Abstract
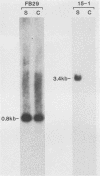
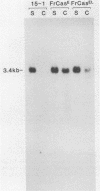
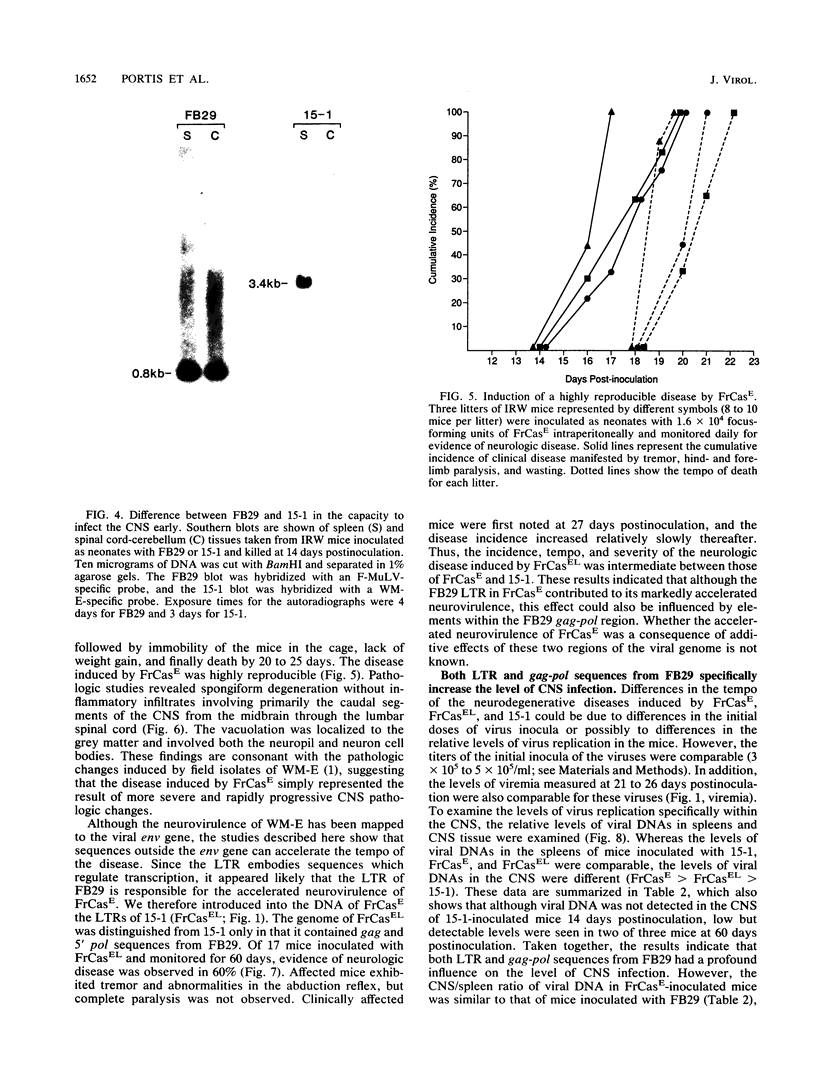
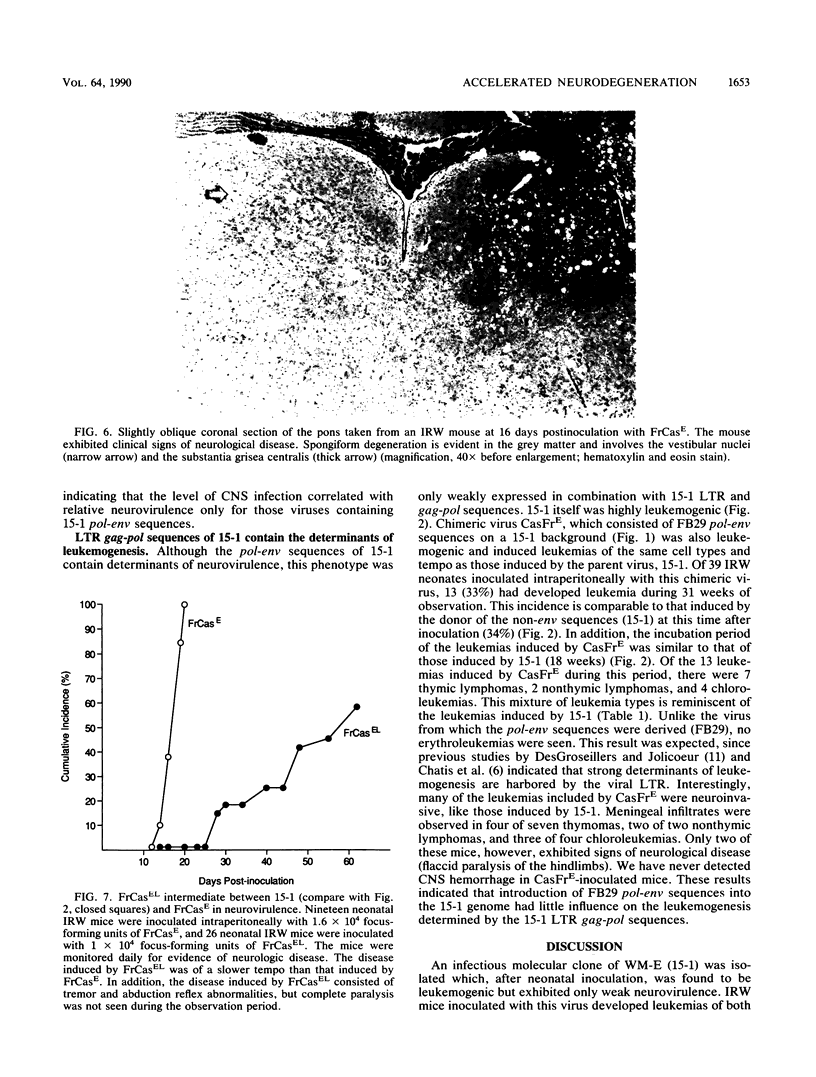
The wild mouse ecotropic retrovirus (WM-E) induces a spongiform neurodegenerative disease in mice after a variable incubation period of 2 months to as long as 1 year. We isolated a molecular clone of WM-E (15-1) which was weakly neurovirulent (incidence, 8%) but was highly leukemogenic (incidence, 45%). Both lymphoid and granulocytic leukemias were observed, and these leukemias were often neuroinvasive. A chimeric virus was constructed containing the env and 3' pol sequences of 15-1 and long terminal repeat (LTR), gag, and 5' pol sequences from a clone of Friend murine leukemia virus (FB29). FB29 has been shown previously to replicate to high levels in the central nervous system (CNS) but is not itself neurovirulent. This finding was confirmed at the DNA level in the current study. Surprisingly, intraperitoneal inoculation of neonatal IRW mice with the chimeric virus (FrCasE) caused an accelerated neurodegenerative disease with an incubation period of only 16 days and was uniformly fatal by 23 days postinoculation. Introduction of the LTR of 15-1 into the FrCasE genome yielded a virus (FrCasEL) with a degree of neurovirulence intermediate between those of 15-1 and FrCasE. No differences were found in the levels of viremia or the relative levels of viral DNA in the spleens of mice inoculated with 15-1, FrCasE, or FrCasEL. However, the levels of viral DNA in the CNS correlated with the relative degrees of neurovirulence of the respective viruses (FrCasE greater than FrCasEL greater than 15-1). Thus, the env and 3' pol sequences of WM-E (15-1) were required for neurovirulence, but elements within the LTR and gag-pol regions of FB29 had a profound influence on the level of CNS infection and the rate of development of neurodegeneration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
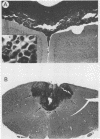
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Brodsky I., Kahn S. B., Ross E. M., Petkov G., Braverman S. D. Prelymphoid leukemia phase of Rauscher virus infection. J Natl Cancer Inst. 1967 May;38(5):779–787. [PubMed] [Google Scholar]
