Abstract
A more complete biomechanical understanding of a combined posterior cruciate ligament and posterolateral corner knee reconstruction may help surgeons develop uniformly accepted clinical surgical techniques that restore normal anatomy and protect the knee from premature arthritic changes. We identified the in situ force patterns of the individual components of a combined double-bundle posterior cruciate ligament and posterolateral corner knee reconstruction. We tested 10 human cadaveric knees using a robotic testing system by sequentially cutting and reconstructing the posterior cruciate ligament and posterolateral corner. The knees were subjected to a 134-N posterior tibial load and 5-Nm external tibial torque. The posterior cruciate ligament was reconstructed with a double-bundle technique. The posterolateral corner reconstruction included reattaching the popliteus tendon to its femoral origin and reconstructing the popliteofibular ligament. The in situ forces in the anterolateral bundle were greater in the posterolateral corner-deficient state than in the posterolateral corner-reconstructed state at 30° under the posterior tibial load and at 90° under the external tibial torque. We observed no differences in the in situ forces between the anterolateral and posteromedial bundles under any loading condition. The popliteus tendon and popliteofibular ligament had similar in situ forces at all flexion angles. The data suggest the two bundles protect each other by functioning in a load-sharing, codominant fashion, with no component dominating at any flexion angle. We believe the findings support reconstructing both posterior cruciate ligament bundles and both posterolateral corner components.
Introduction
Despite advances in knowledge of the anatomy and biomechanics of the posterior cruciate ligament (PCL), effective surgical management of PCL injuries remains a challenge in orthopaedic practice. Variable clinical outcomes after nonoperative and surgical treatment have contributed to the controversy surrounding the correct approach to this injury [2–5, 29]. Contributing to the unsatisfactory results from PCL reconstruction is the failure to address associated injuries to supporting structures such as those of the posterolateral corner (PLC) of the knee [6, 8, 18, 25]. Numerous studies describe the importance of these structures and their synergistic relationship with the PCL in controlling knee translation and rotation [15, 17, 22, 30, 32]. In addition, failure to treat injuries to the PLC places the knee at risk for continued instability and development of premature degenerative arthritis, even with a surgically reconstructed PCL [12, 15, 25, 30].
In one small cadaveric study, combined reconstruction of the PCL and PLC successfully restored knee kinematics at Time zero to within 1.2 mm of normal posterior translation and 1.1° of normal external rotation with loads applied at fixed flexion angles [28]. This study also showed that double-bundle PCL reconstruction alone, without simultaneous reconstruction of the PLC, may result in significant increases in posterior tibial translation, external tibial rotation, and in situ forces in the PCL grafts. The ability of a PLC reconstruction to limit external tibial rotation and affect forces in a PCL graft in response to various loading conditions has been studied [13, 15, 17, 20, 21, 28]. All previous studies, however, have reported the effects of the loading conditions on the PCL or PLC as a whole, without focusing attention on the specific components of the reconstruction.
Previous anatomic studies have mechanically characterized the individual bundles of the PCL, showing the normal anterolateral (AL) bundle is more taut in flexion and relatively more slack in extension [10, 31]. The reverse is true for the posteromedial (PM) bundle, which is relatively more taut in extension and more slack in flexion [10, 31]. Consequently, it has been postulated that the AL bundle functions independently in knee flexion and the PM bundle functions independently in knee extension. However, recent studies have suggested that based on length and orientation relationships, the bundles of the PCL may play a more synergistic relationship [1, 26]. Specifically, although the AL bundle becomes more taut with increasing knee flexion, it also becomes more vertically oriented, which decreases its ability to resist posterior tibial translation. Conversely, with increasing knee flexion, the less taut PM bundle becomes more horizontally oriented, increasing its ability to resist posterior tibial translation. However, it remains unclear how this orientation and tension relationship in the individual bundles of the PCL contribute to the in site forces in the reconstruction and to overall knee stability. Also, a recent cadaveric study of the structures of the native PLC found no significant differences between the mean load responses of the intact popliteus tendon (PT) and popliteofibular ligament (PFL) in response to an external tibial torque at all flexion angles [19].
