Abstract
The mechanism of the T-cell response and cytokine induction to restrict human immunodeficiency virus 1 (HIV-1) infection is not clear. During early infection, HIV-infected individuals have a high frequency of virus-specific cytotoxic T lymphocytes (CTLs) that effectively reduces the viral load. However, the CTLs are unable to clear the virus at later stages of infection, leading to disease progression. Dysregulation of cytokines like interleukin-12 (IL-12) and interferon-γ (IFN-γ) as a result of the interaction of HIV-1-specific T cells with antigen-presenting cells is one of the possible causes of CTL dysfunction. Secretion of IL-12 is reduced with the progression of HIV infection, correlating with impaired CTL function; however, the role of IL-12 in CTL regulation awaits elucidation. Here, we have studied the role of IL-12 in CTL dysfunction by using DNA immunization of wild-type (WT) and IL-12-deficient mice with HIV-1 gp120 complementary DNA. It was observed that the CTL response in IL-12-deficient mice was significantly less than that in WT mice. Our results further demonstrated that coimmunization with IL-12 vector restored the impaired CTL response in IL-12-deficient mice. However, immunization with IL-12 vector failed to rescue the CTL response in IFN-γ deficient mice, suggesting that the CTL-promoting function of IL-12 is IFN-γ-mediated. Our data suggest a phase-specific role of IL-12 in the CTL response, specifically in the priming of CD4+ T cells that provide help to CD8+ T cells. Our results also suggest that IL-12 is vital for the priming of antigen-specific T cells and plays an essential role in IFN-γ induction in T cells.
Keywords: cytokine, cytotoxic T lymphocytes, gp120, human immunodeficiency virus 1, interleukin-12
Introduction
The immune system comprises a composite array of cells that preserve the integrity of the organism by eliminating the pathogens. Both the innate and adaptive arms play a vital role in clearance of pathogens. The key players of adaptive cellular immune response are T lymphocytes, which include cells with a cytotoxic effector function that are called cytotoxic T lymphocytes (CTLs).1 The CTLs kill the target cells by one of at least three distinct pathways. Two of them involve direct cell–cell contact between effector and targets. One is binding of Fas ligand (expressed on effector CTLs) to Fas receptor, which is present on target cells; this leads to apoptosis of the target cells.2 The second pathway involves the release of perforin and granzyme from the CTLs. Perforin is a soluble pore-forming cytolytic protein that is sequestered into cytotoxic granules. Upon the formation of an immunological synapse between target and CTL, cytotoxic granules fuse with the plasma membrane of the CTL and release their content (which also includes granzyme) into the synapse. In the synapse, perforin functionally synergizes with granzyme into the target cells, which leads to target cell death.3–5 The third pathway is mediated through interferon-γ (IFN-γ) and tumour necrosis factor-α, which are produced by effector cells after T-cell receptor stimulation and which act on the target cell.
Several studies suggest that CTLs play a critical role in antiviral immunity. Direct evidence for the protective role of CD8+ T cells was provided in a simian immunodeficiency virus model in Rhesus macaques, in which elimination of CD8+ T cells resulted in a dramatic increase in viral load.6 However, in spite of the presence of human immunodeficiency virus (HIV) -specific CTLs in infected individuals, virus production continues at a high level in the later stages of the infection. It is unclear why CD8+ T cells provide only partial protection and are unable to prevent progression to acquired immunodeficiency syndrome (AIDS). There could be several possible explanations for this dysfunction; depletion of essential helper CD4+ T cells by HIV may be one important cause for the progressive loss of CTL function. Second, HIV-1 infection also results in the overexpression of immunosuppressive agents and concomitant impairment of type 1 cytokine production from accessory and effector cells.7–9
Generation of effector CTLs appears to require three chronological signals: T-cell receptor ligation, costimulatory signal (B7 with CD28 and CTLA410) and interaction of the CD40 ligand (CD40L) present on the T cell with the CD40 that is expressed on antigen-presenting cells (APCs). Studies have demonstrated that interaction of CD40 with CD40L (CD154) induces secretion of interleukin-12 (IL-12) from APCs.11 Priming of the CTL response depends upon CD40 signalling.12 Interleukin-12 is a critical factor in the immune response against HIV because it is important for regulating IFN-γ production by T cells and natural killer cells, antigen presentation and accessory cell function by macrophages and dendritic cells, and cytolytic activities of CTLs and natural killer cells. Studies on the status of IL-12 during HIV infection indicate that although there is an increase in IL-12 synthesis in early infection,13,14 the secretion of IL-12 is gradually impaired with the progress of infection,15,16 which correlates with CTL dysfunction in the later stages of infection. Studies of the role of endogenous IL-12 using IL-12-deficient (IL-12−/−) mice have demonstrated the significance of IL-12 in IFN-γ expression and CTL generation. However, conflicting reports exist in the literature showing that IL-12−/− mice have no defect in IFN-γ secretion and are able to clear lymphocytic choriomeningitis virus infection,17 whereas other studies have shown that IFN-γ expression is defective in IL-12−/− mice and they are thereby more susceptible to infection.18
Several reports are available showing that the immune response can be induced against different HIV-1 proteins by DNA immunization.19,20 However, the role of cytokines in such a system is still not clearly understood. In the present work, we have examined the role of IL-12 in the induction of CTL generation by the HIV-1 Env protein gp120 as a model antigen, using DNA immunization in mice. Although the role of IL-12 in the generation of CTLs has been described, our results demonstrate for the first time that IL-12 is essential for the priming of CD4+ T cells. This induces the differentiation of CD8+ T cells into gp120-specific CTL effectors and the production of IFN-γ, suggesting that impairment of CD4+ T cells in the absence of IL-12 could be the possible cause of CD8+ T-cell dysfunction, as observed during HIV infection.
