Abstract
Synovial sarcoma is a rare sarcoma of unknown histologic origin. We previously reported the gene expression profile of synovial sarcoma was closely related to that of malignant peripheral nerve sheath tumors, and the fibroblast growth factor (FGF) signal was one of the main growth signals in synovial sarcoma. Here we further demonstrate the neural origin of synovial sarcoma using primary tumors and cell lines. The expression of neural tissue-related genes was confirmed in synovial sarcoma tumor tissues, but the expression of some genes was absent in synovial sarcoma cell lines. Treatment of synovial sarcoma cell lines with BMP4 or FGF2 enhanced or restored the expression of neural tissue-related genes and induced a neuron-like morphology with positive Tuj-1 expression. Treatment with all-trans-retinoic acid also induced the expression of neural tissue-related genes in association with growth inhibition, which was not observed in other cell lines except a malignant peripheral nerve sheath tumor cell line. A growth-inhibitory effect of all-trans-retinoic acid was also observed for xenografted tumors in athymic mice. The simultaneous treatment with FGF signal inhibitors enhanced the growth-inhibitory effect of all-trans-retinoic acid, suggesting the combination of growth signaling inhibition and differentiation induction could be a potential molecular target for treating synovial sarcoma.
Introduction
Synovial sarcomas (SSs) account for approximately 7% to 10% of all human soft tissue sarcomas and predominantly affect the extremities but can occur in any part of the body, including the head and neck [20, 49]. Despite its name, SS arises from tissues other than normal synovial lining. Ultrastructural studies of epithelium-like cells in biphasic synovial sarcomas revealed characteristics of a true glandular epithelium, which were not observed in either superficial or deeper cells of synovium [21]. Further, immunohistochemical studies have demonstrated keratins and epithelial membrane antigen-positive epithelium-like cells in biphasic synovial sarcomas and unlike those found in the lining cells of synovium [43]. Therefore, the name “synovial sarcoma” is believed inappropriate for this class of tumor, and the cell of origin of SS is still unknown [35].
Genetically, SSs are characterized as tumors harboring the reciprocal chromosomal translocation t(18;X) producing an SYT-SSX fusion gene [31]. The myf5 gene is an important transcription factor regulating the differentiation of cells along the myogenic lineage [5]. A recent report using a conditional knock-in mouse model using the promoter of the myf5 gene as a driver of the SYT-SSX gene demonstrated sarcomatous tumors strongly resembling human SS [22]. This suggested the precursor of SS was cells of the myogenic lineage. The global gene expression profile of the SS-like tumors that developed in these knock-in mice, however, differed considerably from that reported in human SS [20], possibly as a result of the difference in cell origin.
We previously performed gene expression profiling of 47 soft tissue sarcomas using a genome-wide cDNA microarray and reported SS was clustered into the same group with malignant peripheral nerve sheath tumor (MPNST), the precursor cells of which are neural crest-derived cells [36]. Among the products of the 26 genes whose expression was upregulated in SS, collagen Type IX is one of the major components of cartilage [17], neurofilament heavy polypeptide (NFH) has important roles in the assembly of filaments and in the radial growth of axons [28], and endothelin 3 is an important signal for melanocytes and enteric neurons [7]. In addition, the expression of the chondrogenic transcription activator SOX9 [9] is upregulated in SSs [2]. These results suggest the cellular origin of SS is neural crest-derived cells capable of differentiating in multiple directions. Neural crest-derived cells are precursors of neuroblastomas [41, 42], malignant melanomas [18], and the Ewing family of tumors [46], which are sarcomas harboring a tumor-specific fusion oncoprotein (EWS-Fli1 or EWS- ERG) [34]. SS is known to develop at unusual anatomic sites such as the pleurae [6], visceral organs [10], bone marrow [24], and peripheral nerves [14]. Molecular diagnoses using the SYT-SSX fusion transcript as a marker have proved some renal tumors previously diagnosed as different diseases were in fact SSs [4]. This diverse distribution may favor the notion that SS originate from neural crest-derived cells, which migrate into many types of tissues.
The goal of this study was to demonstrate the neural origin of SS using primary tumors and cell lines and also to apply the differentiation induction therapy for the treatment of SS in combination with a growth signal inhibitor.
Materials and Methods
Using SS tumor tissues and cell lines, the hypothesis of neural origin of SS was investigated by mRNA and protein expression analyses of a set of neural tissue genes such as nestin, which is expressed in undifferentiated cells [42]. Also, several neural inducers were used to induce the neural phenotype in cell lines, and the therapeutic application was examined by in vitro and in vivo growth inhibition assay.