The clinical basis for further investigation of the biomechanics of a combined double-bundle PCL and PLC knee reconstruction is that a better understanding may serve to establish a more anatomic, reproducible surgical approach to this combined injury pattern. Ultimately, anatomic reconstruction may better protect the joint surface from premature arthritic changes and the grafts from failure [24]. Based on the findings in previous studies that showed increased forces in the entire PCL graft in the PLC-deficient state [15, 28], a more taut AL bundle in flexion [10, 31], and similar load responses in the components of the native PLC through the range of motion (ROM) [19], we arrived at our specific hypotheses.
We hypothesized: (1) the in situ forces in the individual AL and PM bundles of the PCL reconstruction are greater in the PLC-deficient knee; (2) the AL bundle has greater forces than the PM bundle toward 90° flexion, and the opposite is true toward full extension; and (3) the PT and PFL have similar in situ forces through the ROM.
Materials and Methods
To test our hypotheses, a robotic/universal force-moment sensor (UFS) testing system was used to evaluate a double-bundle PCL and PLC knee reconstruction in a cadaveric model. This testing system was capable of measuring 5-degrees-of-freedom knee kinematics and the in situ forces in the intact PCL and PLC and their grafts with the knee at any chosen flexion angle under external loading conditions. Based on our previous data, we conducted an a priori power analysis (power, 0.80; significance level, 0.05) to ensure differences of 10 N for in situ force measurements could be detected [15, 27, 28, 32]. A difference of 10 N was selected as clinically relevant based on previous biomechanical investigations of forces generated in the PCL and PLC under physiologic conditions and previous in vitro testing [15, 19, 20, 22, 28, 32, 33]. Although no studies have specifically investigated the effect of 10 N differences on in vivo behavior or clinical outcomes in PCL-PLC reconstructions, the native components of the PLC assume loads as low as 15 N under physiologic external tibial torque in a cadaveric model [19]. Also, differences in PLC graft tensions as low as 10 N may lead to significant increases in the PCL graft forces compared with the intact PCL under physiologic loading [20]. From the power analysis, we determined 10 knee specimens would be required for this study.
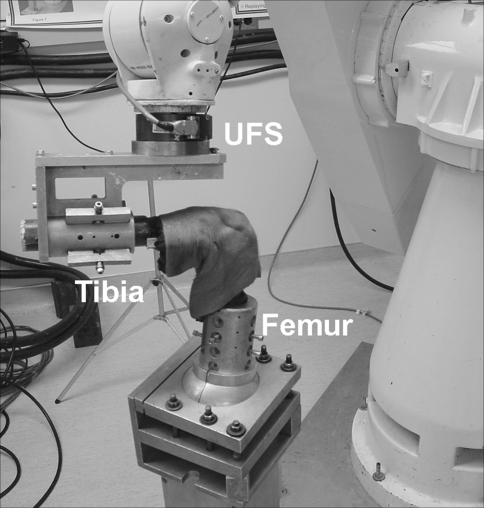
We enclosed fresh human cadaveric knees in two separate airtight plastic bags and stored them at −20°C. After the specimens were thawed overnight at room temperature, they were examined physically and radiographically. We excluded those with ligamentous or bony abnormalities. Ten tibial and femoral diaphyses (age, 38–71 years) were cut 20 cm from the joint line. We rigidly fixed the fibula with a cortical screw to the tibia to prevent motion during the testing. All soft tissues overlying the bone were removed 10 cm from the joint line superiorly and inferiorly, preserving the soft tissue surrounding the knee. The specimens were potted in an epoxy compound (Bond-Tite Products, Cleveland, OH) and rigidly mounted in thick aluminum cylinders/clamps. We mounted the femoral clamp to the base of the robotic manipulator (Puma Model 762; Unimate Inc, Pittsburgh, PA). The tibial clamp was mounted to the end effector of the robotic manipulator through the UFS (Model 4015; JR3 Inc, Woodland Hills, CA) (Fig. 1).
Fig. 1.
The robotic system is capable of operating in position-controlled mode in which the joint is moved in space to a desired position in six-degrees-of-freedom while the UFS measures the resulting external forces and moments acting on the joint [7, 9, 27]. The robot also can be operated in a force-controlled mode using force-moment feedback from the UFS so a desired force can be applied while the resulting changes in kinematics are recorded.
We first determined the path of passive flexion-extension for the intact knee from 0° to 90° knee flexion. To find this path, the robot flexed the knee in 1°-increments and recorded the position at which all external forces and moments were minimized to less than 2 N and 0.2 Nm, respectively, at each angle. This path served as the starting position for application of external loads to the knee and a reference to which knee kinematics were measured. The path then was cycled 10 times to minimize the viscoelastic effects of the soft tissue.