Materials and methods
Plasmid preparation
The gp120 sequence was amplified from a subtype C Indian isolate IN301904 (NIH AIDS Research and Reagent Program, USA21) by polymerase chain reaction and was cloned into pCDNA3.1 vector (Clontech, Mountain View, CA). The cloning of gp120 in pCDNA3.1 was confirmed by restriction digestion and sequence analysis. Expression of gp120 by pCDNAgp120 was confirmed both in vitro and in vivo (data not shown). The pMZ-Z1-mIL-12 vector, expressing the p35 and p40 subunits of IL-12, was obtained from Invivogen (San Diego, CA). Endotoxin-free plasmids were prepared using QIAGEN columns according to the manufacturer’s protocol (Qiagen, Valencia, CA).
Mice and immunization
BALB/c, CD40 and IL-12−/− (6- to 8-week-old) mice on a BALB/c background were obtained from Jackson Laboratories (Bar Harbor, ME) and were maintained in the Experimental Animal Facility of the National Centre of Cell Science, Pune. Mice were injected with 0·025% bupivacaine intramuscularly in the quadriceps muscle 48 hr before DNA immunization. Mice were later immunized intramuscularly using a 26-gauge needle with three doses of 100 μg pCDNAgp120 either alone or with pMG-Z1-mIL-12 on days 0, 15 and 30. The spleen was removed 10 days after the last immunization and the cells were used for the assays described below. The experiments accorded with the policies of the institutional committee for the purpose of control and supervision of experiments on animal approved protocols.
Enzyme-linked immunosorbent assay (ELISA) for gp120
Sera were collected from immunized mice 10 days after the last immunization. Direct ELISA was used to measure the antibody response against gp120. Briefly, the ELISA plate (Costar, Corning, NY) was coated overnight at 4° with 50 μl of 5 μg/ml gp120 protein in phosphate-buffered saline (PBS) obtained from Dr Ian M Jones (University of Reading, UK)22,23 Following washing with PBS containing 0·05% Tween-20, the wells were blocked for 2 hr with 5% bovine serum albumin (Amersham, Piscataway, NJ) and 0·05% Tween-20 in PBS. Sera were diluted in 5% bovine serum albumin/0·05% Tween-20 and added to the ELISA wells. Following incubation at 37°, the plate was washed five times and incubated with a 1 : 500 dilution of peroxidase-conjugated rabbit anti-mouse secondary antibody (KPL, Gaithersburg, MD). After washing, the presence of gp120 antibody was checked by measuring the development of colour with ABTS substrate (Roche Biochemicals, Mannheim, Germany). The reaction was stopped with 0·33 m HCl and analysed at 450 nm on an ELISA reader.
Preparation of murine splenocytes for CTL assay
Ten days after the last immunization mice were killed and their spleens were removed aseptically. A single-cell suspension was prepared by crushing the spleen with frosted-end slides. Red blood cells were removed by treating the spleen cells with Gey’s solution24 for 5 min at 4° followed by two washes with RPMI-1640.
T-cell proliferation assay
The 3[H]thymidine (TdR) uptake assay was used to measure the proliferation of splenocytes after antigenic stimulation. Splenocytes from immunized mice were resuspended at a concentration of 2 × 105 cells/200 μl in RPMI-1640 containing 10% fetal calf serum (FCS) and antibiotics. Three Subtype C gp120 peptides were synthesized encompassing both T-helper and CTL epitopes based on the HIV Molecular Immunology Database of Los Alamos National Laboratory, NM. The peptides are 335–349 KENWTDTLQRVSKKL, 307–321 SIRIGPGQTFYATGE, 101–115 NQMHEDVISLWDQSL (Sigma, St Louis, MO). The peptides were added at a final concentration of 10 μg/ml. After 60 hr, 1 μCi 3[H]TdR (BRIT, Mumbai, India) was added to each well and incubated for 12 hr at 37° in 5% CO2. The cells were harvested on glass fibre filter paper using a Packard cell harvester and the TdR uptake was counted in a Top count microplate counter (Perkin Elmer, Waltham, MA).