Tumor tissues were obtained at either biopsy or resection surgery and kept at −80°C. Informed consent was obtained from each patient and tumor samples were approved for analysis by the Ethics Committee of the Faculty of Medicine, Kyoto University. Five human SS cell lines (YaFuSS, HS-SY-II, SYO-1, Fuji, 1273/99) were used in this study. YaFuSS and HS-SY-II cells have the SYT-SSX1 fusion gene, and the others have the SYT-SSX2 fusion gene (data not shown). The properties of YaFuSS were described previously [26]. HS-SY-II was a gift from H. Sonobe (Kochi Medical School, Japan) [44], SYO-1 from A. Kawai (National Cancer Center, Japan) [29], Fuji from S. Tanaka (Hokkaido University, Japan) [37], and 1273/99 from O. Larsson (Karolinska Institute, Stockholm, Sweden). NMS-2 (MPNST) was a gift from A. Ogose (Niigata University, Japan) [25]. Saos2 (osteosarcoma), HT1080 (fibrosarcoma), COLO205 (colon cancer), SW480 (colon cancer), and NT2/D1 (neurogenic clone of pluripotent embryonal carcinoma [3]) were purchased from the American Type Culture Collection (Manassas, VA). Adult human mesenchymal stem cells were obtained from BioWhittaker (Walkersville, MD) and maintained in MSCGM™ medium (BioWhittaker). Tumor cell lines Fuji, SW480, and COLO205 were maintained in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO) with 10% fetal bovine serum (FBS; HyClone, Logan, UT). Other tumor cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma) with 10% FBS. OPTI-MEM® I, which contains insulin and transferrin as protein supplements (Invitrogen, Carlsberg, CA), was used in serum-free cultures.
Anti-neuron-specific class III β-tubulin antibody (Tuj-1) and recombinant human bone morphogenetic protein 4 (rhBMP4) were purchased from R&D Systems (Minneapolis, MN). Recombinant human fibroblast growth factor 2 (rhFGF2) was obtained from Oncogene Research Products (San Diego, CA).
The expression of neural tissue-related genes was analyzed by reverse transcription (RT)-PCR in 29 sarcoma tumor samples, including 18 SSs and also 12 tumor cell lines including five SS cell lines. Total RNA was extracted using TRIzol® reagent (Invitrogen) following the manufacturer’s instructions. After treatment with DNase I (Nippon Gene, Osaka, Japan), 1 mg of total RNA was reverse-transcribed into single-stranded cDNA using the oligo(dT) primer and SuperScript™ II reverse transcriptase (Invitrogen). PCR was performed using 1 mL of RT product in a final volume of 25 mL containing 20 pmol each of the sense and antisense primers, 2.5 mmol/L MgCl2, 0.2 mmol/L of each dNTP, and 1 unit of rTaq polymerase (Toyobo, Osaka, Japan). All PCRs were performed using GeneAmp® 9700 (PE Applied Biosystem, Foster City, CA). Information on the primers is available on request.
To investigate whether SS cell lines are able to differentiate into the neural cell lineage, cells were treated with ATRA, rhFGF2, or rhBMP4, all known as inducers of neural differentiation [12, 27, 33]. Cytoplasmic staining of Tuj-1 and the mRNA expression of MASH1 were used as markers for neural induction by these treatments. For neurogenic induction, cells (2.5 × 103) in DMEM with 10% FBS were seeded in an eight-well chamber slide (Permanox® type; Nalgene Nunc International, Rochester, NY) and cultured overnight. Then the medium was replaced with serum-free OPTI-MEM® I medium with vehicle (0.1% dimethyl sulfoxide), all-trans-retinoic acid (ATRA) (1 mmol/L), rhFGF2 (100 ng/mL), or rhBMP4 (50 ng/mL) and cultured for 14 days (for rhBMP4) or 7 days (for the others). The cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature, permeabilized, blocked with phosphate-buffered saline (PBS) containing 0.1% Triton® X-100 (Sigma) and 0.5% bovine serum albumin for 1 hour, and incubated with Tuj-1 (1:500) followed by tetramethylrhodamine isothiocyanate-conjugated antimouse immunoglobulin (1:100; DakoCytomation, Glostrup, Denmark). After three washes with PBS, the cells were incubated with 4′,6-diamidino-2-phenylindole. To check the expression of mammalian achaete-scute homolog 1 (MASH1), cells were treated with 1 mmol/L ATRA (for 7 days), 100 ng/mL rhFGF2 (for 48 hours), or 50 ng/mL rhBMP4 (for 48 hours) and total RNA was extracted as described previously.
