Abstract
Mannose-binding lectin (MBL) exists in the serum as a complex with MBL-associated serine protease (MASP). A recent paper described how MASP-free recombinant rat MBL stimulates the phagocytosis of Escherichia coli and Staphylococcus aureus by rat Kupffer cells through an increase in the level of a phagocytosis receptor. We have examined the effect of human MBL on the phagocytic action of human macrophages. Purified recombinant human MBL stimulated the phagocytosis of E. coli by THP-1 macrophages, leaving that of latex beads, apoptotic human cells, zymosan particles or S. aureus unchanged. This stimulatory effect was observed when either phagocytes or targets were preincubated with MBL. Furthermore, MBL bound to THP-1 macrophages as well as to E. coli, but not to S. aureus, through lipid A. These results indicated that human MBL in the absence of MASP stimulates macrophage phagocytosis of E. coli by bridging targets and phagocytes.
Keywords: macrophages, mannose-binding lectin, microbial pathogens, phagocytosis
Introduction
Failure in the expeditious removal of invading microbes or altered self cells impairs the development, as well as increases the risk of infectious diseases, inflammation and autoimmunity. These ‘foreign’ or ‘unwanted’ cells are selectively recognized and engulfed by phagocytes.1 The selectivity in phagocytosis is achieved through specific molecular recognition by receptors residing at the surface of phagocytes and their ligands or markers of phagocytosis on the surface of target cells.1,2 The markers are either surface constituents of the target cells or soluble molecules in body fluid that specifically bind to the targets. Bacteria are mostly phagocytosed by neutrophils and macrophages, often with the aid of proteins present in serum, such as immunoglobulins, complement components, collectin and thrombospondin.1–3 These serum proteins bind to bacteria and at the same time to the phagocytosis receptors represented by Fc receptors, complement receptors, collectin receptors and integrins; the acting of serum proteins as a bridge between phagocytes and targets is called opsonization.1–4
Mannose-binding lectin (MBL) belongs to a collectin family of proteins that possess two distinct functional domains; the collagenous domain located near the N terminus and the carbohydrate recognition domain at the C terminus.5,6 Three identical polypeptide chains of MBL are cross-linked covalently with disulphide bonds at the N terminus. This ‘structural subunit’ further forms oligomers (dimer to hexamer) in serum, and a higher order structure is considered necessary for MBL to accomplish its actions. MBL has two major roles in the immune system; the activation of complement components and the opsonization of unwanted cells.5–10 The carbohydrate recognition domain of MBL serves as a calcium-dependent lectin that binds to the 3-hydroxyl and 4-hydroxyl groups of sugars, including N-acetyl-D-glucosamine, mannose, N-acetyl-mannosamine, fucose and glucose. As an opsonin, MBL bridges targets and phagocytes by simultaneously binding to sugars on the surface of target cells using the carbohydrate recognition domain and to the complement receptor CR1 or the collectin receptor C1qR present on phagocytes, presumably using the collagenous domain. The phagocytosis of a variety of bacteria, including Staphylococcus aureus,11Mycobacterium bovis12 and Neisseria meningitidis,13 by neutrophils and macrophages in vitro is stimulated by MBL. In contrast, there are reports that MBL does not influence the phagocytosis in vitro of Mycobacterium avium14 and Candida albicans.15 Also, MBL seems to be involved in the phagocytic elimination of altered cells, i.e. apoptotic cells, with an opsonin-like action.16–18 Furthermore, a deficiency of MBL makes mice more susceptible to infections from S. aureus, accompanied by an increase in the number of bacteria in blood and organs,19,20 and impairs the clearance of apoptotic cells in the peritoneum.21 In the circulation, MBL is found in association with a protease called MBL-associated serine protease (MASP), which is responsible for the cleavage of C4, and thus the activation of complement. In most experiments cited above, MBL prepared from the sera of humans or rats, which is thus a complex with MASP, were used. Although Bonar et al. showed that recombinant human MBL stimulates the phagocytosis of mycobacteria by neutrophils in vitro, the stimulation was observed only when the reaction was supplemented with serum.12 Recently, Ono and colleagues reported that a MASP-free recombinant rat MBL stimulated the phagocytosis of S. aureus and Escherichia coli by rat Kupffer cells through an increase in the expression of class A scavenger receptor, a phagocytosis receptor for certain types of bacteria.22 This report suggests that MBL modulates host immune responses to invading microbes without the aid of MASP. In the present study, we examined whether MASP-free human MBL regulates the phagocytic action of human macrophages and, if so, how it does so in comparison with its effect on Kupffer cell actions.