T-cell purification
Red-blood-cell-depleted cells were incubated in nylon wool (Robbins Scientific, Sunnyvale, CA) column for 90 min at 37° in 5% CO2 under sterile conditions. Cells were eluted and spun down at 225 g at 4°. Cells were incubated with rat serum for 30 min on ice and then CD4+ or CD8+ T-cell enrichment cocktail (Stem Cell Technology, Vancouver, BC, Canada) was added and the mixture was kept for 15 min on ice. Cells were then washed with PBS containing 1% FCS. The pellet was incubated with M-280 Dynal beads for 45 min with constant mixing at 4°. After incubation, cells were kept on a magnet (Dynal, Carlsbad, CA) for separation. Unwanted cells were bound to the magnet, while the desired cells came out in flow through. The flow through was spun down at 225 g at 4° and the resulting pellet contained purified CD8+ or CD4+ T cells.
CTL assay by JAM TEST
The CTL assay was performed following the method developed by Matzinger.25 Naive splenocytes were incubated with 10 μm of a pool of the three gp120 peptides described above for in vitro stimulation. Incubation was for 2 hr at 37° and then the cells were irradiated in a gamma-chamber. Splenocytes (2 × 106) from immunized mice were stimulated with 1 × 106 peptide-pulsed, irradiated, normal syngeneic splenocytes in 24-well tissue culture plates (Nunc, Rochester, NY). All cultures were incubated in RPMI-1640 supplemented with 10% heat inactivated FCS and 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). After 5 days, dead cells were removed on Histopaque (Sigma). The viable T cells were counted by a trypan blue exclusion method. These effector cells were used in CTL assays. The p815 cell line was pulsed with a pool of three gp120 peptides for 2 hr at 37° in 5% CO2. The target cells p815 were pulsed over night with 1 μCi [3H]TdR (BRIT) at 37° under sterile conditions. After two washes, the radiolabelled target cells were plated in complete RPMI-1640 at a concentration of 2·5 × 104 cells/100 μl. The effector cells were plated in various ratios with the target cells in a total volume of 100 μl in triplicate into the wells of a 96-well, U-bottomed tissue culture plate (Greiner, Frickenhausen, Germany). After 3 hr of incubation at 37°, cells were harvested in a cell harvester (Packard, Waltham, MA) and counts were analysed on a Top count microplate counter (Perkin Elmer).
Where, E = experimentally retained DNA in presence of Effectors (in counts/min) and S = retained DNA in the absence of Effectors.
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated from APC–T-cell coculture using Trizol (Invitrogen) according to the manufacturer’s instructions. Five micrograms RNA was used for first-strand cDNA synthesis using Moloney murine leukaemia virus reverse transcriptase. The cDNA was then used as a template for PCR amplification of mouse IFN-γ perforin and granzyme B. The primers used for PCR were: IFN-γ forward 5′-AACGCTACACACTGCATCTTGG-3′ and reverse 5′-CTCATGAATGCATCCTTTTTCG-3′; perforin forward 5′-CTCGCA TGTACAGTTTTCGCCTGG-3′ and reverse 5′-TGTGAGCCCATTCAGGGTCAGCTG-3′; granzyme B forward 5′-CTCGACCCTACATGGCCTTAC-3′ and reverse 5′-CCAgCCACATAGCACACATC-3′; and β-actin forward 5′-GTGGGCCGCTCTAGGCACCA-3′ and reverse 5′-TGGCCTTAGGGTTCAGGGGG-3′. Each sample was amplified for mouse β-actin to ensure equal input.
Statistical analysis
Each individual experiment was repeated at least three times. The error bars represent the mean ± SD of triplicate cultures in vitro. For in vivo experiments, error bars represent the mean ± SD, which is a minimum of four mice per group. Statistical analysis of the experimental data was conducted using Student’s t-test with the levels of significance defined as P< 0·05.
Results
gp120-mediated immune response in wild-type and IL-12−/− mice
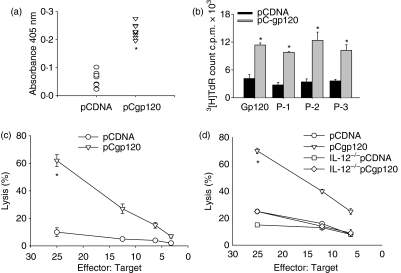
To study the immune response generated against gp120 using DNA immunization the gp120-specific antibody response was analysed in the serum. T helper and CTL assays were performed with splenocytes isolated from immunized mice. Our results clearly show elicitation of a gp120-specific antibody response in pCgp120-immunized mice as compared to pCDNA-immunized mice (Fig. 1a). A gp120-specific T helper proliferative response and CTL lysis were also observed in pCgp120-immunized mice as compared to pCDNA-immunized mice (Fig. 1b and c). To investigate the role of IL-12 in gp120-specific CTL responses, we immunized BALB/c WT and IL-12−/− mice with empty and gp120-expressing vectors and analysed the gp120-specific CTL response. CTL lysis was impaired in IL-12−/− mice immunized with pCgp120 compared with WT mice, indicating the role of IL-12 in the generation of gp120-specific CTL responses (Fig. 1d).