We tested each knee under a 134-N posterior tibial load and a 5-Nm external tibial torque at 0°, 30°, and 90° knee flexion (Table 1). To achieve these loading conditions, the posterior load or the external tibial torque was applied to the tibia incrementally (to minimize viscoelastic effects of the tissue) at a rate of 20 mm per minute. During this loading, when the UFS measured any other forces or moments on the joint, the robot changed the joint position to minimize them. After the 134-N posterior force or the 5-Nm external tibial torque was achieved, the UFS recorded the forces and moments and immediately returned the knee to the starting position.
Table 1.
Study protocol and data obtained
| Action | Data obtained |
|---|---|
| Path of passive flexion-extension (0°–90°) | Reference positions for intact knee |
| Intact knee | |
| A. 134 N posterior tibial load | Intact knee kinematics under A |
| B. 5 Nm external tibial torque | Intact knee kinematics under B |
| Section PLC | In situ forces in the PLC |
| PLC-deficient knee | |
| C. 134 N posterior tibial load | PLC-deficient knee kinematics under C |
| D. 5 Nm external tibial torque | PLC-deficient knee kinematics under D |
| Section PCL | In situ forces in the PCL in the intact and PLC-deficient knees |
| PLC- and PCL-deficient knee | |
| E. 134 N posterior tibial load | PCL- and PLC-deficient knee kinematics under E |
| F. 5 Nm external tibial torque | PCL- and PLC-deficient knee kinematics under F |
| Reconstruct PCL and PLC | |
| PLC- and PCL-reconstructed knee | |
| G. 134 N posterior tibial load | PCL- and PLC-deficient knee kinematics under G |
| H. 5 Nm external tibial torque | PCL- and PLC-deficient knee kinematics under H |
| Release PT & PFL components in alternating order | In situ forces in PT and PFL components in the PCL-reconstructed state |
| PCL-reconstructed knee | |
| I. 134 N posterior tibial load | PCL-reconstructed knee kinematics in the PLC-deficient knee under I |
| J. 5 Nm external tibial torque | PCL-reconstructed knee kinematics in the PLC-deficient knee under J |
| Release AL & PM bundles in alternating order | In situ forces in the AL & PM bundles in the PLC-reconstructed and PLC-deficient states |
We then transected the PCL through a medial parapatellar arthrotomy and reapplied the loads to the PCL-deficient knee. Next, we released the PLC, which consisted of detaching the PT from its femoral insertion and transecting the PFL. After reapplying the loads, we performed the reconstructions as described below. Finally, we released the reconstructions and reapplied the same loading conditions after each step (Table 1). The order of release was randomized.
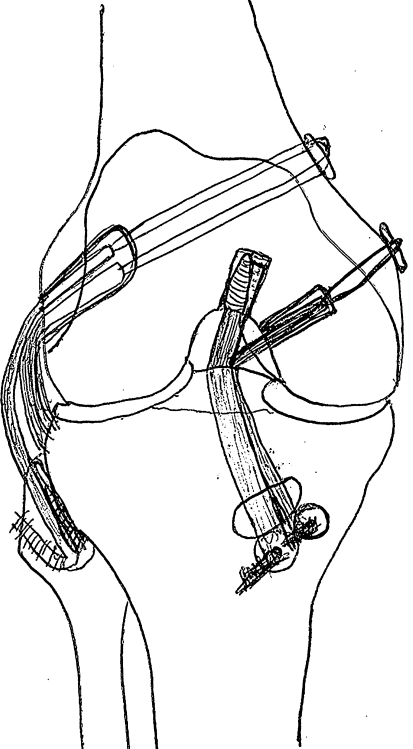
The surgical technique used in this study is used clinically for restoration of normal anatomy based on previous anatomic and biomechanical studies of the PCL and PLC (Fig. 2) [10, 11, 14, 23, 28]. We performed the PCL reconstruction using an 11-mm Achilles tendon (AL bundle) and a 7- to 8-mm doubled semitendinosus tendon (PM bundle) [14]. We drilled the femoral tunnel for the AL graft using an inside-out technique through the insertion site of the AL bundle of the PCL. Similarly, the femoral PM graft tunnel also was drilled from inside out through the insertion site of the PM bundle of the PCL. We drilled one 10-mm tibial tunnel using a PCL tibial drill guide and placement of a Kirschner wire through the center of the tibial PCL insertion site. Femoral fixation of the AL graft was performed using a 9 × 25-mm metal interference screw to secure the bone block of the Achilles tendon graft from inside out. We achieved fixation of the PM graft using a 20-mm closed-loop EndoButton (Smith & Nephew, Inc, Andover, MA). Both PCL grafts then were pulled through the tibial tunnel for later tensioning and fixation.