Materials and methods
Materials
Phorbol 12-myristate 13-acetate (PMA), methotrexate, mannose-conjugated agarose and zymosan particles were purchased from Sigma-Aldrich (St Louis, MO). Q-Sepharose Fast Flow was obtained from Amersham Biosciences (Uppsala, Sweden), fluorescein isothiocyanate (FITC) was from Molecular Probes (Eugene, OR), and FITC-labelled latex beads (Polybead Microparticles, 1·72 μm in diameter) were from Polyscience (Warrington, PA). Native human MBL was purified from human sera as described previously.23 Recombinant human MBL was expressed in a cell line derived from Chinese hamster ovary cells and chromatographically purified as described previously,24 except that the final purification step was avoided and a mixture of proteins of different sizes was used in the experiments (see the text). Anti-human MBL rabbit antiserum, anti-human MBL mouse monoclonal antibody, and an enzyme-linked immuosorbent assay kit for human MBL used to determine the amount of MBL were purchased from Dobeel Corp. (Seongnam, Republic of Korea). Lipopolysaccharide (LPS) was obtained from E. coli K12 (rough-type LPS) or E. coli O18 (smooth-type LPS) as described previously.25 Lipid A of E. coli was purchased from the Peptide Institute (Minoh, Osaka, Japan). Calreticulin prepared from human sera and anti-human calreticulin rabbit antiserum26 were provided by Dr Shunji Natori (RIKEN, Wako, Japan). Recombinant human C1q was purchased from Complement Technology (Tyler, TX), and the peptides GRGDSP and GRGESP were obtained from Takara Bio (Otsu, Shiga, Japan).
Cell culture
The human monocyte-derived cell line THP-1 was maintained with RPMI-1640 medium containing 10% volume by volume (v/v) heat-inactivated fetal bovine serum at 37° with 5% (v/v) CO2 in air. THP-1 cells were used as phagocytes after differentiating into macrophages through incubation with PMA (160 nm) for 72 hr at 37°. The human leukaemic cell line Jurkat was cultured with the same medium containing 10% fetal bovine serum and induced to undergo apoptosis by incubation with the anticancer drug doxorubicin (0·3 μg/ml) for 30–36 hr. The extent of apoptosis was determined by measuring the ratio of cells with externalized phosphatidylserine and condensed chromatin, as described previously.27Escherichia coli (strain W3110) and S. aureus (strain Smith), obtained from Dr Kazuhisa Sekimizu (the University of Tokyo, Tokyo, Japan), were cultured with Luria broth at 37° until they reached a logarithmic phase of growth, and were washed successively with phosphate-buffered saline (PBS) containing 5 mm ethylenediaminetetraacetic acid (EDTA) (pH 8·0) and PBS alone. The harvested bacteria were incubated with 0·1 m sodium carbonate buffer (pH 9·5) containing FITC for fluorescence labelling and were used as target cells in the assay of phagocytosis.
Assay for phagocytosis
Phagocytosis reactions were conducted in the absence of serum using PMA-treated THP-1 cells as phagocytes and latex beads, apoptotic Jurkat cells, zymosan, E. coli or S. aureus as targets, essentially as described previously.27,28 Either phagocytes or targets were preincubated with MBL or left untreated as a control, and used in the reaction. For the phagocytosis of latex beads or zymosan, FITC-labelled latex beads (at a ratio of 50 targets to one phagocyte) or zymosan particles labelled with 5-carboxyfluorescein (Molecular Probes) (at a ratio of 10 targets to one phagocyte) were mixed with phagocytes, and the mixture was incubated at 37° for 2 hr. The samples were then washed with PBS, fixed, and examined by fluorescence microscopy. Apoptotic Jurkat cells were mixed with phagocytes (10 targets to one phagocyte), and the mixture was incubated at 37° for 30 min. The cells were then washed with PBS and fixed with paraformaldehyde, and their membranes were permeabilized with methanol. The samples were finally stained with haematoxylin and examined by light microscopy. The FITC-labelled bacteria were mixed with macrophages (at a ratio of 1000 for E. coli or 100 for S. aureus to one macrophage, unless otherwise stated in the text), which had been washed with PBS containing 5 mm EDTA (pH 8·0) then with PBS, and the mixture was incubated at 37° for 1 hr unless otherwise stated in the text. After the incubation for phagocytosis, the mixture was agitated by pipetting to remove bacteria that lightly attached to macrophages. The remaining macrophages were fixed with PBS containing 2% weight by volume (w/v) paraformaldehyde, 0·1% (w/v) glutaraldehyde and 0·05% (v/v) Triton X-100, and rinsed with PBS. The final samples in all the phagocytosis reactions with fluoresceinating targets were mounted with non-fluorescent glycerol and examined under a fluorescence–phase contrast microscope to determine the extent of phagocytosis. The numbers of macrophages with engulfed targets and of engulfed cells present in each macrophage were determined and expressed relative to the total number of macrophages (in percentage) and per 100 macrophages, respectively. To determine the extent of phagocytosis of bacteria by flow cytometry, FITC-labelled bacteria were mixed with phagocytes (at a ratio of 100 bacteria to one phagocyte) in RPMI-1640 medium at 37° for 45 min. The samples were then supplemented with an ice-cold solution consisting of 0·1 mm sodium acetate buffer (pH 4·5) and trypan blue (2 mg/ml), respectively, to terminate the reaction and quench unincorporated targets, and finally analysed by flow cytometry.