Figure 1.
Immune response against gp120 in mice using DNA immunization. (a) Analysis of gp120 antibody response from pCDNA-immunized and pCgp120-immunized mice. The sera from pCDNA- and pCgp120-immunized wild-type (WT) mice were assayed at a dilution of 1 : 250 for a gp120-reactive antibody response on gp120-protein-coated enzyme-linked immunosorbent assay plates as described in the text. (b) gp120-specific T helper cell proliferation in immunized mice. Splenocytes from pCDNA-injected and pCgp120-injected mice were plated at 2 × 105 cells/well in 96-well plates and pulsed with 10 μg gp120 peptides or without antigen (medium). Proliferation was assessed by [3H]thymidine-incorporated assay. (c) gp120-specific cytotoxic T-lymphocyte (CTL) response in pCDNA- and pCgp120-immunized WT mice. BALB/c mice were immunized with pCDNA and pCgp120. Splenocytes from pCDNA- and pCgp120-injected mice were plated at 2 × 106 cells/well in 24-well plates with 1 × 106 irradiated, gp120-peptide-pulsed naive splenocytes. After 5 days of culture, viable CD8+ T cells were harvested and plated against [3H]thymidine-incorporated, gp120-peptide-pulsed p815 cells and tested for their cytolytic activity in standard 3½ hr in a JAM test. The Effector : Target ratios used are shown in the figure. Each data point is the mean of triplicate samples. (d) A gp120-specific CTL response in pCDNA- and pCgp120-immunized, IL-12-deficient mice. WT and IL-12 mice were immunized with pCDNA and pCgp120 and a gp120-specific CTL assay was performed as described above. The results represent three individual experiments and error bar represent the mean ± SD of a given group. The results are statistically significant as indicated by *P< 0·05.
IL-12 is required during the priming of CTLs and APC-derived IL-12 during restimulation is not useful for elicitation of gp120-specific CTLs
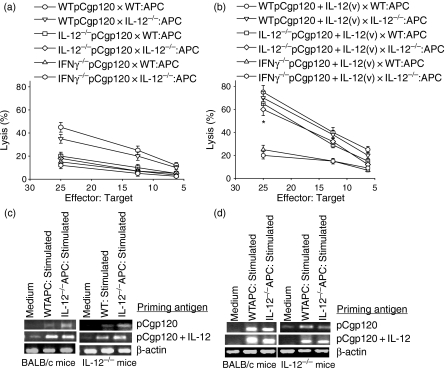
There are several reports demonstrating that IL-12 induces IFN-γ, which is required for effective CTL lysis. However, the stage at which IL-12 is required for IFN-γ production during CD8+ T-cell differentiation is not clearly understood. Wild-type, IL-12−/− and IFN-γ−/− mice were immunized with pCgp120 alone or pCgp120 along with plasmid encoding IL-12. The gp120-specific CTL lysis was significantly reduced in IL-12−/− and IFN-γ−/− mice (Fig. 2a) compared with WT mice. To analyse the role of APCs in restimulation, CD8+ T cells were stimulated by both WT and IL-12−/− APCs. Our results demonstrate that there was no significant difference in CTL response using either WT or IL-12−/− APCs, suggesting thereby that IL-12 is not required during in vitro restimulation of CTLs. Copriming with IL-12 vector rescued the CTL activity in IL-12−/−mice (Fig. 2b) but not in IFN-γ−/− mice, indicating the significance of IL-12 in the priming of CTLs but probably not in the effector function of CTLs. These results further suggest that IFN-γ, induced by IL-12 during priming, might help in CTL maturation. The RT-PCR profile for IFN-γ gene expression shows that expression was enhanced when IL-12 vector was injected along with pCgp120 in WT and IL-12−/− mice (Fig. 2c). The impaired functional activity of cytotoxic T lymphocytes during HIV-1 infection has been recently attributed to decreased intracellular levels of perforin26 in addition to the antigen-specific defects reported earlier.27 Therefore, we investigated expression of the perforin gene and demonstrated that copriming with the IL-12 vector induced perforin in both WT and IL-12−/− mice (Fig. 2d), reiterating the role of IL-12.
Figure 2.
