Abstract
The mechanism of differentiation of naïve T cells to a variety of effector lineages, but particularly to T helper type 1 (Th1) and Th2 cells, has been the subject of intense scrutiny over the past two decades. Studies have revealed that the expression of cytokines, receptors, signalling molecules, transcription factors, DNA methylating enzymes and histone-modifying enzymes is altered during the process and has been shown to play a co-ordinated role to facilitate expression of the cytokines interleukin-4 (IL-4), IL-5 and IL-13 in Th2 cells, or interferon-γ in Th1 cells. Regulation of IL-4 expression has been of particular interest for two main reasons: first because IL-4 acts as a growth factor for Th2 cells, and second because of its role in the induction of immunoglobulin class switching to immunoglobulin E, which plays a critical role in mediating allergic responses. Study of the pathways that promote this tissue-restricted expression of IL-4 may highlight potential areas for therapeutic intervention.
Keywords: c-Maf, cytokine, GATA3, T helper type 2 cell, transcription
Introduction
Naive CD4+ T cells are capable of differentiating into a variety of effector lineages in response to changes in their environment. These cells are capable of expressing low levels of both interleukin-4 (IL-4) and interferon-γ (IFN-γ), but as differentiation takes place, signature cytokine expression becomes polarized. How then are these changes implemented? It is now apparent that a number of distinct biological pathways co-ordinate to orchestrate efficient differentiation. Many reviews have addressed these topics in detail, here we highlight some of the more recent data sets that provide this complex picture with additional detail.
Signals – from cell surface to nucleus
T-cell differentiation is thought to take place in three phases. A commitment phase, where an initial differentiative decision is made under the influence of ligation of cytokine and notch receptors, is followed by a reinforcement phase, where the effector responses of individual cells are amplified as epigenetic alterations at specific gene loci combine to permit enhanced cytokine transcription and silencing of the other differentiation limbs. Finally, once the epigenetic programmes are complete and target loci are fully reorganized, cytokine expression is maintained under the influence of T-cell-receptor-mediated signals such as activating protein 1 (AP-1) and nuclear factor-κB (NF-κB).1
T helper type 2 (Th2) differentiation is initiated by the binding of IL-4 to either type I (IL-4 Rα/γc) or type II (IL-4Rα/IL-13Rα1) receptor complexes containing the IL-4Rα subunit and either the common γ chain or the IL-13Rα1 chain respectively. The type II receptor can also be recognized by IL-13, which explains some of the functional overlap between the cytokines. The crystal structures of these receptor–ligand complexes have recently been solved.2 The IL-13Rα1 contains an N-terminal immunoglobulin-like domain that is not present in other γc superfamily receptors and is required for IL-13 but not IL-4 signalling.3 The assembly energetics of the heterodimeric type II receptors differs depending on whether IL-4 or IL-13 is the ligand. Heterodimeric receptor assembly initiates with ligand binding to its preferential receptor module (known as the ‘driver’), followed by recruitment of the heterodimeric partner, the ‘trigger’. For IL-4, IL-4Rα constitutes the ‘driver’ and IL-13Rα1 constitutes the ‘trigger’. For IL-13, the ‘driver’ and ‘trigger’ receptor modules are reversed; this assembly difference causes different signalling potencies and kinetics.2
Heterodimerization of the IL-4 receptor on the cell surface leads to phosphorylation of the cytoplasmic C-terminal tails by the Janus Kinase (JAK) family of tyrosine kinases, which in turn leads to recruitment and phosphorylation of the signal transducer and activator (STAT6). Phosphorylation of STAT6 induces a conformational change enabling dimerization, nuclear translocation, DNA binding and transcriptional activation of a variety of target genes including IL-4, IL-5 and IL-13,4 and the Th2-specific transcription factors GATA3 and c-Maf.5 Conversely, STAT6 and GATA3 also act to silence the expression of genes such as IFNγ in Th2 cells.6 STAT6 is thought to provide an instructional signal, which must subsequently be reinforced to establish high-level cytokine transcription. STAT6 is known to recruit the histone acetyl transferase Creb-binding protein (CBP) as a cofactor, but a novel factor, collaborator of STAT6 (CoaSt6) has been described. CoaSt6 is a poly(ADP-ribosyl) polymerase, and inhibition of its activity blocks IL-4-dependent transcription.7
