AIA is by upregulating IL-17 production by effector T cells. Recent studies have demonstrated a pathogenic role of IL-17 in the development and progression of joint inflammation and damage in various models of RA, including AIA, CIA and adjuvant arthritis.19–21,31 Blockade of IL-17 during reactivation of AIA as well as neutralization of IL-17 in the absence of IFN-γ has previously prevented the progression of disease.29,31 IL-17 may contribute to joint inflammation as well as cartilage and bone degradation by stimulating various cell types including fibroblasts, macrophages, chondrocytes and osteoblasts to release proinflammatory cytokines such as IL-1 and TNF, and destructive mediators such as MMPs, as well as to upregulate the expression of receptor activator of nuclear factor-κB ligand (RANKL) thus promoting osteoclastogenesis.18
Studies using mice that were deficient in both CD80 and CD86 have shown that these costimulatory molecules also play an important role in the homeostasis of CD4+ CD25+ Treg cells.43,44 Depletion and adoptive transfer studies have demonstrated that Treg cells, in turn, play a suppressive role in joint inflammation and damage during the onset of AIA.32 In the current study, however, it is unlikely that the development and/or function of Treg cells was attenuated as the result of CD80 and/or CD86 blockade because these molecules were inhibited only during the effector phase of the immune response and, more importantly, arthritis severity and accumulation of effector T cells in joints were actually decreased, rather than increased, by all mAb treatments.
CD80 and CD86 have also been shown to play a critical role in the development of humoral immune responses to various antigens by affecting antibody production and isotype switching.39,42 However, in the present study, none of the mAb treatments affected circulating levels of mBSA-specific total IgG or IgG subclasses, IgG1 and IgG2a. This is most likely because of the inhibition of CD80 and/or CD86 late in the immune response, after the establishment of humoral immunity.
The current studies provide evidence that CD80 and CD86 play a critical pathogenic role in the effector phase of AIA. In support of our results, inhibition of CD80 and CD86 after the onset of CIA has also been demonstrated to be beneficial.49,55 Furthermore, CTLA-4–immunoglobulin, which blocks both CD80 and CD86, is currently being used in clinical trials and has reduced symptoms of RA.54 The present investigations provide further insight into the role of CD80 and CD86 during the effector arthritogenic T-cell responses that induce joint inflammation and destruction. While inhibition of either CD80 or CD86 significantly reduced arthritis severity, our results demonstrate a critical, previously unrecognized role of CD86, and not CD80, in upregulating effector T-cell production of IL-17, a recently discovered cytokine with increasing importance in the pathogenesis of RA. They also show that inhibition of CD86 is more effective than blockade of CD80 in reducing the accumulation of pathogenic effector T cells in inflamed joints. These results, together with the majority of evidence demonstrating higher expression of CD86 than CD80 in joints of patients with RA,52,53 raise the possibility that CD86 may be a more appealing therapeutic target than CD80 for the treatment of human RA and may suggest further specific approaches to fine-tuning costimulation blockade.
In summary, the current studies provide further evidence that CD80 and CD86 play an important in vivo role during the effector phase of T-cell-mediated immunity. They demonstrate that both CD80 and CD86 have important pathogenic, but distinct, roles in the effector CD4+ T-cell responses that induce joint inflammation and damage in AIA. CD80 contributes to arthritis severity by decreasing systemic levels of the anti-inflammatory cytokine, IL-4, as well as increasing CD4+ T-cell accumulation in joints, without affecting IL-17 production. In contrast, CD86 promotes joint inflammation and destruction by upregulating production of IL-17 and enhancing accumulation of pathogenic effector T cells in joints, without affecting Th1 or Th2 development. Furthermore, these results provide new in vivo evidence demonstrating that CD80 and CD86 have distinct roles in the regulation of Th17 function during the effector phase of the immune response, with CD86, and not CD80, playing a critical role in the production of IL-17 by effector/memory T cells.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia. The authors would like to thank April Dacumos for technical assistance and Dr David Tarlinton (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for providing the 16-10A1 and GL1 hybridomas.