- Brooks B. R., Swarz J. R., Narayan O., Johnson R. T. Murine neurotropic retrovirus spongiform polioencephalomyelopathy: acceleration of disease by virus inoculum concentration. Infect Immun. 1979 Feb;23(2):540–544. doi: 10.1128/iai.23.2.540-544.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. L., Klement V. Clonal heterogeneity of wild mouse leukemia viruses: host ranges and antigenicity. Virology. 1976 Sep;73(2):532–536. doi: 10.1016/0042-6822(76)90415-3. [DOI] [PubMed] [Google Scholar]
- Carlberg K., Ryden T. A., Beemon K. Localization and footprinting of an enhancer within the avian sarcoma virus gag gene. J Virol. 1988 May;62(5):1617–1624. doi: 10.1128/jvi.62.5.1617-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Dennis L. H., Brodsky I. Thrombocytopenia induced by the Friend leukemia virus. J Natl Cancer Inst. 1965 Dec;35(6):993–999. [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform encephalopathy: the 3'-end long terminal repeat-containing viral sequences influence the incidence of the disease and the specificity of the neurological syndrome. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8818–8822. doi: 10.1073/pnas.82.24.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Morrey J. D. Tissue-specific replication of Friend and Moloney murine leukemia viruses in infected mice. J Virol. 1987 May;61(5):1350–1357. doi: 10.1128/jvi.61.5.1350-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson T. N., Langdon W. Y., Hoffman P. M., Hartley J. W., Morse H. C., 3rd Histologic and cell surface antigen studies of hematopoietic tumors induced by Cas-Br-M murine leukemia virus. J Natl Cancer Inst. 1984 Feb;72(2):447–454. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Rongey R. W., Estes J. D., Huebner R. J. Spontaneous tumors of aging wild house mice. Incidence, pathology, and C-type virus expression. J Natl Cancer Inst. 1973 Mar;50(3):719–734. doi: 10.1093/jnci/50.3.719. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Petersen L. L. An improved method for the isolation of supercoiled DNA molecules using ion-exchange column chromatography. Gene Anal Tech. 1987 Jan-Feb;4(1):5–8. doi: 10.1016/0735-0651(87)90006-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Morse H. C., 3rd Host genetic determinants of neurological disease induced by Cas-Br-M murine leukemia virus. J Virol. 1985 Jan;53(1):40–43. doi: 10.1128/jvi.53.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Robbins D. S., Morse H. C., 3rd Role of immunity in age-related resistance to paralysis after murine leukemia virus infection. J Virol. 