Recent data suggest that a second route of Th2 commitment can occur independently of IL-4 signalling as engagement of Notch receptors on naïve cells with their ligands (Jagged 1 and 2 or Delta-like 1, 3 and 4) causes release of notch intracellular domain (NICD) from the T-cell membrane and its subsequent nuclear translocation.8,9 Notch signalling preferentially induces expression of the developmentally regulated GATA3 transcript, and the ensuing increase in GATA3 activity was required for Notch-mediated induction of IL-4 expression.10,11
Transcriptional regulation-genes
The Th2 cytokine locus is structurally conserved across species; the IL-4, IL-5 and IL-13 genes lie in close proximity to one another on human and murine chromosomes 5 and 11, respectively, also located within this locus are the genes KIF3A, Rad50 and IRF1.12KIF3A and RAD50 encode a kinesin involved in microtubule function, and a protein involved in DNA double-strand break repair respectively. The IRF1-encoded protein, interferon regulatory factor 1, whose gene juxtaposes IL-5, serves to activate expression of IFN-α and IFN-β transcription and also functions as a transcriptional activator of genes that are induced by IFN-α, IFN-β and IFN-γ. Spilianakis et al. (Flavell and colleagues) have studied how this locus interacts with other chromosomes using chromosome conformation capture assays (3C). These studies have shown that the IFNγ and Th2 cytokine loci directly interact in naïve murine T cells and comprised a chromatin hub in which the cytokine genes are poised for rapid expression. Upon the initiation of differentiation from naïve to effector cells, this interchromosomal association was significantly reduced, being replaced by intrachromosomal interactions as lineage-specific restriction of cytokine expression commences.13
Orchestrating Th2 differentiation
Two transcription factors, T-bet and GATA3, have been demonstrated to function as master regulators of Th1 and Th2 differentiation, respectively, and to orchestrate the complex programme of lineage restriction that facilitates preferential expression of the signature cytokines and, to varying degrees, silencing of the opposite differentiation limb. Expression of IL-4 is reliant upon a further factor, c-Maf, which is thought to function within a chromatin environment that is established under the influence of GATA3 (Fig. 1). The range of cofactors that GATA3, T-bet, c-Maf and their ancillary transcription factors recruit to implement the restrictive gene expression programmes is still subject to investigation.
Figure 1.
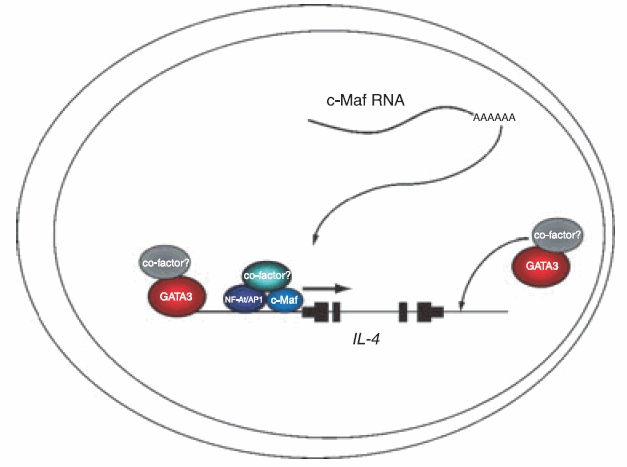
Mechanisms of activation of the interleukin-4 gene IL-4. GATA3 acts as the master regulator of T helper type 2 differentiation and facilitates IL-4 transcription by coordinating chromatin remodelling within the 5q locus. c-Maf functions within this chromatin context along with the proteins nuclear factor of activated T cells (NF-AT) and activating protein-1, acting to mediate upregulation of IL-4. The majority of the cofactors that are recruited to facilitate gene expression remain to be determined.
GATA3
GATA3 expression is essential for CD4+ cell generation because its downstream targets include genes encoding the T-cell receptor chains required for cell selection and proliferation. Expression of GATA3 is low in naïve T cells but is selectively upregulated in Th2 cells and downregulated in Th1 cells with kinetics that correlates with the time–course of Th1/Th2 cell differentiation.14,15 Increased GATA3 expression is brought about via a STAT6-independent positive autoregulatory loop.16,17In vivo, conditional GATA3 knockout mice failed to develop appropriate Th2 cytokine expression in response to parasitic infection but IL-4, IL-5 and IL-13 showed differential responsiveness to GATA3.18,19 This led to the conclusion that GATA3 is essential for the induction of Th2 differentiation and initial Th2 cytokine expression, while not being required for the maintenance of IL-4 expression in mature cells, a role that has been proposed to be carried out by the Th2-restricted transcription factor c-Maf.