Abbreviations
- AIA
antigen-induced arthritis
- CFA
complete Freund’s adjuvant
- CIA
collagen-induced arthritis
- CTLA-4
cytotoxic T-lymphocyte-associated antigen-4
- ELISA
enzyme-linked immunosorbent assay
- HPF
high-power field
- HRP
horseradish peroxidase
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- mBSA
methylated bovine serum albumin
- MMP
matrix metalloproteinase
- RA
rheumatoid arthritis
- Th
T helper
- TNF
tumour necrosis factor
- Treg
T regulatory
References
- 1.Kinne R, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester G. Macrophages in rheumatoid arthritis. Arthritis Rheum. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillinger M, Abramson S. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:691–714. [PubMed] [Google Scholar]
- 3.Weyand C, Seyler T, Goronzy J. B cells in rheumatoid synovitis. Arthritis Res Ther. 2005;7:S9–12. doi: 10.1186/ar1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skapenko A, Leipe J, Lipsky P, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7:S4–14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarvak T, Natvig J. Cell–cell interactions in synovitis. Antigen presenting cells and T cell interaction in rheumatoid arthritis. Arthritis Res. 2000;3:13–7. doi: 10.1186/ar135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978;298:869–71. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- 7.Van Boxel J, Paget S. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975;293:517–20. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- 8.Paulus H, Machleder H, Levine S, Yu D, MacDonald N. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977;20:1249–62. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- 9.Panayi G, Tugwell P. The use of cyclosporin A in rheumatoid arthritis: conclusions of an international review. Br J Rheumatol. 1994;33:967–99. doi: 10.1093/rheumatology/33.10.967. [DOI] [PubMed] [Google Scholar]
- 10.Raza K, Fulciani F, Curnow S, et al. Early rheumatoid arthritis is characterised by a distinct and transient synovial fluid cytokine profile of T-cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miltenburg A, van Laar J, de Kuiper R, Daha M, Breedveld F. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–10. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 12.Schulze-Koops H. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Practice Res Clin Rheumatol. 2001;15:677–91. doi: 10.1053/berh.2001.0187. [DOI] [PubMed] [Google Scholar]
- 13.Constantin A, Loubet-Lescoulie P, Lambert N, Yassine-Diab B, Abbal M, Mazieres B, de Preval C, Cantagrel A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse-transcriptase-polymerase chain reaction. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Almawi W, Melemedjian O, Rieder M. An alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-secreting profile. Clin Transplant. 1999;13:365–74. doi: 10.1034/j.1399-0012.1999.130501.x. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Li Z, Yang X, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toh M-L, Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol. 2007;19:284–8. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 17.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubberts E, Koenders M, van den Berg W. The role of T cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2004;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bush K, Farmer K, Walker J, Kirkham B. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–5. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 20.Lubberts E, Koenders M, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo C, Joosten L, van den Berg W. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–9. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 21.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 22.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4 + CD25 + T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Amelsfort J, van Roon J, Noordegraaf M, Jacobs K, Bijlsma J, Lafeber F, Taams L. Proinflammatory mediator-induced reversal of CD4 + ,CD25 + regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–42. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 24.Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–9. [PubMed] [Google Scholar]
- 25.Wong P, Quinn J, Sims N, van Nieuwenhuijze A, Campbell I, Wicks I. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158–68. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 26.Petrow P, Thoss K, Katenkamp D, Brauer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol Invest. 1996;25:341–53. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- 27.Pohlers D, Nissler K, Frey O, Simon J, Petrow P, Kinne R, Brauer R. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen-induced arthritis: influence on T helper cell activation. Clin Exp Immunol. 2004;135:409–15. doi: 10.1111/j.1365-2249.2003.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshino S, Yoshino J. Enhancement of T-cell-mediated arthritis in mice by treatment with a monoclonal antibody against interleukin-4. Cellular Immunol. 1998;185:153–7. doi: 10.1006/cimm.1998.1291. [DOI] [PubMed] [Google Scholar]
- 29.Irmler I, Gajda M, Bräuer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–36. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 30.Williams AS, Richards PJ, Thomas E, et al. Interferon-gamma protects against the development of structural damage in experimental arthritis by regulating polymorphonuclear neutrophil influx into diseased joints. Arthritis Rheum. 2007;56:2244–54. doi: 10.1002/art.22732. [DOI] [PubMed] [Google Scholar]