Fig. 2.
The PLC was reconstructed using a 5- to 6-mm doubled gracilis tendon for the PFL and by reattaching the PT to its femoral origin. The double-bundle PCL reconstruction was performed using an 11-mm Achilles tendon (AL bundle) and a 7- to 8-mm doubled semitendinosus tendon (PM bundle).
We reconstructed the PLC using a 5- to 6-mm doubled gracilis tendon for the PFL and by reattaching the PT to its femoral origin. The single femoral tunnel for the PLC reconstruction was drilled through the insertion site of the PT, just anterior to the femoral attachment of the lateral collateral ligament. This tunnel was directed toward the flare of the medial femoral condyle metaphysis to avoid colliding with the PCL femoral tunnels. We drilled the fibular tunnel of the PFL graft from posterior to anterior at the native insertion of the PFL. The PFL graft was fixed in the femoral tunnel by tying the sutures from one end of the graft (Number 2 braided, nonabsorbable, Krackow stitch) over a button at the medial side of the tunnel.
We then placed the knee and maintained it in a neutral position for tensioning and final fixation of the grafts. The PT was pulled into the same femoral tunnel and tensioned to 67 N using a spring scale as the robot preconditioned the graft by moving the knee five times through the ROM. With tension maintained and the knee at 30° flexion, we fixed the PT in the femoral tunnel (with the PFL graft) by tying the sutures from the end of the tendon (Number 2 braided, nonabsorbable, Krackow technique) over a button at the medial side of the tunnel. The PFL graft then was passed into its fibular tunnel from posterior to anterior and tensioned to 67 N while the robot preconditioned the graft as previously described. We achieved fixation using a 7 × 20-mm metal interference screw with the knee in 30° flexion while 67 N of graft tension was maintained. The PFL graft then was pulled back over the lateral edge of the fibular tunnel and sutured to the surrounding tissues for additional fixation.
The AL PCL graft then was tensioned to 88 N and preconditioned as previously described. The graft tension was maintained and the graft was fixed to the anterior tibia at 90° knee flexion as the robotic manipulator applied a 134-N anterior tibial load to reduce posterior subluxation. We obtained fixation using a 9 × 13-mm soft tissue washer (Linvatec Inc, Largo, FL) and a cortical screw. The PM PCL graft was tensioned to 67 N and preconditioned. We fixed the graft to the tibia at 15° knee flexion using a 9 × 13-mm soft tissue washer (Linvatec Inc) and a cortical screw while a 134-N anterior tibial load was applied and 67 N of graft tension maintained.
The dependent variables analyzed included the in situ forces in the reconstructed AL and PM bundles in the PLC-reconstructed and PLC-deficient states and the in situ forces in the reconstructed PT and PFL in the PCL-reconstructed state under the specified external loading conditions. Because the individual bundles of the PCL and the individual components of the PLC grafts were released sequentially and the previously determined kinematics were repeated after the release of each component, the in situ forces could be determined (Table 1). We made this determination by calculating the difference in in situ forces measured by the UFS before and after release of the component. By the principle of superposition, this difference is the in situ forces that can be attributed to that component [27].
Because we used the same specimen for all testing conditions, we used a two-way repeated-measures analysis of variance for statistical analysis. The two factors investigated were knee condition (intact, PLC-deficient, PLC- and PCL-deficient, PLC- and PCL-reconstructed, PCL-reconstructed) and knee flexion angle (0°, 30°, 90°). We performed multiple contrasts to evaluate the effects of knee condition at specific flexion angles.