c-Maf
c-Maf was isolated in a yeast two-hybrid screen to find Th2-specific interaction partners of nuclear factor of activated T cells (NF-ATc), a Maf response element (MARE) was identified within the proximal IL-4 promoter adjacent to a NF-AT site and within the region specified as essential for conferring Th2 specificity.5 Purified naïve CD4+ T cells and splenocytes from c-Maf−/− mice demonstrated impaired production of IL-4 and an inability to polarize to a Th2 phenotype. Culture of these cells in the presence of exogenous IL-4 restored IL-5 expression to wild-type levels, confirming the previous findings from overexpression studies that c-Maf selectively regulates IL-4 expression in Th2 cells.20
While numerous studies have demonstrated a role for c-Maf in the transactivation and expression of IL-4, some caveats remain. Initial studies of c-Maf showed that it was expressed at low levels in naïve spleen Th precursor (Thp) cells yet ligation of the T-cell receptor under Th2 conditions only resulted in expression of c-Maf by day 8 of the primary stimulation and by 20 hr of the secondary stimulation.5 Since IL-4 expression is rapidly induced following T-cell receptor stimulation, the lack of c-Maf expression at earlier time-points may reflect a role only in maintenance of IL-4 expression rather than induction. c-Maf is also subject to potent posttranslational regulation because detectable protein levels fall rapidly following activation of primary human Th2 cells.21
NF-AT
Establishment of a transcriptionally permissive chromatin environment precedes high-level cytokine expression, however, even these differentiated T cells remain relatively quiescent in the absence of T-cell receptor ligation and costimulation, thereafter, there is a rapid induction of activating transcription factors such as AP-1, NF-κB and NF-AT, which act in concert to upregulate cytokine gene transcription. A number of distinct NF-AT/AP-1 sites are present within the proximal promoter of IL-4 and chromatin immunoprecipitation studies have demonstrated binding of NF-AT1 to the IL-4 promoter and enhancer regions in activated murine Th2 cells but not Th1 cells, supporting the hypothesis of a positive regulatory role in IL-4 expression.22,23 Transcriptional activation by NF-AT proteins was found to involve recruitment of the transcriptional coactivator CBP/p300 (now KAT3A/B),24 to its N-terminal activation domain,25 thereby linking NFAT recruitment to DNA with the changes in histone acetylation that accompany transcriptional upregulation.26,27
Transcriptional silencing of IL-4 in Th1 cells
Expression of IL-4 is actively silenced in Th1 cells, at least two types of mechanisms can bring about this repression, as exemplified by T-bet and IRF1 (Fig. 2).
Figure 2.
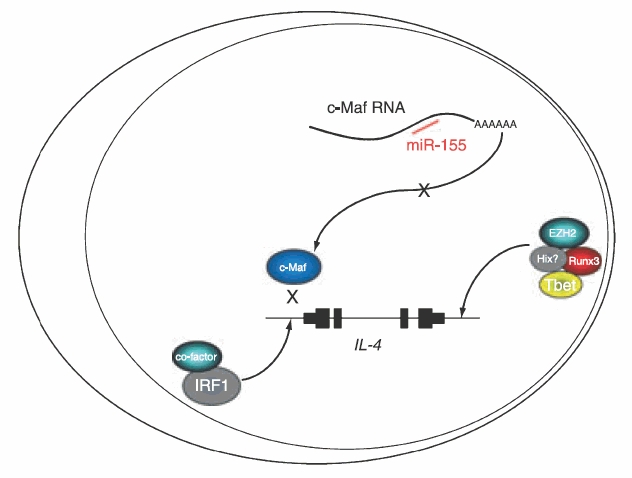
Mechanisms of repression of the interleukin-4 gene IL-4. T-bet acts to silence IL-4 expression in T helper type 1 cells via recruitment of the histone methyl transferase EZH2. Interferon regulatory factor 1 has been described as a repressor of IL-4 transcription that binds to the IL-4 promoter. miR-155 is a microRNA that is thought to inhibit translation of c-Maf RNA.