- 31.Koenders M, Lubberts E, Oppers-Walgreen B, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–9. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey O, Petrow P, Gajda M, et al. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4 + CD25 + T cells. Arthritis Res Ther. 2005;7:R291–301. doi: 10.1186/ar1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nissler K, Pohlers D, Huckel M, Simon J, Brauer R, Kinne R. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen induced arthritis: influence on macrophage activation. Ann Rheum Dis. 2004;63:1470–7. doi: 10.1136/ard.2003.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawlor K, Wong P, Campbell I, van Rooijen N, Wicks I. Acute CD4 + T lymphocyte-dependent interleukin-1-driven arthritis selectively requires interleukin-2 and interleukin-4, joint macrophages, granulocyte-macrophage colony-stimulating factor, interleukin-6, and leukemia inhibitory factor. Arthritis Rheum. 2005;52:3749–54. doi: 10.1002/art.21495. [DOI] [PubMed] [Google Scholar]
- 35.Simon J, Surber R, Kleinstauber G, Petrow P, Henzgen S, Kinne R, Brauer R. Systemic macrophage activation in locally-induced experimental arhtritis. J Autoimmun. 2001;17:127–36. doi: 10.1006/jaut.2001.0534. [DOI] [PubMed] [Google Scholar]
- 36.Salomon B, Bluestone JA. Complexities of CD28/B7:CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 37.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 39.Odobasic D, Kitching A, Tipping P, Holdsworth S. CD80 and CD86 costimulatory molecules regulate crescentic glomerulonephritis by different mechanisms. Kidney Int. 2005;68:584–92. doi: 10.1111/j.1523-1755.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161:2762–71. [PubMed] [Google Scholar]
- 41.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–93. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 42.Borriello F, Sethna M, Boyd S, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal centre formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 43.Greenwald R, Freeman G, Sharpe A. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 44.Salomon B, Lenschow D, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J. B7/CD28 costimulation is essential for the homeostasis of the CD4 + CD25 + immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 46.Chang T, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–40. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odobasic D, Kitching A, Semple T, Timoshanko J, Tipping P, Holdsworth S. Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J Am Soc Nephrol. 2005;16:2012–22. doi: 10.1681/ASN.2004060437. [DOI] [PubMed] [Google Scholar]
- 48.Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–8. [PubMed] [Google Scholar]
- 49.Webb lM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26:2320–8. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 50.Knoerzer D, Karr R, Schwartz B, Mengle-Gaw L. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96:987–93. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tellander A, Pettersson U, Runstrom A, Andersson M, Michaelsson E. Interference with CD28, CD80, CD86 or CD152 in collagen-induced arthritis. Limited role of IFN-gamma in anti-B7-mediated suppression of disease. J Autoimmun. 2001;17:39–50. doi: 10.1006/jaut.2001.0527. [DOI] [PubMed] [Google Scholar]
- 52.Balsa A, Dixey J, Sansom D, Maddison P, Hall N. Differential expression of the costimulatory molecules B7.1 (CD80) and B7.2 (CD86) in rheumatoid synovial tissue. Br J Rheumatol. 1996;35:33–7. doi: 10.1093/rheumatology/35.1.33. [DOI] [PubMed] [Google Scholar]
- 53.Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis: role of CD86 in the synovium. J Immunol. 1996;156:3074–86. [PubMed] [Google Scholar]
- 54.Kremer J, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 55.Quattrocchi E, Dallman M, Feldmann M. Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum. 2000;43:1688–97. doi: 10.1002/1529-0131(200008)43:8<1688::AID-ANR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitching AR, Tipping PG, Huang XR, Mutch D, Holdsworth SR. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 1997;52:52–9. doi: 10.1038/ki.1997.303. [DOI] [PubMed] [Google Scholar]
- 58.Veihelmann A, Harris A, Krombach F, Schutze E, Refior H, Messmer K. In vivo assessment of synovial microcirculation and leukocyte–endothelial cell interaction in mouse antigen-induced arthritis. Microcirculation. 1999;6:281–90. [PubMed] [Google Scholar]
- 59.Abrams J, Kelley S, Hayes E, Kikuchi T. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells and endothelial cells. J Exp Med. 2000;192:681–93. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herold KC, Lu J, Rulifson IC, Vezys V, Taub D, Grusby MJ, Bluestone JA. Regulation of C-C chemokine production by murine T cells by CD28/B7 costimulation. J Immunol. 1997;159:4150–3. [PubMed] [Google Scholar]
- 61.Carroll R, Riley J, Levine B, Feng Y. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4 + T cells. Science. 1997;276:273–6. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 62.Natesan M, Razi-Wolf Z, Resier H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783–91. [PubMed] [Google Scholar]
- 63.Oosterwegel MA, Mandelbrot DA, Boyd SD, Lorsbach RB, Jarrett DY, Abbas AK, Sharpe AH. The role of CTLA-4 in regulating Th2 differentiation. J Immunol. 1999;163:2634–9. [PubMed] [Google Scholar]
- 64.Kim S, Evans C, Kim S, oligino T, Ghivizzani S, Robbins P. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000;2:293–302. doi: 10.1186/ar104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen J, Wong H, Costa G, Bienkowski M, Wahl S. Suppression of monocyte function and differential upregulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344–51. [PubMed] [Google Scholar]
- 66.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid arthritis. Arthritis Rheum. 1992;35:874–83. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- 67.Borghaei R, Rawlings P, Mochan E. Interleukin-4 suppression of interleukin-1-induced transcription of collagenase (MMP-1) and stromelysin (MMP-3) in human synovial fibroblasts. Arthritis Rheum. 2004;41:1398–406. doi: 10.1002/1529-0131(199808)41:8<1398::AID-ART8>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bober L, Rojas-Triana A, Jackson J, Leach M, Manfra D, Narula S, Grace M. Regulatory effects of interleukin-4 and interleukin-10 on human neutrophil function ex vivo and on neutrophil influx in a rat model of arthritis. Arthritis Rheum. 2000;43:2660–7. doi: 10.1002/1529-0131(200012)43:12<2660::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]