We have reported the FGF signaling pathway is important for growth in SS cells [26]. Treatment with a FGF receptor (FGFR) induced growth arrest but failed to induce cell death, either necrotic or apoptotic [26]. For in vitro treatment with ATRA and an FGFR inhibitor, cells (1.0 × 104) were seeded on collagen-coated 60-mm dishes, and after their adhesion to the dishes, DMEM with 10% FBS was exchanged for DMEM with 10% FBS containing ATRA (1 or 10 μmol/L) and/or a FGFR-specific inhibitor, PD166866 (100 or 500 nmol/L) provided by Pfizer Global Research and Development (Groton, CT). In the control, DMEM with 10% FBS was changed every 2 to 3 days. The counting of cells was performed once every 2 days until Day 17.
To investigate whether these growth-inhibitory effects occur in vivo, treatment with ATRA of human SS xenograft in nude mice was performed using SYO-1 cells. For in vivo treatment with ATRA, the procedures were performed as previously described [38]. Briefly, SYO-1 (5 × 106) cells suspended in 100 mL of PBS were subcutaneously injected into the hind flank of male BALB/c nu/nu athymic mice at 5 weeks of age (Japan SLC, Hamamatsu, Japan). Mice were randomly assigned to ATRA-treated, vehicle-treated (sesame oil), and untreated groups (two mice per group). Starting on Day 2, the day after the inoculation of SYO-1 cells (Day 1), ATRA (20 mg/kg) was given to the mice by mouth through a gastric tube in 0.1 mL of purified sesame oil (Sigma) six times a week. Tumor size was measured with Vernier calipers, and the volume was calculated as π/6 x length x width x height on Days 3, 7, 10, 14, and 17.
The data are shown as mean ± standard deviation. The one-factor analysis of variance (ANOVA) test was used to determine differences in the effect of ATRA in vitro. The repeated-measure ANOVA test was used to determine the difference in the effect of ATRA in vivo and in the effect of a combination of ATRA and PD166866 in vitro. All statistical analyses were performed using Statcel software (OMS Publishing Inc, Tokyo, Japan).
Results
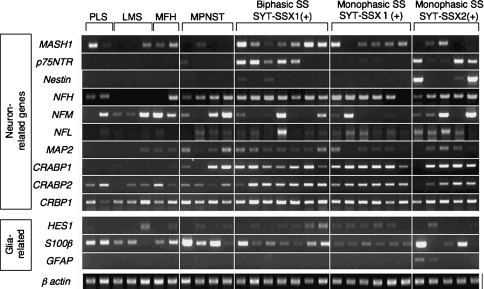
Among genes related to neurons, the nerve growth factor receptor (p75NTR) gene was expressed in five of seven biphasic and three of 11 monophasic SS tissues, whereas none of the other tumors expressed the p75NTR gene (Fig. 1). Expression of the cellular retinoic acid binding protein 1 (CRABP1) gene was also specific to SSs and the MPNST as we reported previously. Although the frequency was low (five of 18), the expression of the nestin gene was also specific to SS. SSs expressed the neurogenic transcription factor MASH1 (14 of 18 cases), NFH (17 of 18), CRABP2 (17 of 18), cellular retinol binding protein 1 (CRBP1) (17 of 18), and microtubule-associated protein 2 (MAP2) (14 of 18) genes, which were, however, also observed in other tumors. As for glia-related genes, HES1 (13 of 18 cases) and S100β (16 of 18) were expressed in SSs but also in several other types of sarcomas, and no SS or other tumor expressed glial fibrillary acidic protein (GFAP), which is a marker for terminally differentiated glial cells. There was no apparent relationship between the expression of any particular gene and histologic (monophasic or biphasic) or genetic (SYT-SSX1 or SYT-SSX2) features of SS.
Fig. 1.
The mRNA expression of neuron- and glia-related genes was analyzed by reverse transcription-PCR in tissue samples of 29 sarcomas, including 18 synovial sarcomas (SSs). Among the SS tumors, seven were SYT-SSX1-positive biphasic, six were SYT-SSX1-positive monophasic, and five were SYT-SSX2-positive monophasic. PLS = pleomorphic liposarcoma; LMS = leiomyosarcoma; MFH = malignant fibrous histiocytoma; MPNST = malignant peripheral nerve sheath tumor.