Assay for binding of MBL to bacteria and phagocytes
To examine the binding of MBL to bacteria, E. coli or S. aureus (1 × 108) that had been washed successively with PBS containing 5 mm EDTA and PBS, were suspended with PBS (0·1–0·3 ml) containing or not containing 10 mm EDTA and supplemented with recombinant human MBL (50 μg). The mixtures were incubated for 1 hr at 37° and washed three times with PBS. The washed bacteria were lysed with a buffer consisting of 63 mm Tris–HCl (pH 6·8), 2·5% (w/v) sodium dodecyl sulphate (SDS), and 1% (v/v) protease inhibitor cocktail (Sigma-Aldrich), and then solubilized proteins were resolved by SDS–7·5% (w/v) polyacrylamide gel electrophoresis (PAGE). The separated proteins were electrophoretically transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with 5% (w/v) dried skimmed milk and successively reacted with anti-MBL antiserum and horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody. Signals were visualized by chemiluminescence reactions using Western Lightening System (PerkinElmer, Boston, MA), and data were processed using Fluor-S Multiimager (Bio-Rad Laboratories, Hercules, CA). To examine the binding ability of MBL to LPS and lipid A, 96-well plates (MS-8496F; Sumitomo Bakelite, Tokyo, Japan) were coated by incubation with rough-type or smooth-type E. coli LPS (20 μg/ml in PBS) or lipid A (10 μg/ml in PBS) overnight at 4°. The plates were washed three times with PBS containing 0·05% (v/v) Tween-20 and incubated with PBS containing 1% (w/v) bovine serum albumin (BSA) for 1 hr at room temperature for blocking. Native or recombinant human MBL diluted with a buffer consisting of 50 mm Tris–HCl (pH 8), 0·2 m NaCl, 20 mm CaCl2, and 1% BSA was added, and the plates were incubated for 3 hr at room temperature. The plates were then washed three times with Tween-20-containing PBS and reacted successively with anti-human MBL monoclonal antibody and horseradish peroxidase-conjugated anti-mouse IgG. The samples were washed three times with Tween-20-containing PBS and subjected to a colorimetric reaction using tetramethyl benzidine solution (Invitrogen-Zymed, San Francisco, CA), and the amount of converted substrates was determined by measuring the absorbance at 450 nm. To examine the binding of MBL to human cells, THP-1 cells (1 × 106) before and after PMA treatment or apoptotic Jurkat cells were washed successively with PBS containing 0·05 mm EDTA and PBS alone, and incubated with recombinant human MBL (10 μg) in PBS for 10 min on ice. The cells were then washed with PBS and reacted with either anti-human MBL rabbit antiserum or normal rabbit serum by incubation for 10 min on ice, and supplemented with biotinylated anti-rabbit IgG antibody and Alexa Fluor 488-conjugated streptavidin (Molecular Probes). The level of MBL bound to cells was finally examined by either flow cytometry or fluorescence microscopy.
Immunochemical detection of calreticulin
THP-1 macrophages, which had been fixed and membrane-permeabilized by successive treatments with 3% paraformaldehyde and methanol or left untreated, were incubated with PBS containing 0·1% BSA for blocking. The cells were then reacted with anti-human calreticulin antiserum diluted with PBS containing 0·1% BSA, washed with PBS, and incubated with biotinylated anti-rabbit IgG antibody followed by the addition of Alexa Fluor 488-labelled streptavidin. The final samples were examined on a confocal laser-scanning microscope (LSM510; Carl Zeiss, Jena, Germany).
Data processing and statistical analysis
Data from the assay for phagocytosis are representative of three (for the reactions with latex beads, zymosan, or apoptotic cells as targets) or five (for the reactions with bacteria) independent experiments that yielded similar results, and those from quantitative analyses are expressed as the mean ± standard deviations (n > 3). Statistical analyses were performed using Student’s t-test, and P values of less than 0·05 were considered significant. The data significantly different from controls were marked with asterisks.