Results
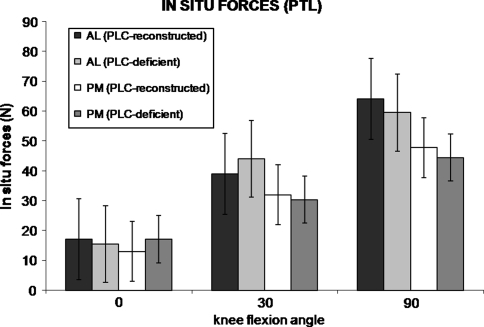
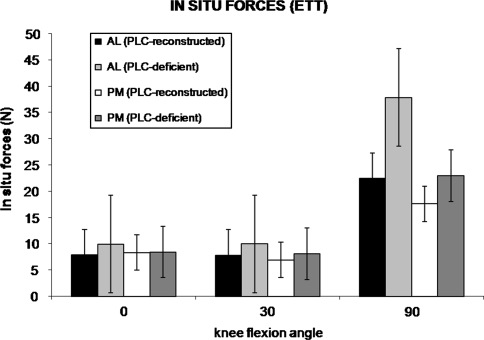
The in situ forces in the AL bundle of the PCL reconstruction were greater in the PLC-deficient state than in the PLC-reconstructed state. Specifically, the in situ forces were greater in the AL bundle at 30° under the 134-N posterior tibial load (p = 0.036) and at 90° in response to the 5-Nm external tibial torque (p = 0.006) (Figs. 3, 4). We observed no differences in the AL bundle at the other flexion angles under either loading condition (0.165 < p < 0.591). There were no differences in in situ forces in the PM bundle between the PLC-reconstructed and PLC-deficient states at any flexion angles under either the 134-N posterior tibial load or the 5-Nm external tibial torque (0.388 < p < 0.968).
Fig. 3.
In situ forces in the AL and PM bundles in the PLC-reconstructed and PLC-deficient states (mean ± standard error of the mean) under a 134-N PTL are shown. The in situ forces in the AL bundle were greater (p = 0.036) in the PLC-deficient state than in the PLC-reconstructed state at 30°. There were no differences in the in situ forces between the AL and PM bundles at any flexion angle in either PLC state.
Fig. 4.
In situ forces in the AL and PM bundles in the PLC-reconstructed and PLC-deficient states (mean ± standard error of the mean) under a 5-Nm external tibial torque (ETT) are shown. The in situ forces in the AL bundle were higher (p = 0.006) in the PLC-deficient state than in the PLC-reconstructed state at 90°. There were no differences in the in situ forces between the AL and PM bundles at any flexion angle in either PLC state.
When the in situ forces in the PM bundle were compared with those in the AL bundle in the PLC-reconstructed and PLC-deficient states, we observed no differences under either loading conditions at any flexion angle (0.215 < p < 0.886).
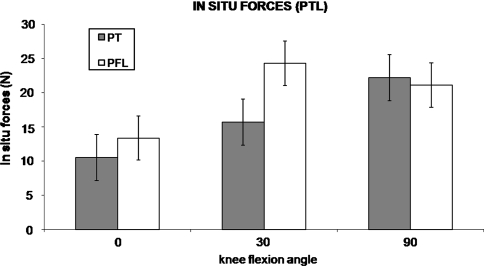
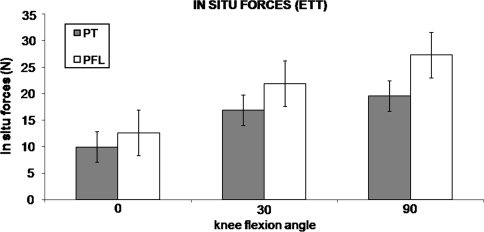
We observed no differences (0.244 < p < 0.724) between the PT and PFL at any flexion angle under either loading condition (Figs. 5, 6).
Fig. 5.
In situ forces in the PT and PFL in the PCL-reconstructed state (mean ± standard error of the mean) under a 134-N PTL are shown. There were no differences detected between the PT and PFL at any flexion angle.
Fig. 6.
In situ forces in the PT and PFL in the PCL-reconstructed state (mean ± standard error of the mean) under a 5-Nm external tibial torque (ETT) are shown. There were no differences detected between the PT and PFL at any flexion angle.