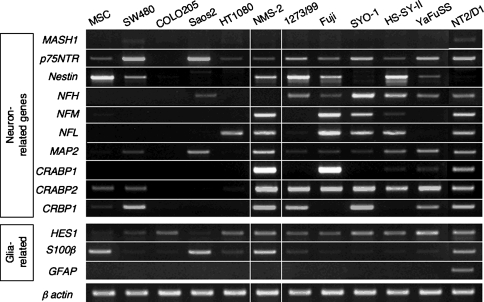
Similar to the primary tumors, the SS cell lines expressed a number of neuron-related genes such as CRABP1, which were not detected in other cell lines (Fig. 2). There was, however, a difference between the expression profiles of the primary tumors and cell lines. The expression of the MASH1 was lost in all cell lines, which was detected in the primary tumors (14 of 18 cases). In contrast, expression of the nestin gene, which was detected strongly in only two of 18 tumor tissues, was relatively strong in four of five cell lines. The expression of neural filaments and retinoic acid-related genes was almost identical, and the expression of glia-related genes was also similar. Therefore, SS cell lines partially retained the original expression profile.
Fig. 2.
The mRNA expression of neuron- and glia-related genes was analyzed by reverse transcription-PCR in 12 tumor cell lines, including five synovial sarcoma (SS) cell lines (YaFuSS, HS-SY-II, SYO-1, Fuji, 1273/99). The SS cell lines expressed a number of neuron-related genes such as CRABP1, which was not detected in other cell lines. The SS cell lines partially retained the original expression profile of the primary SS tumors shown in Figure 1.
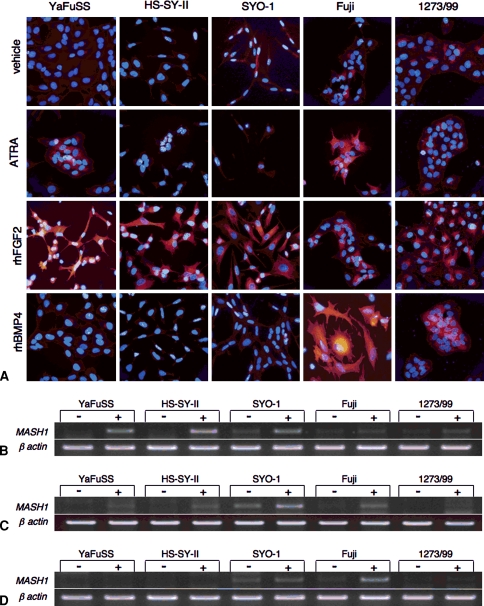
On treatment with ATRA, the staining of Tuj-1 was observed in YaFuSS and Fuji cells (Fig. 3A) and the expression of the MASH1 gene was induced in YaFuSS, HS-SY-II, and SYO-1 (Fig. 3B). On treatment with FGF2, all the cell lines except Fuji showed dendrite-like processes with strong Tuj-1 staining, and the expression of MASH1 was slightly upregulated in all cell lines (Fig. 3C). These features were particularly prominent in YaFuSS, the growth of which was severely inhibited by the treatment with FGF2 as we previously reported. The treatment with BMP2 strongly induced the staining of Tuj-1 with morphologic changes and mRNA expression in Fuji (Figs. 3A, 3D). These results indicate, although there was a considerable difference among cell lines, all the SS cell lines possessed the ability to respond to the neural induction.
Fig. 3A–D.
The neuronal differentiation of synovial sarcoma (SS) cells is shown. (A) Immunostaining for Tuj-1 (red) after treatment with vehicle, ATRA, rhFGF2, or rhBMP4 is shown. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Original magnification was x100 in all figures. The mRNA expression of the MASH1 gene in SS cells is shown after treatment with (B) ATRA, (C) rhFGF2, or (D) rhBMP4.
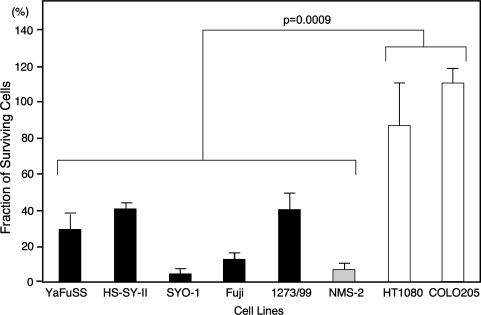
During the neural induction experiments, we noticed the growth of SS cells was inhibited by treatment with ATRA. Treatment with ATRA (10 μmol/L) failed to inhibit the growth of fibrosarcoma (HT1080) or colon carcinoma (COLO208) cells but suppressed (p = 0.0009) the growth of all five SS cell lines as well as the MPNST (NMS-2) (Fig. 4). Growth-inhibitory effects were dose-dependent and some SS cell lines such as SYO-1 and Fuji showed growth reduction at 1 mmol/L with morphologic change (data not shown).