Results
Stimulation of phagocytosis of E. coli, but not of other target cells, by MBL
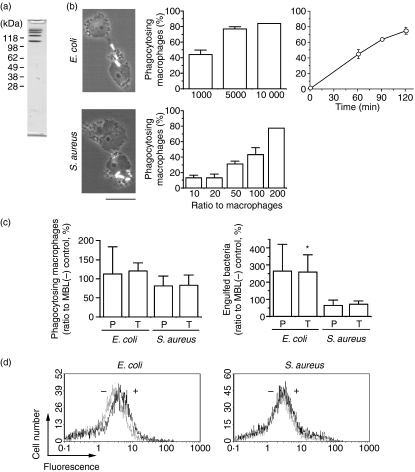
To obtain MASP-free human MBL, we expressed human MBL cDNA in Chinese hamster ovary cells and purified MBL by affinity chromatography using mannose-conjugated agarose. The materials bound to and eluted from the affinity column contained proteins with different molecular masses of larger than 100 kDa as revealed by SDS–PAGE (Fig. 1a). It is not known why MBL was obtained as a mixture of proteins, but presumably it formed multimers during preparation. We decided to use this preparation as recombinant human MBL in further experiments, because the same preparation has been shown to be functional in terms of the activation of human endothelial cells as well as the activation of complement when supplemented with MASP.24 We first tested the effect of MBL on the phagocytosis of bacteria by macrophages. Phagocytosis reactions were conducted with THP-1 macrophages as phagocytes and Gram-positive S. aureus or Gram-negative E. coli, which were labelled at the surface with FITC, as targets. We found that THP-1 macrophages effectively phagocytosed E. coli and S. aureus in a dose- and time-dependent manner (Fig. 1b). To see the effect of MBL, either phagocytes or target bacteria were preincubated with MBL before the phagocytosis reactions, and the level of phagocytosis was determined by fluorescence microscopy or flow cytometry. Pretreatment of neither phagocytes nor targets with recombinant MBL significantly influenced the ratio of phagocytes that had phagocytosed bacteria (left panel in Fig. 1c), but the same treatment increased the number of E. coli, but not of S. aureus, that had been incorporated into phagocytes (right panel in Fig. 1c). The extent of this stimulation was almost the same when either phagocytes or target bacteria were preincubated with MBL. Data from a flow cytometric analysis also revealed a stimulatory effect of recombinant MBL on the phagocytosis of E. coli but not of S. aureus by THP-1 macrophages (Fig. 1d).
Figure 1.
Effect of recombinant human mannose-binding lectin (MBL) on the phagocytosis of bacteria by macrophages. (a) Protein composition of recombinant human MBL preparation. The major fraction obtained after affinity chromatography was analysed by sodium dodecyl sulphate–polyacrylamide gel electropharesis, and the separated proteins were visualized by staining with Coomassie Brilliant Blue. The positions of molecular mass markers are indicated at the left. (b) Phagocytosis of bacteria by THP-1 macrophages. Phorbol myristate acetate-treated THP-1 macrophages were incubated with either Escherichia coli or Staphylococcus aureus, and the extent of phagocytosis was determined. The ratios (in percentage) of macrophages that have accomplished phagocytosis are shown as ‘phagocytosing macrophages’. Data shown as the mean ± standard deviations (n = 3) are representative of three independent experiments. Fluorescence-phase contrast micrographs of the macrophages with engulfed bacteria (seen in white) are shown at the left. Scale bar = 10 μm. (c) Stimulation of phagocytosis of E. coli by MBL. Either THP-1 macrophages (P) (2 × 106) or bacteria (T) (1 × 107) were preincubated with MBL (10 μg) before the assay of phagocytosis. Mean values in the control reaction with no added MBL are 39 (with E. coli) and 45 (with S. aureus) for ‘phagocytosing macrophages’, and 102 (with E. coli) and 259 (with S. aureus) for ‘engulfed bacteria’ (per 100 macrophages). (d) Stimulation of phagocytosis of E. coli by MBL. Phagocytosis reactions with THP-1 macrophages (2 × 105) and the indicated bacteria (1 × 106) were conducted in the presence (thicker lines) and absence (thinner lines) of MBL (10 μg), and the level of phagocytosis was determined by flow cytometry. Data are representative of two independent experiments that yielded similar results.
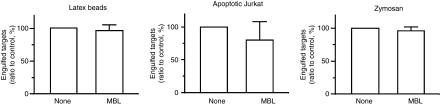
We next tested the effect of recombinant human MBL on the phagocytosis of other targets such as latex beads, apoptotic cells and zymosan. To do so, FITC-labelled latex beads, unlabelled Jurkat cells undergoing doxorubicin-induced apoptosis, and 5-carboxyfluorescein-labelled zymosan particles were used as targets for phagocytosis by PMA-stimulated THP-1 cells. We found that MBL had no effect on the ratio of phagocytes that had accomplished phagocytosis (data not shown) as well as the number of targets engulfed by each phagocyte (Fig. 2). All these results indicated that the effect of recombinant human MBL on the phagocytosis by THP-1 macrophages was target specific, and that only the phagocytosis of E. coli was stimulated so far as we tested.
Figure 2.
Effect of recombinant human mannose-binding lectin (MBL) on the phagocytosis of various target particles. THP-1 macrophages and the indicated target materials were incubated in the presence and absence of MBL (10 μg), and the extent of phagocytosis was determined by fluorescence microscopy. Mean values in the control reactions with no added MBL were 340 (with latex beads), 27 (with apoptotic Jurkat cells), and 106 (with zymosan).