Discussion
Variable clinical outcomes, which often include the development of premature arthritis, after treatment of combined PCL and PLC injuries have led to controversy surrounding the correct approach to this injury pattern [12, 24, 25]. A better understanding of the biomechanics of the components of a combined PCL and PLC reconstruction may help to establish a more anatomic, reproducible surgical approach that better protects the joint from premature arthritic changes and the grafts from failure [24]. Based on previous studies that showed increased forces in the entire PCL graft in the PLC-deficient state [15, 28], a more taut AL bundle in flexion [10, 31], and similar load responses in the components of the native PLC through the ROM [19], we hypothesized in this study: (1) the in situ forces in the individual AL and PM bundles of the PCL reconstruction are greater in the PLC-deficient knee; (2) the AL bundle has greater forces than the PM bundle toward 90° flexion, and the opposite is true toward full extension; and (3) the PT and PFL have similar in situ forces through the ROM.
The techniques chosen for reconstruction of both structures in this study are based on clinically used techniques for restoration of normal anatomy [16]. The instability pattern of injury to the PFL and PT without injury to the lateral collateral ligament helped maintain consistency with previous studies [13, 15, 17]. A major limitation of this study design is it does not account for biologic remodeling or graft elongation, which may change the behavior of the grafts with time in vivo. In addition, it does not account for the dynamic stabilizing effects of contracting muscles such as that which may occur with popliteus muscle contraction. Furthermore, because this study examined only three flexion angles between 0° and 90°, it may be useful to evaluate these knee conditions at higher flexion angles. However, the utility of this study design is that we were able to precisely evaluate the in situ forces in the individual components of a complex, clinically used knee reconstruction. We also were able to compare the in situ forces of the individual components in the same knee, thus minimizing interspecimen variability. Measurement of the individual graft performance at initial reconstruction in a cadaveric model provides valuable insight into the behavior of reconstructions that are used clinically, but cannot be evaluated biomechanically in this manner in vivo. To supplement this controlled laboratory evaluation, additional studies are necessary to examine further the long-term clinical outcomes of the double-bundle PCL and PLC-reconstructed knee.
Our findings support our first hypothesis that the in situ forces in the individual AL and PM bundles of the PCL reconstruction are greater in the PLC-deficient knee. Specific to this hypothesis, we found that under a posterior tibial load, the in situ forces in the AL bundle were greater at 30° in the PLC-deficient state than in the PLC-reconstructed state. Also, under an external tibial torque, the in situ forces in the AL bundle were greater at 90° in the PLC-deficient state. These data are consistent with those of previous studies that reported single-bundle and double-bundle PCL graft forces are greater in knees with a deficient PLC than in those with an intact PLC [15, 28]. In this PLC-deficient knee, the AL bundle may be at an increased risk of graft failure because of increased in situ forces [13, 15, 28, 30, 32]. This increase in AL bundle forces in the PLC-deficient knee further emphasizes the importance of reconstructing the PLC to protect the AL bundle from increased forces under external loading conditions. The finding that the forces in the AL bundle were greater in the PLC-deficient state under an external tibial torque also confirms the role of the PLC in providing rotational stability of the knee at 90° flexion [17, 20].
Contrary to our second hypothesis, the AL bundle did not have greater in situ forces than the PM bundle toward 90° flexion, and the PM bundle did not have greater in situ forces toward full extension. In fact, there were no differences in the in situ forces between the AL and PM bundles at any flexion angles. By analyzing the in situ forces in the individual components of the reconstruction, we were able to establish whether either component functions more independently at certain flexion angles, as previously suggested [10, 31]. The in situ forces show each bundle’s contribution to knee stability when the knee is subjected to a posterior tibial load or an external tibial torque [27]. Our finding that there were no significant differences in in situ forces between the bundles at any flexion angle suggests that both bundles function through the ROM in a codominant fashion. Although contrary to our hypothesis, this finding is consistent with the conclusions of other recent studies that evaluated the bundles in terms of length and orientation [1, 26]. Ahmad et al., in a small cadaveric study investigated the role each bundle contributes to posterior knee stability by studying the orientation and length characteristics of the individual bundles in a series of cadaveric knees [1]. They reported that with increasing knee flexion, the AL bundle becomes tighter [1]. This finding is consistent with historic data [10, 31] and would increase its ability to resist posterior tibial translation. However, they also reported that with increasing knee flexion, the AL bundle became more vertically oriented, decreasing its ability to resist posterior tibial translation [1]. The PM bundle, conversely, became more horizontal with increasing knee flexion. This orientation increased the ability of the PM bundle to resist posterior tibial translation [1]. Because of this codominance, neither bundle functioned independently in restraining posterior tibial translation at specific knee flexion angles. Another recent study used MRI and a dual-orthogonal fluoroscopic system to measure the length, elevation, deviation, and twist of the PCL bundles during knee flexion in living subjects [26]. Papannagari et al. reported that both bundles elongated and changed their orientations up to 120° knee flexion. They concluded that the bundles do not behave in the reciprocal fashion that traditionally had been postulated [10, 31]. Rather, both elongate and function through the ROM. Therefore, reconstruction should mimic the native anatomy with anatomic tunnel placement of two bundles.