Fig. 4.
A graph illustrates the growth-inhibitory effect of ATRA in vitro. Cells (1.0 × 104) were seeded on 60-mm dishes and cultured in standard medium containing 10% FBS with or without ATRA (10 mmol/L). The cells were counted on Day 17, and the fraction of surviving cells (number of treated cells divided by number of untreated cells) was calculated. Treatment with ATRA failed to inhibit the growth of fibrosarcoma (HT1080) or colon carcinoma (COLO208) cells but suppressed the growth of all five synovial sarcoma cell lines as well as the MPNST (NMS-2). p = 0.0009.
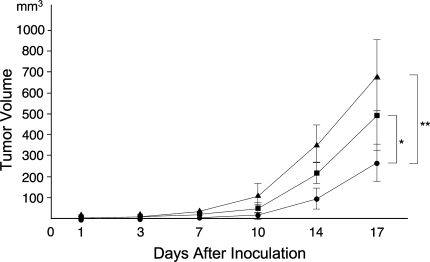
In vivo treatment with ATRA on SS xenograft (SYO-1) achieved a growth-inhibitor effect at Day 17 (p = 0.026 versus no treatment; p = 0.034 versus vehicle) (Fig. 5). The inhibitory effect, however, was not complete, and the tumor started to regrow with the same kinetics as pretreated cells (Fig. 5). Dried skin and body weight reduction were observed in ATRA-treated mice, indicating the limitation of dose. Therefore, the growth-inhibitory effect of ATRA in vivo was limited.
Fig. 5.
A graph illustrates the growth-inhibitory effect of ATRA in vivo. SYO-1 (5 × 106) cells suspended in 100 mL phosphate-buffered saline were subcutaneously injected into the hind flank of male BALB/c nu/nu athymic mice. Mice were randomly assigned to ATRA-treated (●), vehicle-treated (■), and untreated (▴) groups (five mice per group). ATRA treatment (20 mg/kg) was started on Day 2, and tumor volume was measured on Days 3, 7, 10, 14, and 17. In vivo treatment with ATRA achieved growth-inhibitor effect at Day 17 (*p = 0.026 versus vehicle; **p = 0.034 versus no treatment). The inhibitory effect, however, was not complete, and the tumor started to regrow with the same kinetics as pretreated cells.
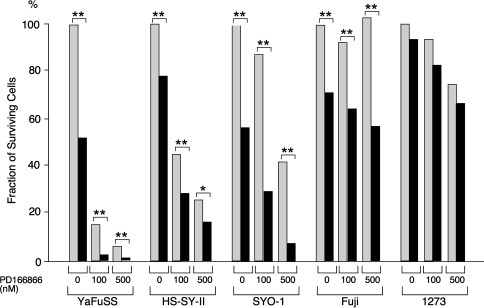
The differentiation induction experiments suggested the differentiation-inducting effect of ATRA was more prominent in growth-arrested cells than proliferating cells (data not shown). Therefore, we analyzed whether the combination of ATRA and FGFR inhibitor facilitates the growth-inhibitory effect of each. In the case of YaFuSS, the simultaneous administration of ATRA (1 mmol/L) and PD166866 (100 nmol/L) had much greater inhibitory effects than the single administration of each drug (Fig. 6). Similar additive effects were observed in HS-SY-II, SYO-1, and Fuji cells, suggesting the coadministration of PD166866 may enable the dose of ATRA to be reduced.
Fig. 6.
The graph illustrates the growth-inhibitory effect of ATRA combined with an FGFR inhibitor in vivo. Cells (1.0 × 104) were seed on 60-mm dishes and cultured in standard medium containing 10% FBS with the indicated concentrations of PD166866 and ATRA (gray, 0 mmol/L; black, 1 mmol/L). The cells were counted on Day 17, and the fraction of surviving cells (number of treated cells divided by number of untreated cells) was calculated. In the case of YaFuSS, the simultaneous administration of ATRA (1 mmol/L) and PD166866 (100 nmol/L) had much greater-inhibitory effects than the single administration of each drug. Similar additive effects were observed in HS-SY-II, SYO-1, and Fuji cells. *p < 0.05; **p < 0.01.