Binding of MBL to targets and phagocytes
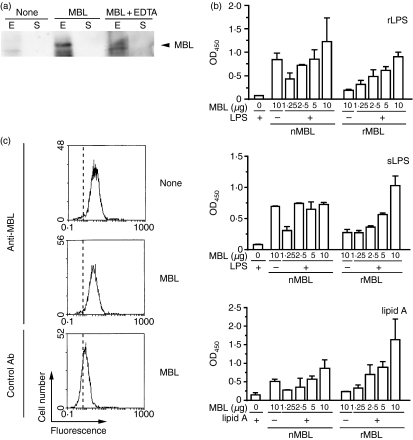
We then asked whether MBL binds to the target cells. We first examined the binding of recombinant human MBL to E. coli and S. aureus. Bacteria were incubated with MBL and washed to remove MBL unbound or lightly associated with bacteria, and their lysates were examined for the presence of bound MBL by Western blotting. The results indicated that recombinant human MBL binds to E. coli, but not to S. aureus (Fig. 3a). The amount of MBL bound to E. coli was reduced by the presence of EDTA, suggesting that the Ca2+-dependent carbohydrate-binding activity of MBL is responsible for the binding. We then tested whether or not MBL binds to LPS of E. coli. For this purpose, we examined the binding of MBL to culture plates that had been coated with either rough-type or smooth-type E. coli LPS. We found that recombinant human MBL binds to either type of LPS while no significant binding was observed for native human MBL (upper and middle panels in Fig. 3b). When MBL that had bound to LPS-coated plates was detached from the plates and analysed by Western blotting with anti-MBL antibody, all the polypeptides present in the MBL preparation (see Fig. 1a) were detected (data not shown). This indicated that all the proteins in the MBL preparation were capable of binding to LPS and suggested that they were all recombinant human MBL. Furthermore, recombinant MBL was found to possess affinity for lipid A (lower panel in Fig. 3b). On the other hand, the same MBL preparation did not bind to apoptotic Jurkat cells as assessed by flow cytometry (Fig. 3c). These results indicated that recombinant human MBL bound to E. coli but not to the other target cells, and explained why it selectively stimulated the phagocytosis of E. coli by THP-1 macrophages.
Figure 3.
Binding of mannose-binding lectin (MBL) to lipopolysaccharide (LPS). (a) Binding of MBL to bacteria. Escherichia coli (E) or Staphylococcus aureus (S) were incubated with MBL in the absence and presence of ethylenediaminetetraacetic acid (EDTA), and the pelleted bacteria were lysed and analysed by Western blotting for the presence of bound MBL. Only portions of the data containing signals of MBL are shown. Data are representative of three independent experiments that yielded similar results. (b) Binding of MBL to LPS and lipid A. The indicated amounts of recombinant human MBL (rMBL) or native human MBL (nMBL) were incubated in culture containers whose surfaces had been coated with rough-type LPS (rLPS), smooth-type LPS (sLPS), or lipid A of E. coli, and the amount of bound MBL remaining in the containers after washes was determined by an enzyme-linked immunosorbent assay. Data shown as the mean ± standard deviations (n= 3) are representative of three (with LPS) or two (with lipid A) independent experiments that yielded similar results. (c) No binding of MBL to apoptotic Jurkat cells. Doxorubicin-treated Jurkat cells (1 × 106) were incubated with MBL (10 μg), and the level of MBL bound to Jurkat cells was determined immunochemically by flow cytometry. The vertical broken line in each panel indicates the position of a peak in the reaction with no added antibody. Data are representative of three independent experiments that yielded similar results.
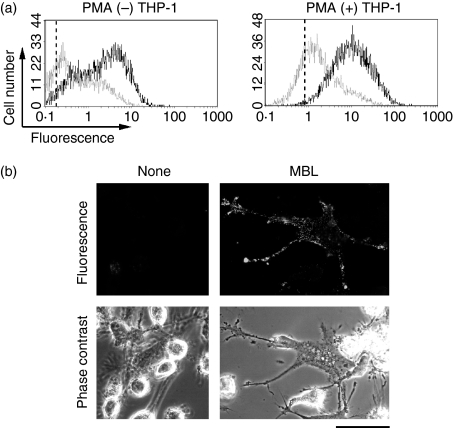
We next tested whether MBL binds to THP-1 macrophages as it does to E. coli. THP-1 cells were incubated with recombinant MBL, and the amount of MBL bound to the cells was determined immunochemically by flow cytometry. Recombinant human MBL bound to THP-1 cells, and the level did not significantly differ before and after PMA treatment (Fig. 4a). This suggested that a putative receptor for MBL already existed in THP-1 cells before the differentiation into macrophages. The treatment with PMA caused, in some THP-1 cells, portions of the plasma membrane to protrude, producing a structure known as a filopodium. When those cells were incubated with MBL and immunocytochemically examined for the distribution of MBL, MBL seemed to be preferentially associated with the filopodia (Fig. 4b). Taking these results and those shown above into consideration, recombinant MASP-free human MBL stimulated the phagocytosis of E. coli, most probably by serving as a bridge between E. coli and THP-1 macrophages.