T-bet
T-bet was originally cloned in an attempt to isolate transcription factors that caused the tissue-specific expression of Th1 cytokines.28T-bet−/− mice display defects in Th1 cell development and IFNγ expression, and have an expanded Th2 cell compartment.29,30 Overexpression of T-bet in developing primary Th2 cells by retroviral transduction led to the activation of endogenous IFNγ expression and a reduction of IL-4 levels, thereby impeding Th2 differentiation. In fully differentiated Th2 cells, retrovirally transduced T-bet was less able to diminish Th2 cytokine gene expression.28 T-bet function is influenced by two further transcription factors, Runx331 and Hlx.32,33 Expression of these proteins is induced by T-bet which then directly interacts with both to synergistically induce maximal IFNγ expression, thereby reinforcing Th1 differentiation. In addition to promoting IFNγ expression, a complex containing T-bet, Runx3 and its obligate partner core binding factor (CBF)β are thought to participate in silencing IL-4 expression31 and have been detected by chromatin immunoprecipitation at hypersensitive site IV (HSIV), the IL-4 silencer, in Th1 cells along with enhancer of zeste homolog 2 (EZH2) [now lysine methyl transferase (KMT)6].
IRF1
Expression of IRF1 and IRF2 is induced by IFN-γ, and both proteins can act as regulators of IFN-γ-inducible genes. IRF1 and IRF2 can also mediate transcriptional repression and have been demonstrated to bind to three IRF binding sites in the IL-4 promoter.34 One of these sites, NRE1/IRF, was previously identified as an element conferring repressive activity to the IL-4 gene.35 Mice deficient in IRF1 produce increased levels of IL-4 coupled with reduced IFNγ expression. Taken together, these data suggest that IRF1, induced by IFN-γ, can act to silence IL-4 expression. The location of the IRF1 gene, which juxtaposes that encoding IL-5, raises questions about whether the chromatin changes that facilitate Th2 cytokine expression may also act to silence IRF1 expression and thereby relieve its influence on IL-4 transcription.
microRNAs
The last few years have seen an explosion in the identification and reports of function of specific micro-RNAs (miRNAs), this class of small RNAs has been shown to function predominantly by mediating the inhibition of translation through binding to conserved sequences in target mRNAs. Over 500 miRNA genes have been described in humans and one of these, miR-155/bic is of particular importance for the regulation of IL-4. miR-155 maps to an exon of the untranslated RNA bic.36Bic/miR-155 transcription is enhanced in B and T cells,37 and in macrophages during the inflammatory response.38 Overexpression has also been observed in B-cell lymphomas and in some solid tumours.39 Bradley and colleagues40 generated bic-deficient mice that developed a lung pathology with age. These mice displayed increased bronchiolar subepithelial collagen deposition, lung myofibroblast proliferation and airways smooth muscle (ASM) accumulation. They also had increased numbers of leukocytes present in bronchiolar lavage fluid compared to normal littermates and the expression of a number of cytokines, chemokine and transcription factors in T cells from these mice was found to be aberrant. c-Maf was identified as a miR-155 target in microarray experiments, and Maf RNA was upregulated in bic null Th2 cells so it was suggested that modulation of c-Maf levels by bic/miR155 might contribute towards Th2 responses in vivo.
Epigenetic modifications
Much of the knowledge of how transcription factors regulate gene expression through epigenetic changes comes either from studies of their influence upon the establishment of chromatin environments, from in vitro differentiation experiments using retrovirally transduced transcription factors or from experiments where target sequences for the transcription factors are deleted in transgenic mice. The three main data sets from these types of experiments are analysis of DNase1 hypersensitive site (DHS) formation, DNA methylation changes and alterations in histone tail modifications.
DNase1 hypersensitivity
DNase1 hypersensitivity studies have been used to identify regions of DNA in which nucleosomal compaction has been relieved and so indicate DNA domains that may be accessible to regulatory transcription factors. Such studies have revealed that a number of distinct clusters of DHSs are present within the IL-4 locus in both mice and humans.22,41–44 Naïve and Th1 cells show almost identical patterns of hypersensitive sites across the IL-4 locus, but additional DHSs were shown to be induced in Th2 cells, these include IL-4 HSI, II, III and V and IL-13 HSI, II and III41 and HSS2 and HSS344 (Fig. 3). These studies confirmed a reorganization of the locus during Th2 differentiation, subsequently IL-4 HSII and HSIII have been shown to function as enhancers in Th2 cells.45 The influence of GATA3 in establishing this altered Th2 chromatin environment was confirmed by ectopic expression of GATA3 in committed Th1 cells. This reactivated Th2 cytokine expression and induced the Th2-specific sites HSII within the IL-4 gene,46 HSS2 within CNS-147 and HSVA within CNS-2.22
Figure 3.