Our study supports our third hypothesis that the PT and PFL have similar in situ forces through the ROM, as we found no differences in the in situ forces between these two components at any flexion angle. This finding is consistent with a biomechanical study of the native PLC [19]. In that study, the PT and the PFL were found to have similar loading patterns in response to an external rotation torque, with their mean load responses generally increasing with increasing knee flexion angle, before decreasing slightly after 90°. We found a similar increase in the in situ forces in the PT and PFL under external tibial torque with increasing flexion angle. These findings suggest that both components play complementary roles as stabilizers to external rotation of the knee and are functionally important throughout the range of knee flexion tested.
We believe the findings in this study support reconstructing both PCL bundles and both PLC components in a combined PCL and PLC injury pattern. Consistent with previous studies, these data suggest that in the PLC-deficient knee, the AL bundle may be at an increased risk of graft failure because of increased in situ forces. Also, the findings of this study suggest that the two PCL bundles function in a load-sharing, codominant fashion, with no component dominating at any flexion angle. Although this finding is contrary to historically accepted theory, it is consistent with a more recent understanding of the role of the orientation and length of the two bundles in optimizing the graft’s ability to resist an external load throughout the full ROM. Finally, the finding that the PFL and PT had similar in situ forces shows that both PLC components may play equally important roles in restoring knee stability in the combined PCL and PLC-reconstructed knee.
Acknowledgments
We thank the Musculoskeletal Research Center, University of Pittsburgh, and the Aircast Foundation for support.
Footnotes
One or more of the authors (CDH) have received funding from the Aircast Foundation, Pittsburgh, PA.
Each author certifies that his or her institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ahmad CS, Cohen ZA, Levine WN, Gardner TR, Ateshian GA, Mow VC. Codominance of the individual posterior cruciate ligament bundles: an analysis of bundle lengths and orientation. Am J Sports Med. 2003;31:221–225. [DOI] [PubMed]
- 2.Bianchi M. Acute tears of the posterior cruciate ligament: clinical study and results of operative treatment in 27 cases. Am J Sports Med. 1983;11:308–314. [DOI] [PubMed]
- 3.Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24:306–310. [DOI] [PubMed]
- 4.Cross MJ, Powell JF. Long-term followup of posterior cruciate ligament rupture: a study of 116 cases. Am J Sports Med. 1984;12:292–297. [DOI] [PubMed]
- 5.Dandy DJ, Pusey RJ. The long-term results of unrepaired tears of the posterior cruciate ligament. J Bone Joint Surg Br. 1982;64:92–94. [DOI] [PubMed]
- 6.Fanelli GC, Giannotti BF, Edson CJ. Arthroscopically assisted combined posterior cruciate ligament/posterior lateral complex reconstruction. Arthroscopy. 1996;12:521–530. [DOI] [PubMed]
- 7.Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology:a cadaveric study. Am J Sports Med. 1998;26:395–401. [DOI] [PubMed]
- 8.Freeman RT, Duri ZA, Dowd GS. Combined chronic posterior cruciate and posterolateral corner ligamentous injuries: a comparison of posterior cruciate ligament reconstruction with and without reconstruction of the posterolateral corner. Knee. 2002;9:309–312. [DOI] [PubMed]
- 9.Fujie H, Livesay GA, Woo SL, Kashiwaguchi S, Blomstrom G. The use of a universal force-moment sensor to determine in-situ forces in ligaments: a new methodology. J Biomech Eng. 1995;117:1–7. [DOI] [PubMed]
- 10.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint: anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216–231. [DOI] [PubMed]
- 11.Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15:741–749. [DOI] [PubMed]
- 12.Harner CD, Hoher J. Evaluation and treatment of posterior cruciate ligament injuries. Am J Sports Med. 1998;26:471–482. [DOI] [PubMed]