Figure 4.
Binding of mannose-binding lectin (MBL) to THP-1 macrophages. (a) Flow cytometric analysis. THP-1 cells (1 × 106) before and after the treatment with phorbol 12-myristate 13-acetate (PMA) were incubated in the presence (thicker lines) and absence (thinner lines) of MBL (10 μg), and the level of MBL bound to THP-1 cells after washes was analysed immunochemically by flow cytometry. The vertical broken line in each panel indicates the position of a peak in the reaction with no added antibody. Data are representative of two independent experiments that yielded similar results. (b) Microscopic analysis. PMA-treated THP-1 macrophages (1 × 106) were incubated with MBL (10 μg) and examined by fluorescence microscopy. Fluorescence and phase-contrast views of the same microscopic fields of THP-1 macrophages that have extended filopodia are shown. Scale bar = 50 μm.
Calreticulin as a candidate MBL receptor of THP-1 macrophages
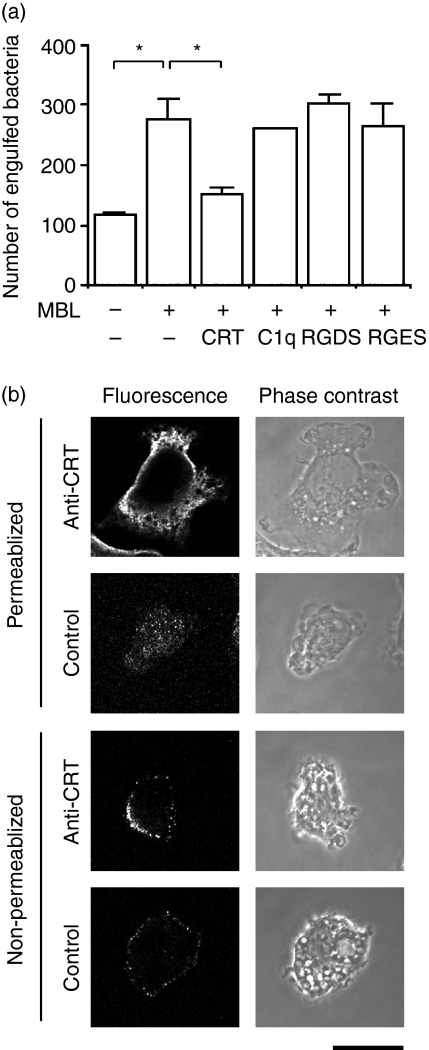
We next tried to obtain a clue as to the receptor(s) of THP-1 macrophages responsible for MBL’s stimulation of the phagocytosis of E. coli. Previous reports have suggested C1qR, CR1, or calreticulin as the receptor for the collagenous domain of MBL.5–10 An assay for the phagocytosis of E. coli by THP-1 macrophages was conducted in the absence and presence of human C1q or human calreticulin as a competitive inhibitor of MBL. The addition of calreticulin reduced the level of phagocytosis to that in the absence of MBL, while C1q did not influence the phagocytosis (Fig. 5a). A peptide that neutralizes the action of integrin seemed to have no effect on the action of MBL (Fig. 5a). These results indicated that calreticulin sequestered the stimulatory effect of MBL on the phagocytosis of E. coli by THP-1 macrophages. We then immunocytochemically examined the presence of calreticulin at the surface of THP-1 macrophages (Fig. 5b). When membrane-permeabilized cells were analysed, we found that the dominant location of calreticulin was the cytoplasm, in particular places near the plasma membrane (upper four panels). Examination of the same cells without treatment for membrane permeabilization revealed that calreticulin was also widely distributed as speckles at the surface of THP-1 macrophages (lower four panels) as had been previously observed with Jurkat cells.29 These results suggested that calreticulin served as a MBL receptor of THP-1 macrophages, most likely binding to the collagenous domain of MBL, and was responsible for the action of MBL to stimulate the phagocytosis of E. coli.
Figure 5.
Search for a candidate receptor of mannose-binding lectin (MBL). (a) Inhibition of phagocytosis by calreticulin. Phagocytosis of Escherichia coli (1 × 108) by THP-1 macrophages (1 × 105) in the presence of MBL (50 μg) was examined with the indicated additives. Data shown as the mean ± standard deviations (n= 3) are representative of three independent experiments that yielded similar results. CRT, human calreticulin (50 μg); C1q, human C1q (50 μg); RGDS, peptide containing the RGDS sequence (0·5 mm); RGES, peptide containing the RGES sequence (0·5 mm). (b) Presence of calreticulin at the surface of THP-1 macrophages. Phorbol 12-myristate 13-acetate (PMA) -treated THP-1 cells with or without membrane permeabilization were immunocytochemically analysed for the presence of calreticulin with anti-human calreticulin antiserum (anti-CRT) or normal rabbit serum (control). Phase-contrast and fluorescence views of the same microscopic fields are shown. Scale bar = 10 μm. Data are representative of two independent experiments that yielded similar results.
