Abstract
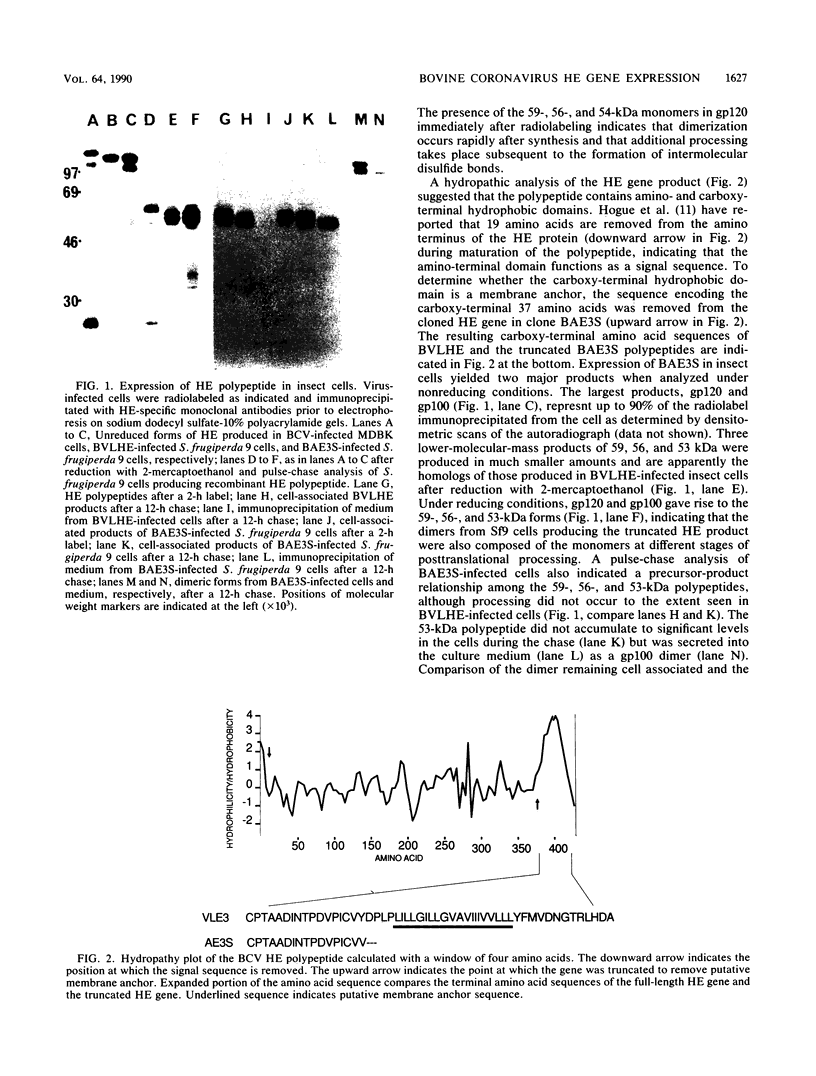
A cDNA fragment representing the hemagglutinin-esterase (HE) gene of bovine coronavirus (BCV) was inserted into the genome of Autographa californica nuclear polyhedrosis virus. Infection of insect cells with the recombinant virus resulted in the production of a 120-kilodalton disulfide-linked dimeric form of the BCV HE polypeptide. Deletion of the carboxy-terminal hydrophobic domain from the HE polypeptide resulted in secretion of a dimeric form of the truncated HE polypeptide. The acetylesterase activity of the BCV HE was detectable in insect cells expressing the BCV hemagglutinin and was inhibited by two monoclonal antibodies which also inhibit hemagglutination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butters T. D., Hughes R. C. Isolation and characterization of mosquito cell membrane glycoproteins. Biochim Biophys Acta. 1981 Feb 6;640(3):655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- Callebaut P. E., Pensaert M. B. Characterization and isolation of structural polypeptides in haemagglutinating encephalomyelitis virus. J Gen Virol. 1980 May;48(1):193–204. doi: 10.1099/0022-1317-48-1-193. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P. J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986 Jul;67(Pt 7):1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Roy R. S., Begin M. E. Bovine coronavirus isolation and cultivation in continuous cell lines. Am J Vet Res. 1980 Jan;41(1):30–38. [PubMed] [Google Scholar]
- Deregt D., Babiuk L. A. Monoclonal antibodies to bovine coronavirus: characteristics and topographical mapping of neutralizing epitopes on the E2 and E3 glycoproteins. Virology. 1987 Dec;161(2):410–420. doi: 10.1016/0042-6822(87)90134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregt D., Gifford G. A., Ijaz M. K., Watts T. C., Gilchrist J. E., Haines D. M., Babiuk L. A. Monoclonal antibodies to bovine coronavirus glycoproteins E2 and E3: demonstration of in vivo virus-neutralizing activity. J Gen Virol. 1989 Apr;70(Pt 4):993–998. doi: 10.1099/0022-1317-70-4-993. [DOI] [PubMed] [Google Scholar]
- Deregt D., Sabara M., Babiuk L. A. Structural proteins of bovine coronavirus and their intracellular processing. J Gen Virol. 1987 Nov;68(Pt 11):2863–2877. doi: 10.1099/0022-1317-68-11-2863. [DOI] [PubMed] [Google Scholar]
- Herrler G., Dürkop I., Becht H., Klenk H. D. The glycoprotein of influenza C virus is the haemagglutinin, esterase and fusion factor. J Gen Virol. 1988 Apr;69(Pt 4):839–846. doi: 10.1099/0022-1317-69-4-839. [DOI] [PubMed] [Google Scholar]
- Hogue B. G., Brian D. A. Structural proteins of human respiratory coronavirus OC43. Virus Res. 1986 Aug;5(2-3):131–144. doi: 10.1016/0168-1702(86)90013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue B. G., Kienzle T. E., Brian D. A. Synthesis and processing of the bovine enteric coronavirus haemagglutinin protein. J Gen Virol. 1989 Feb;70(Pt 2):345–352. doi: 10.1099/0022-1317-70-2-345. [DOI] [PubMed] [Google Scholar]