Discussion
Synovial sarcoma is a rare sarcoma of unknown histologic origin. Despite its name, evidence suggests SS arises from tissues other than normal synovial lining. Based on the gene expression profile data in our previous study [36], we hypothesized SS stemmed from cells in the neural lineage, and the neural induction may be applied as a new therapeutic modality for SS.
Our study has a number of limitations. First, the mRNA expression analyses in this study were qualitative rather than quantitative, and tumors other than SS also expressed several neural tissue-related genes (Figs. 1, 2). The expression of particular genes such as p75NTR and CRABP1, however, was observed only in SS and MPNST, suggesting the common characteristics of these two tumors. There was a considerable heterogeneity with respect to the expression profile of neural tissue genes among SS, irrespective of morphologic or genetic classification (Figs. 1, 2). Quantitative analyses using a large number of tumor tissues are required to draw definite conclusions. Second, we used Tuj-1 as an indicator of neural differentiation, which was widely used as a neural marker, although it is not specific in cells in neural lineage. Additional experiments using other markers are now undertaken. Finally, the in vivo growth inhibition assay in this study was a preliminary one using only one cell, because SYO-1 was the only line producing tumors in nude mice constantly. Although no severe side effect was observed in this short-term assay, more intensive analyses using other cell lines will be required to confirm the efficacy of ATRA treatment.
Consistent with the results of our previous study [36], here we demonstrated further evidence of the neural origin of SS, particularly neurons, but not glia, and the differentiation induction by ATRA inhibited the growth of SS in vitro and in vivo. Growing evidence suggests the cellular origins of malignant tumors are tissue stem cells, making the neural crest stem cells a possible candidate for the precursor of SS [1, 11, 23, 30]. Neural crest stem cells are regarded as pluripotent cells capable of differentiating into multiple cell lineages, including chondrocytes, osteocytes, neuron, glia, and melanocytes [13, 32]. We have tried to induce the differentiation of SS cells into these lineages and found some SS cells could differentiate in more than one direction but not multiple directions (data not shown). It has been proposed that in between stem cells and terminally differentiated cells, some cells have the ability to differentiate into two or more lineages, some of which might be the precursor of SS cells [32]. Experiments using knock-in mice with a myf5-driven SYT-SSX suggested a myogenic origin of SS [22], but there are neural cells expressing the myf5 gene in muscle tissues [16]. Knock-in mice carrying the SYT-SSX gene driven by the promoter of an appropriate neural crest marker may clarify this point.
Redifferentiation therapy has been widely investigated as an alternative to standard chemotherapy. Retinoic acid has fundamental effects on cellular differentiation, which may accompany the growth arrest of each cell [45]. Retinoic acid and its derivatives are among the most intensively analyzed redifferentiating agents and are used for the treatment of hematologic and solid malignant tumors [38, 39, 50]. Most of the successes with ATRA therapy has been obtained in patients with acute promyelocytic leukemia characterized by the reciprocal chromosomal translocation t(15;17) generating the fusion oncoprotein PML-RARα [38]. More than 90% of patients with acute promyelocytic leukemia undergo complete disease remission when treated with ATRA plus chemotherapy [19]. The prominent effect of ATRA on this type of tumor, however, depended on the ability of ATRA to directly inhibit the function of the PML-RXRα oncoprotein and was not necessarily related to its redifferentiation property [19]. Experimental studies report growth-inhibitory effects on osteosarcoma [47], rhabdomyosarcoma [40], and AIDS-associated Kaposi sarcoma [15]. Approximately 30% of patients with AIDS-associated Kaposi sarcoma have a substantial clinical response to ATRA treatment [8], and one case report suggested ATRA treatment combined with interferon-α was effective against inoperable metastatic osteosarcoma [48]. Our data demonstrate SS cell lines were far more sensitive to treatment with ATRA than the other types of tumors analyzed, and NMS-2, a cell line derived from MPNST, which in clinical practice is resistant to all chemotherapeutic treatments, also showed marked sensitivity to treatment with ATRA. The growth-inhibitory effect of ATRA in vivo, however, was only partial because a high dose could not be administered as a result of toxic side effects (data not shown). Combined with the inhibition of the FGF signal, which is one of the essential growth signals in SS, ATRA seemed as effective at lower concentrations as at high concentrations, suggesting the possibility of a new therapeutic target for SS.