Discussion
We have shown that MASP-free recombinant human MBL stimulates the phagocytosis of bacteria by macrophages. To the best of our knowledge, this provides further evidence that MBL stimulates the phagocytosis of bacteria without the aid of MASP after the recent report by Ono and colleagues22 that recombinant rat MBL stimulated the phagocytosis of E. coli and S. aureus by rat Kupffer cells in the absence of MASP. Our findings indicate that the stimulatory effect of MASP-free MBL on bacterial phagocytosis is not restricted to a certain type of phagocyte. However, the mechanism of MBL’s action revealed in our study is somewhat different from that in the preceding report. In our hands, the purified recombinant human MBL seemed to directly enhance phagocytosis by acting as a bridge between macrophages and the target E. coli, while the other group suggested that MBL stimulates the phagocytosis of bacteria by increasing the level of a receptor for phagocytosis, class A scavenger receptor in this case, at the surface of Kupffer cells.22 This discrepancy could be explained by the difference in the type of phagocyte, macrophages versus Kupffer cells, used in the two studies. According to our model, MBL simultaneously binds to the target bacteria and phagocytes most probably using distinct domains within the molecule. This means that phagocytes need to have a receptor that is specific for one domain of MBL. If Kupffer cells do not possess such a receptor, MBL has no way of serving as a bridge between the bacteria and phagocytes.
The mode of action of MBL revealed in this study resembles that of antibody, which bridges targets and phagocytes by respectively binding to surface antigens and Fc receptors. It is presumed that MBL might bind to bacteria using the lectin domain at the C terminus while it binds to phagocytes using the collagenous domain. We showed that MBL binds to lipid A of E. coli, as Ono and colleagues reported.22 It is thus probable that MBL recognizes E. coli specifically bound to lipid A using the lectin domain. It can therefore be said that MBL behaves as an opsonin-like molecule in the cellular innate immune response, as does antibody in adaptive immunity. An opsonin-like action of MBL has been reported for the phagocytosis of apoptotic cells by macrophages.16–18 An affinity of MBL for lipid A also explains the specificity of MBL in stimulating phagocytosis; MBL raises the level of the phagocytosis of E. coli by THP-1 macrophages but not of the other targets, including S. aureus, so far tested. In contrast to our results, Ono and colleagues showed that MBL stimulates the phagocytosis of not only E. coli but also S. aureus.22 Again this could be explained by a difference in the mode of action of MBL in the studies by the two research groups. As to the binding partner of MBL at the other end of the bridge, several proteins have been proposed as MBL receptors that reside at the surface of phagocytes and bind to its collagenous domain.5–10 We have suggested in this study that calreticulin, a molecular chaperone of the endoplasmic recticulum, is involved in MBL’s stimulation of the phagocytosis of E. coli by macrophages. Calreticulin has been shown to be required for the efficient phagocytosis of apoptotic cells although it is still controversial on which side, target cells or phagocytes, this protein functions.16,29,30 Therefore, whether calreticulin serves as a MBL receptor of THP-1 macrophages still needs to be more directly investigated. Furthermore, calreticulin is not a membrane-bound protein and so is unlikely to directly transmit a signal for inducing phagocytosis. In fact, CD91 or low-density lipoprotein-related protein has been reported to act as a calreticulin-binding phagocytosis receptor of macrophages.16,30 If calreticulin stimulates the phagocytic action of THP-1 macrophages by triggering CD91, this phagocytosis requires dual bridging molecules: MBL on the E. coli side and calreticulin on the phagocyte side.
Kang et al. recently reported that recombinant human MBL stimulates a humoral innate immune response against bacterial invasion by endothelial cells in the absence of MASP.24 MBL could therefore be responsible for many more responses in innate immunity without the aid of MASP. It is important to elucidate roles for MASP-free MBL in innate immune responses to develop a novel strategy against infectious diseases.
Acknowledgments
We thank Drs K. Sekimizu and S. Natori for the materials. We are also grateful to Drs J.-S. Yum and H. M. Moon of Dobeel Corporation for help in preparing the recombinant human MBL. We thank Y. Minami for help in the initial study of bacterial phagocytosis. This study was supported by the Bilateral Programme of Joint Research Project from Japan Society for the Promotion of Science and an institutional research grant for international collaboration from Kanazawa University to Y.N., and also by the programmes of Joint Research Project under The KOSEF-JSPS Cooperative Programme (F01-2006-000-10016-0) of MOST/KOSEF to B.L.L. Part of the study was supported by the Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (Nos. 16570112 and 18570123) and from Ministry of Education, Culture, Sports, Science and Technology (No. 18057009) to A.S.