- Hogue B. G., King B., Brian D. A. Antigenic relationships among proteins of bovine coronavirus, human respiratory coronavirus OC43, and mouse hepatitis coronavirus A59. J Virol. 1984 Aug;51(2):384–388. doi: 10.1128/jvi.51.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Brian D. A. Bovine coronavirus structural proteins. J Virol. 1982 May;42(2):700–707. doi: 10.1128/jvi.42.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Potts B. J., Brian D. A. Bovine coronavirus hemagglutinin protein. Virus Res. 1985 Feb;2(1):53–59. doi: 10.1016/0168-1702(85)90059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Gröner A., Frese K., Drenckhahn D., Hauser C., Rott R., Doerfler W., Klenk H. D. Synthesis of biologically active influenza virus hemagglutinin in insect larvae. J Virol. 1989 Apr;63(4):1677–1685. doi: 10.1128/jvi.63.4.1677-1685.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luytjes W., Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology. 1988 Oct;166(2):415–422. doi: 10.1016/0042-6822(88)90512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macneughton M. R., Davies H. A. Ribonucleoprotein-like structures from coronavirus particles. J Gen Virol. 1978 Jun;39(3):545–549. doi: 10.1099/0022-1317-39-3-545. [DOI] [PubMed] [Google Scholar]
- Mebus C. A. Pathogenesis of coronaviral infection in calves. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):631–632. [PubMed] [Google Scholar]
- Parker M. D., Cox G. J., Deregt D., Fitzpatrick D. R., Babiuk L. A. Cloning and in vitro expression of the gene for the E3 haemagglutinin glycoprotein of bovine coronavirus. J Gen Virol. 1989 Jan;70(Pt 1):155–164. doi: 10.1099/0022-1317-70-1-155. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Massa P. T., ter Meulen V. Characterization of a variant virus isolated from neural cell culture after infection of mouse coronavirus JHMV. Virology. 1986 Nov;155(1):267–270. doi: 10.1016/0042-6822(86)90187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Siddell S. G., Wege H., ter Meulen V. Characterization of a variant virus selected in rat brains after infection by coronavirus mouse hepatitis virus JHM. J Virol. 1985 May;54(2):429–435. doi: 10.1128/jvi.54.2.429-435.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Leider J., Spaan W., Palese P. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J Virol. 1988 Dec;62(12):4686–4690. doi: 10.1128/jvi.62.12.4686-4690.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Muster T., Lauro A. M., Powers J. C., Palese P. Influenza C virus esterase: analysis of catalytic site, inhibition, and possible function. J Virol. 1989 May;63(5):2056–2062. doi: 10.1128/jvi.63.5.2056-2062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Brideau R. J., Thomsen D. R. Immunization of cotton rats with the human respiratory syncytial virus F glycoprotein produced using a baculovirus vector. J Infect Dis. 1989 Feb;159(2):255–264. doi: 10.1093/infdis/159.2.255. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]