Antigen-presenting cell (APC) derived interleukin-12 (IL-12) is not required for restimulation but interferon-γ (IFN-γ) is necessary for the cytotoxic T-lymphocyte (CTL) response. BALB/c, IL-12−/− and IFN-γ−/− mice were immunized with pCgp120 and pCgp120 + IL-12 vector. Splenocytes from pCgp120- and pCgp120 + IL-12-injected wild-type (WT), IL-12−/− and IFN-γ−/− mice were plated at 2 × 106 per well in 24-well plates with 1 × 106 gp120-peptide-pulsed, irradiated naive WT or IL-12−/− splenocytes. After 5 days of culture, cells were harvested and plated against [3H]thymidine-incorporated gp120-pulsed p815 cells and tested for their cytolytic activity in a standard 3½-hr JAM test. The Effector : Target ratios used are shown in the figure. Each data-point is the mean of triplicate samples. The results represent three individual experiments and the error bars represent the mean ± SD of a given group. The results obtained using WT and IL-12−/− APCs were not statistically significant, whereas the data obtained in coimmunization with IL-12 were statistically significant as indicated by *P< 0·05. (a) gp120-specific CTL response in pCgp120 immunized WT, IL-12−/− and IFN-γ−/− mice stimulated with WT or IL-12−/− APCs. (b) gp120-specific CTL response in pCgp120-immunized or pCgp120 + IL-12-immunized WT, IL-12−/− and IFN-γ−/− mice stimulated with WT or IL-12−/− mice. (c) Reverse transcription–polymerase chain reaction (RT-PCR) for IFN-γ was performed with RNA isolated from the stimulated cells using gene-specific primers. (d) RT-PCR for perforin was performed with RNA isolated from the stimulated cells using gene-specific primers.
gp120-specific primed CD4+ T cells are required for CTL effector function
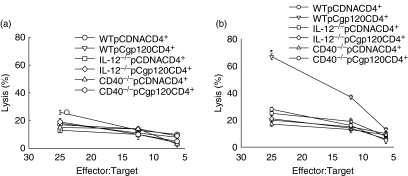
The generation of functional CTL critically depends on IFN-γ produced by CD4+ T cells.28,29 Therefore; we investigated the role of gp120-specific CD4+ T cells in the priming of CD8+ T cells to become effector CTLs. We immunized WT, IL-12−/− and CD40−/− mice with pCDNA and pCgp120 and isolated CD4+ and CD8+ T cells from these mice and cocultured them for 5 days in the presence of gp120-peptide-pulsed, irradiated naive macrophages. CD8+ T cells isolated from pCDNA-immunized WT mice and cocultured with six different group of CD4+ T cells from WT, IL-12−/− and CD40−/− mice did not show CTL lysis as expected (Fig. 3a); however, in the case of CD8+ T cells isolated from pCgp120-immunized mice and cocultured with CD4+ T cells from WT, IL-12−/− and CD40−/− mice, CTL activity was only observed in coculture containing CD4+ T cells from WT pCgp120-immunized mice (Fig. 3b). This result suggests that the presence of antigen-specific primed CD4+ T cells was a crucial requirement for effective CTL lysis and in the absence of IL-12, there is insufficient priming of CD4+ T cells. The unprimed CD4+ T cells fail to provide help to CD8+ T cells, as a result there was no lysis in coculture of CD8+ T cells isolated from pCgp120-immunized WT mice and CD4+ T cells isolated from pCgp120-immunized IL-12−/− and CD40−/− mice.
Figure 3.
CD4+ T cells primed in the presence of interleukin-12 (IL-12) can only provide help to CD8+ T cells for cytotoxic T-lymphocyte (CTL) function. BALB/c wild-type (WT), IL-12−/− and CD40−/− mice were immunized with pCDNA and pCgp120 and CD4+ T cells were isolated. CD8+ T cells were isolated from pCgp120-immunized WT mice. Both CD8+and CD4+ cells from different mice were cocultured with WT gp120-peptide-pulsed macrophages as the antigen-presenting cells (APCs). After 5 days of culture, viable CD8+ T cells were harvested and plated against [3H]thymidine-incorporated, gp120-pulsed p815 cells and tested for their cytolytic activity in a standard 3½-hr JAM test. The Effector : Target ratios used are shown in the figure. Each data-point is the mean of triplicate samples. The results represent three individual experiments and the error bar represents the mean ± SD of a given group. The results obtained with gp120-immunized WT CD4+ T cells are statistically significant, as indicated by *P< 0·05. (a) gp120-specific CTL activity in CD8+ T cells isolated from pCDNA-immunized WT mice. (b) gp120-specific CTL activity in CD8+ T cells isolated from pCgp120-immunized WT mice.