Abbreviations
- BSA
bovine serum albumin
- EDTA
ethylenediaminetetraacetic acid
- FITC
fluorescein isothiocyanate
- LPS
lipopolysaccharide
- MASP
MBL-associated serine protease(s)
- MBL
mannose-binding lectin
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- SDS
sodium dodecyl sulphate
References
- 1.Stuart LM, Ezekowitz RAB. Phagocytosis: elegant complexity. Immunity. 2005;22:539–50. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PR, Martinez-Promares L, Stacey M, Lin H-H, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 5.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–49. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 6.Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin – a soluble pattern recognition molecule. Mol Immunol. 2004;41:113–21. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Ip WKE, Michelow IC, Ezekowitz RAB. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Gupta G, Surolia A. Collectins: sentinels of innate immunity. BioEssays. 2007;29:452–64. doi: 10.1002/bies.20573. [DOI] [PubMed] [Google Scholar]
- 11.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–6. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 12.Bonar A, Chmiela M, Rudnicka W, Rózalska B. Mannose-binding lectin enhances the attachment and phagocytosis of mycobacteria in vitro. Arch Immunol Ther Exp. 2005;53:437–41. [PubMed] [Google Scholar]
- 13.Jack DL, Lee ME, Turner MW, Klein NJ, Read RC. Mannose-binding lectin enhances phagocytosis and killing of Neisseria meningitidis by human macrophages. J Leukoc Biol. 2005;77:328–36. doi: 10.1189/jlb.0604342. [DOI] [PubMed] [Google Scholar]
- 14.Kudo K, Sano H, Takahashi H, et al. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 15.Ip W-K, Lau Y-L. Role of mannose-binding lectin in the innate defense against Candida albicans: enhancement of complement activation, but lack of opsonic function, in phagocytosis by human dendritic cells. J Infect Dis. 2004;190:632–40. doi: 10.1086/422397. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauta AJ, Raaschou-Jensen N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 18.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173:3044–50. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 19.Shi L, Takahashi K, Dundee J, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Shi L, Gowda LD, Ezekowitz RAB. Relative roles of complement factor 3 and mannose-binding lectin in host defense against infection. Infect Immun. 2005;73:8188–93. doi: 10.1128/IAI.73.12.8188-8193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RAB. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 22.Ono K, Nishitani C, Mitsuzawa H, et al. Mannose-binding lectin augments the uptake of lipid A, Staphylococcus aureus, and Escherichia coli by Kupffer cells through increased cell surface expression of scavenger receptor A. J Immunol. 2006;177:5517–23. doi: 10.4049/jimmunol.177.8.5517. [DOI] [PubMed] [Google Scholar]
- 23.Ma YG, Cho MY, Zhao M, Park JW, Matsushita M, Fujita T, Lee BL. Human mannose-binding lectin and L-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J Biol Chem. 2004;279:25307–12. doi: 10.1074/jbc.M400701200. [DOI] [PubMed] [Google Scholar]
- 24.Kang HJ, Lee S-M, Lee H-H, Kim JY, Lee B-C, Yum J-S, Moon HM, Lee BL. Mannose-binding lectin without the aid of its associated serine proteases alters lipopolysaccharide-mediated cytokine/chemokine secretion from human endothelial cells. Immunology. 2007;122:335–42. doi: 10.1111/j.1365-2567.2007.02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju JS, Cho MH, Brade L, et al. A novel 40-kDa protein containing six repeats of an epidermal growth factor-like domain functions as a pattern recognition protein for lipopolysaccharide. J Immunol. 2006;177:1838–45. doi: 10.4049/jimmunol.177.3.1838. [DOI] [PubMed] [Google Scholar]
- 26.Cho J-H, Homma K, Kanegasaki S, Natori S. Activation of human neutrophils by a synthetic anti-microbial peptide, KLKLLLLLKLK-NH2, via cell surface calreticulin. Eur J Biochem. 1999;266:878–85. doi: 10.1046/j.1432-1327.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 27.Shiratsuchi A, Nakanishi Y. Phosphatidylserine-mediated phagocytosis of anticancer drug-treated cells by macrophages. J Biochem. 1999;126:1101–6. doi: 10.1093/oxfordjournals.jbchem.a022555. [DOI] [PubMed] [Google Scholar]
- 28.Shiratsuchi A, Watanabe I, Takeuchi O, Akira S, Nakanishi Y. Inhibitory effect of Toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. J Immunol. 2004;172:2039–47. doi: 10.4049/jimmunol.172.4.2039. [DOI] [PubMed] [Google Scholar]
- 29.Kuraishi T, Manaka J, Kono M, et al. Identification of calreticulin as a marker for phagocytosis of apoptotic cells in Drosophila. Exp Cell Res. 2007;313:500–10. doi: 10.1016/j.yexcr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]