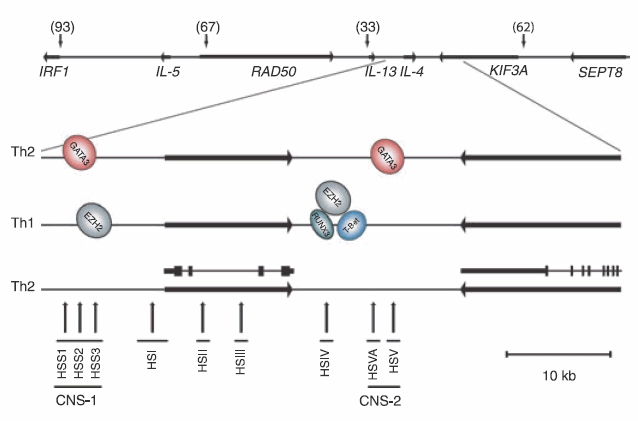
Organization of the interleukin-4 (IL-4)/IL-5/IL-13 locus. Horizontal arrows indicate the position and direction of transcription of genes. CpG islands are indicated by downward-pointing vertical arrows and the numbers of CpG dinucleotides within each cluster are stated. Location of binding sites for transcription factors demonstrated by chromatin immunoprecipitation in T helper type 1 (Th1) and Th2 cells are shown. The positions of DNase I hypersensitive sites in Th2 cells are shown by upward-pointing vertical arrows. The known regulatory regions, CNS-1 and CNS-2, are also indicated.
Experiments conducted in transgenic mice have addressed whether DNA regions containing these DHSs directly contribute to establishment of the Th2 lineage and cytokine gene expression. Mice lacking HSIV have increased levels of IL-4 and IL-13 transcription in naïve cells and caused Th1 cells to express high levels of both IFN-γ and IL-4. This implies that HSIV is critical for silencing IL-4 expression in Th1 cells,48 and indeed, T-bet and Runx3 bind to this silencer in Th1 cells31 to mediate repression.
A Th2 locus control region that influences the expression of all of the Th2 cytokine genes has been identified in the Rad50 3′ flanking DNA. This region contains a number of DHSs, including RHS7 to which GATA3 has also been shown to bind.49 Deletion of RHS7 diminished IL-4 and IL-13 expression in both naïve and Th2 cells.50
DNA methylation
DNA methylation takes place almost exclusively at the CpG dinucleotide. This dinucleotide is generally deficient in the human genome, but clusters within CpG islands (CGIs), approximately half of which are located close to transcriptional start sites in man. A small proportion of these CGIs are methylated and this modification is generally accompanied by silencing of any associated gene.51
Methylated CpG dinucleotides act as a binding substrate for methyl-binding domain (MBD) proteins such as MBD2 and MeCP2;52 these proteins, which may also recruit histone deacetylases, can also contribute towards repression of gene transcription.53 Clusters of methylated CpG dincleotides (CpG islands) have generally been found to associate with silenced genes and a number of such islands are present within the IL-4 locus (Fig. 3). A number of studies have addressed whether such modifications can contribute towards cytokine gene expression.
A limited study of DNA methylation changes at a number of CpG dinucleotides in the human IL-4 gene intronic enhancer and the IL-13 promoter demonstrated the sites to be methylated in naïve and Th1 cells with demethylation occurring in Th2 cells.54 Similar data have also been presented by Makar et al., as naïve cells were differentiated to Th1 populations, the decrease in capacity for IL-4 expression was accompanied by an increase in recruitment of DNA methyl transferases (DNMTs) to the IL-4 and IL-13 promoters and an increase in CpG methylation at these regions. By contrast, these promoters were significantly demethylated in Th2 cells.55
To determine whether increases in DNA methylation at promoters contributes to or is a consequence of silencing, experiments were conducted in murine cells that were devoid of either the DNA methyl transferase DNMT1 or the methyl CpG-binding protein MBD. Naïve T cells from DNMT1−/− mice display increased expression of IL-4, which was accompanied by increased H3K4 methylation, a histone mark generally associated with actively transcribing promoters, thereby illustrating a direct link between DNA and histone methylation.56
The methyl CpG-binding domain protein MBD2 has been proposed to link DNA methylation to silent chromatin.57 T cells from MBD2 null mice exhibit disordered differentiation. Naïve wild-type T cells produce very little IL-4, by contrast, naïve T cells from MBD2 null mice display enhanced IL-4 expression and Th1 cells from these animals show derepressed IL-4. MBD2 silencing renders GATA3 dispensable for IL-4 expression. One function of GATA3, therefore, is to mediate the displacement of MBD2 from DNA,57 the mechanism of this event is, however, unclear.