Acknowledgments
We thank Drs Koichi Nishijo, Kotaro R. Shibata, Yasuko Shima, Yoshiki Kohno, Kenichi Fukiage, Seiji Otsuka, Moritoshi Furu, Kinya Ito, Yonghui Jin, and Yoichiro Kajita for their contribution to this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained. Each author also certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. [DOI] [PMC free article] [PubMed]
- 2.Allander SV, Illei PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, Meltzer PS. Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol. 2002;161:1587–1595. [DOI] [PMC free article] [PubMed]
- 3.Andrews PW, Damjanov I, Simon D, Banting GS, Carlin C, Dracopoli NC, Fogh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2: differentiation in vivo and in vitro. Lab Invest. 1984;50:147–162. [PubMed]
- 4.Argani P, Faria PA, Epstein JI, Reuter VE, Perlman EJ, Beckwith JB, Ladanyi M. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24:1087–1096. [DOI] [PubMed]
- 5.Arnold HH, Braun T. The role of Myf-5 in somitogenesis and the development of skeletal muscles in vertebrates. J Cell Sci. 1993;104:957–960. [DOI] [PubMed]
- 6.Aubry MC, Bridge JA, Wickert R, Tazelaar HD. Primary monophasic synovial sarcoma of the pleura: five cases confirmed by the presence of SYT-SSX fusion transcript. Am J Surg Pathol. 2001;25:776–781. [DOI] [PubMed]
- 7.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. [DOI] [PubMed]
- 8.Bernstein ZP, Chanan-Khan A, Miller KC, Northfelt DW, Lopez-Berestein G, Gill PS. A multicenter phase II study of the intravenous administration of liposomal tretinoin in patients with acquired immunodeficiency syndrome-associated Kaposi’s sarcoma. Cancer. 2002;95:2555–2561. [DOI] [PubMed]
- 9.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. [DOI] [PubMed]
- 10.Billings SD, Meisner LF, Cummings OW, Tejada E. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol. 2000;13:68–76. [DOI] [PubMed]
- 11.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. [DOI] [PubMed]
- 12.Brill G, Vaisman N, Neufeld G, Kalcheim C. BHK-21-derived cell lines that produce basic fibroblast growth factor, but not parental BHK-21 cells, initiate neuronal differentiation of neural crest progenitors. Development. 1992;115:1059–1069. [DOI] [PubMed]
- 13.Bronner-Fraser M. Origins and developmental potential of the neural crest. Exp Cell Res. 1995;218:405–417. [DOI] [PubMed]
- 14.Chu PG, Benhattar J, Weiss LM, Meagher-Villemure K. Intraneural synovial sarcoma: two cases. Mod Pathol. 2004;17:258–263. [DOI] [PubMed]
- 15.Corbell J, Rapaport E, Richman DD, Looney DJ. Antiproliferative effect of retinoid compounds on Kaposi’s sarcoma cells. J Clin Invest. 1994;93:1981–1986. [DOI] [PMC free article] [PubMed]
- 16.Cossu G, Biressi S. Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin Cell Dev Biol. 2005;16:623–631. [DOI] [PubMed]
- 17.Eyre DR, Apon S, Wu JJ, Ericsson LH, Walsh KA. Collagen type IX: evidence for covalent linkages to type II collagen in cartilage. FEBS Lett. 1987;220:337–341. [DOI] [PubMed]
- 18.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. [DOI] [PubMed]
- 19.Fenaux P, Chomienne C, Degos L. All-trans retinoic acid and chemotherapy in the treatment of acute promyelocytic leukemia. Semin Hematol. 2001;38:13–25. [DOI] [PubMed]
- 20.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;2:401–421. [DOI] [PubMed]
- 21.Ghadially FN. Is synovial sarcoma a carcinosarcoma of connective tissue? Ultrastruct Pathol. 1987;11:147–151. [DOI] [PubMed]
- 22.Halder M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR. A conditional mouse model of synovial sarcoma Insights into a myogenic origin. Cancer Cell. 2007;11:375–388. [DOI] [PubMed]
- 23.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. [DOI] [PMC free article] [PubMed]
- 24.Hiraga H, Nojima T, Isu K, Yamashiro K, Yamawaki S, Nagashima K. Histological and molecular evidence of synovial sarcoma of bone: a case report. J Bone Joint Surg Am. 1999;81:558–563. [DOI] [PubMed]
- 25.Imaizumi S, Motoyama T, Ogose A, Hotta T, Takahashi HE. Characterization and chemosensitivity of two human malignant peripheral nerve sheath tumour cell lines derived from a patient with neurofibromatosis type 1. Virchows Arch. 1998;433:435–441. [DOI] [PubMed]