- 13.Harner CD, Hoher J, Vogrin TM, Carlin GJ, Woo SL. The effects of a popliteus muscle load on in situ forces in the posterior cruciate ligament and on knee kinematics: a human cadaveric study. Am J Sports Med. 1998;26:669–673. [DOI] [PubMed]
- 14.Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000;28:144–151. [DOI] [PubMed]
- 15.Harner CD, Vogrin TM, Hoher J, Ma CB, Woo SL. Biomechanical analysis of a posterior cruciate ligament reconstruction: deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med. 2000;28:32–39. [DOI] [PubMed]
- 16.Harner CD, Waltrip RL, Bennett CH, Francis KA, Cole B, Irrgang JJ. Surgical management of knee dislocations. J Bone Joint Surg Am. 2004;86:262–273. [DOI] [PubMed]
- 17.Hoher J, Harner CD, Vogrin TM, Baek GH, Carlin GJ, Woo SL. In situ forces in the posterolateral structures of the knee under posterior tibial loading in the intact and posterior cruciate ligament-deficient knee. J Orthop Res. 1998;16:675–681. [DOI] [PubMed]
- 18.LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee: association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25:433–438. [DOI] [PubMed]
- 19.LaPrade RF, Tso A, Wentorf FA. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32:1695–1701. [DOI] [PubMed]
- 20.Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR. Effects of posterolateral reconstructions on external tibial rotation and forces in a posterior cruciate ligament graft. J Bone Joint Surg Am. 2007;89:2351–2358. [DOI] [PubMed]
- 21.Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR. How well do anatomical reconstructions of the posterolateral corner restore varus stability to the posterior cruciate ligament-reconstructed knee? Am J Sports Med. 2007;35:1117–1122. [DOI] [PubMed]
- 22.Markolf KL, Wascher DC, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part II: The effect of section of the posterolateral structures. J Bone Joint Surg Am. 1993;75:387–394. [DOI] [PubMed]
- 23.Maynard MJ, Deng X, Wickiewicz TL, Warren RF. The popliteofibular ligament: rediscovery of a key element in posterolateral stability. Am J Sports Med. 1996;24:311–316. [DOI] [PubMed]
- 24.Miller MD, Bergfeld JA, Fowler PJ, Harner CD, Noyes FR. The posterior cruciate ligament injured knee: principles of evaluation and treatment. Instr Course Lect. 1999;48:199–207. [PubMed]
- 25.Noyes FR, Barber-Westin SD. Surgical restoration to treat chronic deficiency of the posterolateral complex and cruciate ligaments of the knee joint. Am J Sports Med. 1996;24:415–426. [DOI] [PubMed]
- 26.Papannagari R, DeFrate LE, Nha KW, Moses JM, Moussa M, Gill TJ, Li G. Function of posterior cruciate ligament bundles during in vivo knee flexion. Am J Sports Med. 2007;35:1507–1512. [DOI] [PubMed]
- 27.Rudy TW, Livesay GA, Woo SL, Fu FH. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29:1357–1360. [DOI] [PubMed]
- 28.Sekiya JK, Haemmerle MJ, Stabile KJ, Vogrin TM, Harner CD. Biomechanical analysis of a combined double-bundle posterior cruciate ligament and posterolateral corner reconstruction. Am J Sports Med. 2005;33:360–369. [DOI] [PubMed]
- 29.Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries: a prospective study. Am J Sports Med. 1999;27:276–283. [DOI] [PubMed]
- 30.Skyhar MJ, Warren RF, Ortiz GJ, Schwartz E, Otis JC. The effects of sectioning of the posterior cruciate ligament and the posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1993;75:694–699. [DOI] [PubMed]
- 31.Van Dommelen BA, Fowler PJ. Anatomy of the posterior cruciate ligament: a review. Am J Sports Med. 1989;17:24–29. [DOI] [PubMed]
- 32.Vogrin TM, Hoher J, Aroen A, Woo SL, Harner CD. Effects of sectioning the posterolateral structures on knee kinematics and in situ forces in the posterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2000;8:93–98. [DOI] [PubMed]
- 33.Wascher DC, Markolf KL, Shapiro MS, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part I: The effect of multiplane loading in the intact knee. J Bone Joint Surg Am. 1993;75:377–386. [DOI] [PubMed]