IL-12 vector restores the CD4+ T-cell priming and help of CD8+ T cells
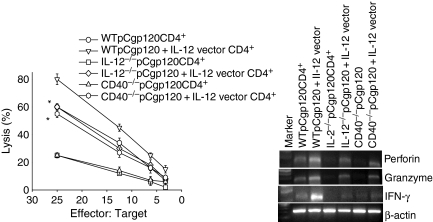
We then immunized WT, IL-12−/− and CD40−/− mice with pCgp120 and pCgp120 along with IL-12 vector on days 0, 15 and 30. The mice were killed 10 days after the last immunization. CD4+ T cells were isolated from these immunized mice. For the same experiment we isolated CD8+ T cells from only WT mice immunized with pCgp120. These CD8+ T cells were cocultured with six groups of CD4+ T cells as mentioned above and CTL assays were performed. The results show that IL-12-coimmunized CD4+ T cells not only induced CTL activity of WT gp120-specific T cells but also rescued the CTL activity in the cocultures of CD4+ T cells from IL-12−/− and CD40−/− mice and CD8+ T cells from WT mice (Fig. 4a). These findings suggest that CD4+ T cells primed with antigen (gp120) in presence of IL-12 are capable of providing help to CD8+ T cells for effective lysis. We also performed RT-PCR for IFN-γ, perforin and granzyme B from cellular RNA isolated from the coculture experiments described above. The results obtained showed up-regulation of IFN-γ, perforin and granzyme, in IL-12-vector-immunized mice (Fig. 4b), which are known to be involved in effective CTL lysis. These results collectively show that IL-12 plays an important role in the priming of antigen-specific CD4+ T cells, which provide help to CD8+ T cells for CTL effector function.
Figure 4.
Interleukin-12 (IL-12) coimmunization restores the CD4+ T-cell priming and help of CD8+ T cells in IL-12−/− and CD40−/− mice. BALB/c wild-type (WT), IL-12−/− and CD40−/− were immunized with pCgp120 or pCgp120 + IL-12 vector and CD4+ T cells were isolated. CD8+ T cells were isolated from pCgp120-immunized WT mice. Both CD8+ and CD4+ cells from different mice were cocultured with WT gp120-peptide-pulsed macrophages as antigen-presenting cells (APCs). After 5 days of culture, viable CD8+ T cells were harvested and plated against [3H]thymidine-incorporated, gp120-pulsed p815 cells and tested for their cytolytic activity in a standard 3½-hr JAM test. The Effector : Target ratios used are shown in the figure. Each data-point is the mean of triplicate samples. The results represent three individual experiments and the error bar represents the mean ± SD of a given group. RNA was isolated and reverse transcription–polymerase chain reaction (RT-PCR) was performed for interferon-γ, perforin and granzyme B using gene-specific primers. (a) gp120-specific CTL activity in CD8+ T cells isolated from pCgp120-immunized mice in the presence of CD4+ cells from IL-12 vector coimmunized mice. The results obtained with IL-12 vector coimmunization are statistically significant, as indicated by *P< 0·05. (b) Perforin, granzyme B and IFN-γ expression by RT-PCR in the coculture described in (a).
Discussion
During HIV infection various cytokines are overproduced in the early stages whereas with the progression of disease, cytokines secreted from T cells become selectively deficient. Peripheral blood mononuclear cells from HIV-infected patients not only show impaired IL-12 induction compared to uninfected controls30–32 but also show a shift from a T helper type 1 to a type 2 cytokine pattern.16 Thus, IFN-γ is also impaired at the later stages of HIV infection.33,34 This suggests the significance of IL-12 because it results in the inability of the patient to mount a strong cellular immune response against the virus.
We have immunized WT and IL-12−/− mice with pCgp120 and observed highly diminished CTL lysis in IL-12−/− mice. Our results indicate the importance of IL-12 in the CTL response. We have also immunized WT, IL-12−/− and IFN-γ−/− mice with pCgp120 or pCgp120 along with IL-12 vector. The CTL response was augmented when IL-12 vector was coimmunized along with pCgp120 compared with pCgp120 alone.35 Our results also show that IL-12 vector rescues the impaired CTL response in IL-12−/− mice; however, immunization of IL-12 vector in IFN-γ−/− mice was unable to restore the CTL response. These observations have suggested that IL-12-mediated IFN-γ is vital for effective CTL. To analyse the role of APC-derived IL-12 for restimulation, we cocultured the primed T cells from immunized mice with APCs from WT and IL-12−/− mice for 6 days and then observed the CTL response. There was no significant difference between WT and IL-12−/− APCs, implying that IL-12 plays a crucial role in the initial priming of T cells and not at the stage of restimulation. This result is consistent with those in a study where HIV-1 protein-loaded DCs augmented the HIV-1-specific CD8+ T-cell response and IFN-γ response from late-stage HIV patients on prolonged highly active anti-retorviral therapy in presence of IL-12.36
The production of IL-12 by phagocytic cells is induced by a variety of mechanisms that may be T-cell-dependent or T-cell-independent. Induction of IL-12 by a T-cell-independent mechanism is the result of interaction of molecules like CD44 with low-molecular-weight fragments of the extracellular matrix glycosamine hyaluronan (LMW-HA), that accumulate during inflammation caused by intracellular pathogens, fungi, viruses or by their biproducts such as lipopolysaccharide and bacterial DNA.37 The T-cell-dependent mechanism of IL-12 production is dependent on the ability of CD40L expressed on activated T cells to interact with CD40 receptor on the surface of monocytes or macrophages and dendritic cells.38 The T-cell-dependent mechanism plays an important part in the immunoregulatory role of IL-12 in the maintenance of a T helper type 1 response.39–41 We have isolated CD4+ T cells from pCgp120-immunized WT, IL-12−/− and CD40−/− mice and cocultured them with CD8+ T cells isolated from pCDNA-immunized and pCgp120-immunized mice and have observed impaired CTL lysis in all groups except WT CD4+ T cells. This observation suggests that gp120 DNA immunization results in T-cell-dependent induction of IL-12 as CTL lysis was impaired in IL-12−/− and CD40−/− mice and it also suggests the requirement of antigen-specific CD4+ T-cell help for the effector function of CTL.