- 26.Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR, Shima Y, Nagayama S, Katagiri T, Nakamura Y, Nakamura T, Toguchida J. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–27012. [DOI] [PubMed]
- 27.Johnson JE, Zimmerman K, Saito T, Anderson DJ. Induction and repression of mammalian achaete-scute homologue (MASH) gene expression during neuronal differentiation of P19 embryonal carcinoma cells. Development. 1992;114:75–87. [DOI] [PubMed]
- 28.Julien JP. Neurofilament functions in health and disease. Curr Opin Neurobiol. 1999;9:554–560. [DOI] [PubMed]
- 29.Kawai A, Naito N, Yoshida A, Morimoto Y, Ouchida M, Shimizu K, Beppu Y. Establishment and characterization of a biphasic synovial sarcoma cell line, SYO-1. Cancer Lett. 2004;204:105–113. [DOI] [PubMed]
- 30.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. [DOI] [PubMed]
- 31.Ladanyi M. Fusion of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:575–562. [DOI] [PubMed]
- 32.Le Douarin NM. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech Dev. 2004;121:1089–1102. [DOI] [PubMed]
- 33.Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol. 1997;7:440–450. [DOI] [PubMed]
- 34.May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, Zucman J, Thomas G, Denny CT. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA. 1993;90:5752–5756. [DOI] [PMC free article] [PubMed]
- 35.Miettinen M, Virtanen I. Synovial sarcoma—a misnomer. Am J Pathol. 1984;117:18–25. [PMC free article] [PubMed]
- 36.Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N, Kusuzaki K, Nakayama T, Tsuboyama T, Nakamura T, Imamura M, Nakamura Y, Toguchida J. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002;62:5859–5866. [PubMed]
- 37.Nojima T, Wang YS, Abe S, Matsuno T, Yamawaki S, Nagashima K. Morphological and cytogenetic studies of a human synovial sarcoma xenotransplanted into nude mice. Acta Pathol Jpn. 1990;40:486–493. [DOI] [PubMed]
- 38.Ohno R, Asou N, Ohnishi K. Treatment of acute promyelocytic leukemia: strategy toward further increase of cure rate. Leukemia. 2003;17:1454–1463. [DOI] [PubMed]
- 39.Petterson F, Colston KW, Dalgleish AG. Retinoic acid enhances the cytotoxic effects of gemcitabine and cisplatin in pancreatic adenocarcinoma cells. Pancreas. 2001;23:273–279. [DOI] [PubMed]
- 40.Ricaud S, Vernus B, Bonnieu A. Response of human rhabdomyosarcoma cell lines to retinoic acid: relationship with induction of differentiation and retinoic acid sensitivity. Exp Cell Res. 2005;311:192–204. [DOI] [PubMed]
- 41.Ross RA, Spengler BA, Domenech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell Growth Differ. 1995;6:449–456. [PubMed]
- 42.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. [DOI] [PubMed]
- 43.Smith ME, Fisher C, Wilkinson LS, Edwards JC. Synovial sarcoma lack synovial differentiation. Histopathology. 1995;26:279–281. [DOI] [PubMed]
- 44.Sonobe H, Manabe Y, Furihata M, Iwata J, Oka T, Ohtsuki Y, Mizobuchi H, Yamamoto H, Kumano O, Abe S. Establishment and characterization of a new human synovial sarcoma cell line, HS-SY-II. Lab Invest. 1992;67:498–505. [PubMed]
- 45.Sporn MB, Roberts AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine. 2nd Ed. New York: Raven Press; 1994.
- 46.Staege MS, Hutter C, Neumann I, Foja S, Hattenhorst UE, Hansen G, Afar D, Burdach SE. DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res. 2004;64:8213–8221. [DOI] [PubMed]
- 47.Thein R, Lotan R. Sensitivity of cultured human osteosarcoma and chondrosarcoma cells to retinoic acid. Cancer Res. 1982;42:4771–4775. [PubMed]
- 48.Todesco A, Carli M, Iacona I, Frascella E, Ninfo V, Rosolen A. All-trans retinoic acid and interferon-alpha treatment of a patient with resistant metastatic osteosarcoma. Cancer. 2000;89:2661–2666. [DOI] [PubMed]
- 49.Weiss SW, Goldblum JR. Enzinger, Weiss’s Soft Tissue Tumors. 4th Ed. St Louis, MO: CV Mosby; 2001.
- 50.Yang Q, Sakurai T, Kakudo K. Retinoid, retinoic acid receptor and breast cancer. Breast Cancer Res Treat. 2002;76:167–173. [DOI] [PubMed]