Histone tail modifications
Over the last decade, major advances have been made in understanding how modifications of histone tails can contribute towards gene expression. It is known that numerous residues on the various histone N-terminal tails may be either singly or multiply altered by the addition of modifications including methyl and acetyl groups, phosphates and ubiquitin chains. These modifications may act to either generate a particular chromatin environment, or to mediate specific biological tasks.58
Genome-wide analysis has demonstrated that histone modification patterns are highly predictive of promoters, transcriptional activity, transcribed and silenced regions, CpG islands, etc.59,60 Genome-wide DHS analysis has also been conducted in resting human CD4+ T cells allowing correlation of these sites with particular histone modifications,61 to date comprehensive methylome analysis has been conducted only using peripheral blood rather than more restricted cell populations.62
IL-4 locus
Studies of epigenetic changes in the murine IL-4 locus during differentiation to Th1 and Th2 cells detected the permissive mark H3K9Ac at HSIV, the IL-4 silencer, in naïve T cells. Upon differentiation to Th1 cells, the mark was modestly enriched at the IL-4 3′, intronic and CNS1 enhancers, and upstream of both IL-4 and IL-13 genes. This correlated with slightly increased IL-4 and IL-13 transcription compared to naïve T cells. As cells were differentiated towards a Th2 lineage, levels of acetylated H3K9/14 became more abundant at these sites compared with Th1 cells. This finding correlated with the enhanced capacity of Th2 cells to express both IL-4 and IL-13.63
Studies by Koyanagi et al. demonstrated that H3K27Me3, a mark usually associated with silenced genes, was also detected at HSIV and HSS3 in naïve cells. This histone methylation was preserved in Th1 cells, but was removed as cells were differentiated to Th2 cells. In Th1 cells, the polycomb protein EZH2, the H3K27 methyl transferase, was also detected at HSIV and at HSS3.64 EZH2 has also been shown to interact with Dnmt1, illustrating the potential link between histone and DNA methylation.65
Conclusion
T-cell differentiation is controlled by factors that influence histone modifications, DNA methylation and DNase1 sensitivity and emerging data suggest that key transcription factors can recruit enzymatic components that can influence each of these pathways. Understanding the range of enzymes that may be recruited to the IL-4 locus during Th2 differentiation will be essential to our understanding of how dysregulation occurs in disease situations.
Acknowledgments
Work in the authors’ laboratory is supported by Asthma UK and the Medical Research Council.
References
- 1.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 2.Laporte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arima K, Sato K, Tanaka G, et al. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem. 2005;280:24915–22. doi: 10.1074/jbc.M502571200. [DOI] [PubMed] [Google Scholar]
- 4.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–83. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Aune TM. Dynamic changes in histone-methylation ‘marks’ across the locus encoding interferon-gamma during the differentiation of T helper type 2 cells. Nat Immunol. 2007;8:723–31. doi: 10.1038/ni1473. [DOI] [PubMed] [Google Scholar]
- 7.Goenka S, Cho SH, Boothby M. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J Biol Chem. 2007;282:18732–9. doi: 10.1074/jbc.M611283200. [DOI] [PubMed] [Google Scholar]
- 8.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 9.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 10.Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–10. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazer KA, Ueda Y, Zhu Y, et al. Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster region. Genome Res. 1997;7:495–512. doi: 10.1101/gr.7.5.495. [DOI] [PubMed] [Google Scholar]
- 13.Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–45. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Jacobson NG, Bhattacharya D, Gorham JD, Fenoglio D, Sha WC, Murphy TL, Murphy KM. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc Natl Acad Sci USA. 1999;96:3888–93. doi: 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 18.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci USA. 2004;101:1993–8. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–65. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 20.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–51. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour J, Cousins DJ, Richards DF, Sattar Z, Lee TH, Lavender P. Regulation of GM-CSF expression by the transcription factor c-Maf. J Allergy Clin Immunol. 2007;120:56–63. doi: 10.1016/j.jaci.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–52. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 23.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–51. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 24.Allis CD, Berger SL, Cote J, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–6. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Avots A, Buttmann M, Chuvpilo S, et al. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–24. doi: 10.1016/s1074-7613(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) J Exp Med. 1998;187:2031–6. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang T, Davis RJ, Chow CW. Requirement of two NFATc4 transactivation domains for CBP potentiation. J Biol Chem. 2001;276:39569–76. doi: 10.1074/jbc.M102961200. [DOI] [PubMed] [Google Scholar]