Recent findings suggest that CD40 engagement enhances antigen presentation in Langerhans cells and primes T cells to induce IFN-γ independent of IL-12.42 To investigate the role of CD40-induced IL-12 in the elicitation of gp120-specific CTL activity, we therefore, isolated CD8+ T cells from pCgp120-immunized WT mice and cocultured them with CD4+ T cells from pCgp120-immunized or pCgp120 + IL-12 vector-immunized WT, IL-12−/− and CD40−/− mice. The results clearly indicated that immunization with IL-12 vector restores the CTL lysis in IL-12−/− and CD40−/− mice and also augments CTL lysis by increased expression of perforin, granzyme B and IFN-γ.
In conclusion, the present data indicate that impaired IL-12-induced priming of antigen-specific CD4+ T cells can lead to CTL dysfunction, implying a possible beneficial effect of IL-12 in AIDS patients to enhance the T helper type 1 response. However, several clinical trials of IL-12 treatment in HIV patients have yielded conflicting results and require further study.43–45 It might also be desirable to reduce overproduction of various cytokines, including IL-12, at the early stage to counteract the excessive immune activation that is known to increase HIV replication.46
Acknowledgments
The work was supported by the Department of Biotechnology, Government. of India. S.G. is a senior research fellow of NCCS. The authors thank the experimental animal facility of NCCS for their help in the investigation. The recombinant gp120 protein was a kind gift from Dr Ian M. Jones of the University of Reading, U.K. The subtype C clone p93IN301904 was obtained from NIH AIDS Research and Reference Reagent Program, USA.
References
- 1.Andersen MH, Schrama D, Thor SP, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S. Fas-mediated apoptosis. Adv Exp Med Biol. 1996;406:119–24. doi: 10.1007/978-1-4899-0274-0_12. [DOI] [PubMed] [Google Scholar]
- 3.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–87. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 4.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 5.Trapani JA, Sutton VR. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr Opin Immunol. 2003;15:533–43. doi: 10.1016/s0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 7.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997;187:187–228. [PubMed] [Google Scholar]
- 8.Shearer GM, Levy RB. Noninfectious cofactors in susceptibility to AIDS: possible contributions of semen, HLA alloantigens, and lack of natural resistance. Ann N Y Acad Sci. 1984;437:49–57. doi: 10.1111/j.1749-6632.1984.tb37121.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee BN, Lu JG, Kline MW, Paul M, Doyle M, Kozinetz C, Shearer WT, Reuben JM. Type 1 and type 2 cytokine profiles in children exposed to or infected with vertically transmitted human immunodeficiency virus. Clin Diagn Lab Immunol. 1996;3:493–9. doi: 10.1128/cdli.3.5.493-499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 11.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan B, Thomas R. CD40 and dendritic cell function. Crit Rev Immunol. 2003;23:83–107. doi: 10.1615/critrevimmunol.v23.i12.50. [DOI] [PubMed] [Google Scholar]
- 13.Rockstroh JK, Kruezer KA, Sauerbruch T, Spengler U. Protein levels of interleukin-12 p70 holomer, its p40 chain and interferon-gamma during advancing HIV infection. J Infect. 1998;37:282–6. doi: 10.1016/s0163-4453(98)92138-7. [DOI] [PubMed] [Google Scholar]
- 14.Vanham G, Penne L, Devalck J, Kestens L, Colebunders R, Bosmans E, Thielemans K, Ceuppens JL. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin Exp Immunol. 1999;117:335–42. doi: 10.1046/j.1365-2249.1999.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–11. [PubMed] [Google Scholar]
- 16.Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–8. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Oxenius A, Karrer U, Zinkernagel RM, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–73. [PubMed] [Google Scholar]
- 18.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Pal R, Mascola JR, et al. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350:34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson AC, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet. 1998;351:1320–5. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 21.Lole KS, Bollinger RC, Paranjape RS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–60. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morikawa Y, Overton HA, Moore JP, Wilkinson AJ, Brady RL, Lewis SJ, Jones MI. Expression of HIV-1 gp120 and human soluble CD4 by recombinant baculoviruses and their interaction in vitro. AIDS Res Hum Retroviruses. 1990;6:765–73. doi: 10.1089/aid.1990.6.765. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Davies AH, Jones IM. Expression and purification of glutathione S-transferase-tagged HIV-1 gp120: no evidence of an interaction with CD26. Virology. 1995;208:142–6. doi: 10.1006/viro.1995.1137. [DOI] [PubMed] [Google Scholar]