1984 Dec;52(3):734–738. doi: 10.1128/jvi.52.3.734-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., DesGroseillers L. Neurotropic Cas-BR-E murine leukemia virus harbors several determinants of leukemogenicity mapping in different regions of the genome. J Virol. 1985 Nov;56(2):639–643. doi: 10.1128/jvi.56.2.639-643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAtee F. J., Portis J. L. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J Virol. 1985 Dec;56(3):1018–1022. doi: 10.1128/jvi.56.3.1018-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Robert-Lezenes J., Mathieu-Mahul D., Gisselbrecht S., Larsen C. J. Isolation and characterization of a gp 70+ non producer Friend tumor cell clone. Biochimie. 1983 Apr-May;65(4-5):259–266. doi: 10.1016/s0300-9084(83)80277-6. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Pitts O. M., Powers J. M., Bilello J. A., Hoffman P. M. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab Invest. 1987 Apr;56(4):401–409. [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Evans L. H. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol. 1985 Sep;55(3):806–812. doi: 10.1128/jvi.55.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L. Wild mouse retrovirus: pathogenesis. Curr Top Microbiol Immunol. 1990;160:11–27. doi: 10.1007/978-3-642-75267-4_2. [DOI] [PubMed] [Google Scholar]
- Rassart E., Nelbach L., Jolicoeur P. Cas-Br-E murine leukemia virus: sequencing of the paralytogenic region of its genome and derivation of specific probes to study its origin and the structure of its recombinant genomes in leukemic tissues. J Virol. 1986 Dec;60(3):910–919. doi: 10.1128/jvi.60.3.910-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Reinsch S. S., Shank P. R. Sequences near the 5' long terminal repeat of avian leukosis viruses determine the ability to induce osteopetrosis. J Virol. 1986 Jul;59(1):45–49. doi: 10.1128/jvi.59.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Jaenisch R., Ruprecht R. M. Retroviruses and mouse embryos: a rapid model for neurovirulence and transplacental antiviral therapy. Science. 1987 Jun 26;236(4809):1671–1674. doi: 10.1126/science.3037694. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F. K., Davison B., Chaffin K. Murine leukemia virus long terminal repeat sequences can enhance gene activity in a cell-type-specific manner. Mol Cell Biol. 1985 Oct;5(10):2832–2835. doi: 10.1128/mcb.5.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]