- 28.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 29.Finotto S, Neurath MF, Glickman JN, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 30.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 31.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–53. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 32.Martins GA, Hutchins AS, Reiner SL. Transcriptional activators of helper T cell fate are required for establishment but not maintenance of signature cytokine expression. J Immunol. 2005;175:5981–5. doi: 10.4049/jimmunol.175.9.5981. [DOI] [PubMed] [Google Scholar]
- 33.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–8. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 34.Elser B, Lohoff M, Kock S, Giaisi M, Kirchhoff S, Krammer PH, Li-Weber M. IFN-gamma represses IL-4 expression via IRF-1 and IRF-2. Immunity. 2002;17:703–12. doi: 10.1016/s1074-7613(02)00471-5. [DOI] [PubMed] [Google Scholar]
- 35.Li-Weber M, Eder A, Krafft-Czepa H, Krammer PH. T cell-specific negative regulation of transcription of the human cytokine IL-4. J Immunol. 1992;148:1913–8. [PubMed] [Google Scholar]
- 36.Tam W. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene. 2001;274:157–67. doi: 10.1016/s0378-1119(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 37.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604–9. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–75. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 42.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the th2 cytokine locus control region. Immunity. 2004;21:865–76. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proc Natl Acad Sci USA. 2004;101:16010–5. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemoto N, Koyano-Nakagawa N, Yokota T, Arai N, Miyatake S, Arai K. Th2-specific DNase I-hypersensitive sites in the murine IL-13 and IL-4 intergenic region. Int Immunol. 1998;10:1981–5. doi: 10.1093/intimm/10.12.1981. [DOI] [PubMed] [Google Scholar]
- 45.Monticelli S, Lee DU, Nardone J, Bolton DL, Rao A. Chromatin-based regulation of cytokine transcription in Th2 cells and mast cells. Int Immunol. 2005;17:1513–24. doi: 10.1093/intimm/dxh329. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O’Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemoto N, Kamogawa Y, Jun Lee H, Kurata H, Arai KI, O’Garra A, Arai N, Miyatake S. Cutting edge: chromatin remodeling at the IL-4/IL-13 intergenic regulatory region for Th2-specific cytokine gene cluster. J Immunol. 2000;165:6687–91. doi: 10.4049/jimmunol.165.12.6687. [DOI] [PubMed] [Google Scholar]
- 48.Ansel KM, Greenwald RJ, Agarwal S, et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nat Immunol. 2004;5:1251–9. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- 49.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5:1017–27. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 50.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat Immunol. 2005;6:42–8. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 51.Illingworth R, Kerr A, Desousa D, et al. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–47. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng HH, Zhang Y, Hendrich B, et al. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 54.Santangelo S, Cousins DJ, Winkelmann NE, Staynov DZ. DNA methylation changes at human Th2 cytokine genes coincide with DNase I Hypersensitive site formation during CD4(+) T cell differentiation. J Immunol. 2002;169:1893–903. doi: 10.4049/jimmunol.169.4.1893. [DOI] [PubMed] [Google Scholar]
- 55.Makar KW, Perez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nat Immunol. 2003;4:1183–90. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 56.Lee GR, Fields PE, Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447–59. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- 57.Hutchins AS, Mullen AC, Lee HW, Sykes KJ, High FA, Hendrich BD, Bird AP, Reiner SL. Gene silencing quantitatively controls the function of a developmental trans-activator. Mol Cell. 2002;10:81–91. doi: 10.1016/s1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- 58.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Birney E, Stamatoyannopoulos JA, Dutta A, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–22. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen L, Kondo Y, Guo Y, et al. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 2007;3:2023–36. doi: 10.1371/journal.pgen.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baguet A, Bix M. Chromatin landscape dynamics of the Il4-Il13 locus during T helper 1 and 2 development. Proc Natl Acad Sci USA. 2004;101:11410–5. doi: 10.1073/pnas.0403334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. J Biol Chem. 2005;280:31470–7. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 65.Vire E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]