- 24.Selected Method in Cellular Immunology. New York: W.H. Freeman and company; 1980. Preparation of mouse cell suspension; pp. 23–4. [Google Scholar]
- 25.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 26.Ang OO, Lin H, Dagarag M, Ng HL, Effros RB, Uittenbogaart CH. Decreased perforin and granzyme B expression in senescent HIV-1-specific cytotoxic T lymphocytes. Virology. 2005;332:16–9. doi: 10.1016/j.virol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wodarz D, Jansen VA. The role of T cell help for anti-viral CTL responses. J Theor Biol. 2001;211:419–32. doi: 10.1006/jtbi.2001.2358. [DOI] [PubMed] [Google Scholar]
- 29.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 30.Chehimi J, Starr SE, Frank I, D’Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura T, Gatanaga H, Borris DL, Connors M, Mitsuya H, Blauvelt A. Decreased stimulation of CD4+ T cell proliferation and IL-2 production by highly enriched populations of HIV-infected dendritic cells. J Immunol. 2003;170:4260–6. doi: 10.4049/jimmunol.170.8.4260. [DOI] [PubMed] [Google Scholar]
- 33.Bailer RT, Holloway A, Sun J, Margolick JB, Martin M, Kostman J, Montaner LJ. IL-13 and IFN-γ secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol. 1999;162:7534–42. [PubMed] [Google Scholar]
- 34.Kostense S, Vandenberghe K, Joling J, Van Baarle D, Nanlohy N, Manting E, Miedema F. Persistent numbers of tetramer+ CD8(+) T cells, but loss of interferon-gamma+ HIV-specific T cells during progression to AIDS. Blood. 2002;99:2505–11. doi: 10.1182/blood.v99.7.2505. [DOI] [PubMed] [Google Scholar]
- 35.Boyer JD, Cohen AD, Ugen KE, et al. Therapeutic immunization of HIV-infected chimpanzees using HIV-1 plasmid antigens and interleukin-12 expressing plasmids. AIDS. 2000;14:1515–22. doi: 10.1097/00002030-200007280-00007. [DOI] [PubMed] [Google Scholar]
- 36.Fan Z, Huang XL, Borowski L, Mellors JW, Rinaldo CR., Jr Restoration of anti-human immunodeficiency virus type 1 (HIV-1) responses in CD8+ T cells from late stage patients on prolonged antiretroviral therapy by stimulation in vitro with HIV-1 protein loaded dendritic cells. J Virol. 2001;75:4413–9. doi: 10.1128/JVI.75.9.4413-4419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodge-Dufour J, Marino MW, Horton MR, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:13806–11. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy M, Picha KS, Fanslow WC, Grabstein KH, Alderson MR, Clifford KN, Chin WA, Mohler KM. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur J Immunol. 1996;26:370–8. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 39.Kelsall BL, Stuber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40–CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996;795:116–26. doi: 10.1111/j.1749-6632.1996.tb52660.x. [DOI] [PubMed] [Google Scholar]
- 40.Stuber E, Strober W, Neurath M. Blocking the CD40L–CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–8. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanham G, Penne L, Devalck J, Kestens L, Colebunders R, Bosmans E, Thielemans K, Ceuppens JL. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin Exp Immunol. 1999;117:335–42. doi: 10.1046/j.1365-2249.1999.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorbachev AV, Fairchild RL. CD40 engagement enhances antigen-presenting langerhans cell priming of IFN-gamma-producing CD4+ and CD8+ T cells independently of IL-12. J Immunol. 2004;173:2443–52. doi: 10.4049/jimmunol.173.4.2443. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson MA, Hardy D, Conninck E, Watson J, Debruin M. Phase I trial of a single dose of recombinant human interleukin-12 in human immunodeficiency virus infected patients with 100–500 CD4 cells/microL. J Infect Dis. 2000;182:1070–6. doi: 10.1086/315819. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson MA, Spritzler J, Landay A, et al. AACTG 325 protocol team. A phase I, placebo-controlled trial of multi-dose recombinant human interleukin-12 in patients with HIV infection. AIDS. 2002;16:1147–54. doi: 10.1097/00002030-200205240-00008. [DOI] [PubMed] [Google Scholar]
- 45.Little RF, Pluda JM, Wyvill KM, Rodriguez-Chavez IR, Tosato G, Catanzaro AT, Steinberg SM, Yarchoan R. Activity of subcutaneous interleukin-12 in AIDS related Kaposi sarcoma. Blood. 2006;107:4650–7. doi: 10.1182/blood-2005-11-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar-Gonzalez JF, Martinez-Maza O, Nishanian P, et al. Increased immune activation precedes the inflection point of CD4 T cells and the increased serum virus load in human immunodeficiency virus infection. J Infect Dis. 1998;178:423–43. doi: 10.1086/515629. [DOI] [PubMed] [Google Scholar]