Abstract
Neoplastic cells growing under hypoxic conditions exhibit a more aggressive phenotype by activating a cascade of molecular events partly mediated by hypoxia-inducible transcription factor (HIF-1α) and vascular endothelial growth factor (VEGF). The roles of these markers have been studied previously in several cancer lines. We ascertained the frequency of HIF-1α expression, VEGF expression, the degree of neovascularization, and cell proliferation in osteosarcoma samples. Samples from osteosarcoma patients were assessed for HIF-1α and VEGF protein expression using immunohistochemistry, neovascularization using antibodies for Factor VIII, and cell proliferation using the Ki-67 labeling index. Associations between these parameters and clinical features were examined. HIF-1α staining was positive in 35% of patients and metastases were present in 61% of these HIF-1α-positive patients. VEGF protein expression was detected in 69% of patients, 92% of whom were female. We observed an insignificant trend for a higher frequency of VEGF expression in the high-grade as compared to low-grade osteosarcoma. We observed no association between vascular density and proliferation index and any clinical parameters. We found an association between HIF-1α expression and metastatic disease and between VEGF expression and female gender.
Introduction
Cancer cells respond similarly to normal cells under hypoxic conditions by activating signaling pathways that induce cell proliferation, angiogenesis, and apoptosis, among others [16]. Under hypoxic conditions, more of the pathways induced in neoplastic cells promote tumor growth than apoptosis. Furthermore, research suggests hypoxic conditions in particular may influence a more aggressive phenotype in neoplastic cells associated with poor patient prognosis [43]. The characteristics of this phenotype include radio- and chemoresistance, increased ability for tumors to metastasize, and the selection of cells resistant to p53-mediated apoptosis [11, 12, 20, 35, 46]. Despite hypervascularization in solid tumors, hypoxic conditions are thought to result in part from an imbalance between the rate of tumor cell proliferation and new endothelial cell formation, as well as disorganization of the new vascular supply [29, 44].
Hypoxia-inducible transcription factor (HIF-1) is an important element in the cellular response to hypoxia. HIF-1 is a heterodimer that consists of a HIF-1α subunit and a HIF-1β subunit. Under normoxic conditions, HIF-1α is degraded and ubiquinated, preventing it from forming a complex with the HIF-1β subunit. However, in response to physiologic hypoxia, both subunits combine to form the HIF-1 complex and bind to specific hypoxia-responsive elements within target genes that activate the transcription of various genes needed to respond to hypoxic conditions [3]. Studies measuring the level of HIF-1α subunit protein expression have helped determine the role of this subunit in cancer and neoplastic growth. HIF-1α subunit is overexpressed in colon, breast, gastric, lung, skin, ovarian, pancreatic, prostate, and renal carcinomas and associated with cell proliferation [50]. Overexpression of HIF-1α has been detected in preneoplastic tumors, including colonic adenoma, breast ductal carcinoma, and prostate intraepithelial neoplasia, but not in benign tumors of the breast and uterus. Further research on breast cancer has demonstrated a higher incidence of overexpression of HIF-1α in metastases versus primary tumor [50].
As previously mentioned, HIF-1 is associated with the activation of various hypoxia-response genes, which include vascular endothelial growth factor (VEGF). VEGF specifically binds to two transmembrane VEGF receptor tyrosine kinases on endothelial cells to initiate intracellular signal transduction pathways that mediate angiogenesis and vascular permeability [19]. Increased VEGF expression is associated with tumor growth and metastasis [8]. Increased VEGF gene expression has been reported in a number of cancer cell lines, as well as in primary tumor tissue from breast, lung, ovary, liver, and colon. Neuroblastoma and osteosarcoma cell lines and primary tumor samples also exhibited higher VEGF expression when compared with normal tissues [6, 18, 23]. The level of circulating VEGF in patients with different tumor types may be predictive of tumor status and prognosis [32].
Among a variety of human tumor types analyzed, tumor hypoxia [9, 13, 14] and proliferation rate [1, 40, 42] may be important determinants of clinical outcome. Cell proliferation, as assessed by Ki-67 labeling index, correlates with HIF-1α overexpression in some human tumors [50].
Limited studies of hypoxia markers in osteosarcoma have revealed important findings. For example, the survival rate of the osteosarcoma patients with tumor VEGF expression was substantially worse than that of the osteosarcoma patients without VEGF expression [18]. More recently, Wu et al. [47] demonstrated hypoxia is associated with increased HIF-1α protein expression and subsequent up-regulation of VEGF expression and angiogenesis in the osteosarcoma cell line SaOS-2. In osteosarcoma, VEGF and neovascularization have been studied previously [18, 24], but the relationship of these parameters with cell proliferation and HIF-1α expression has not been previously described.
We therefore determined the frequency of HIF-1α expression, VEGF expression, degree of vascularization, and cell proliferation in osteosarcoma patients and determined preliminarily if these measures predicted the clinical outcome.
Materials and Methods
We obtained samples from 48 osteosarcomas from patients who underwent surgery at Memorial Sloan-Kettering Cancer Center. Of the 48 patients, 22 were male and 26 female (Table 1). Patients ranged from 7 to 38 years of age (mean, 21 years). The osteosarcomas were subclassified as osteoblastic (n = 25), chondroblastic (n = 11), fibroblastic (n = 2), giant cell rich (n = 1), telangiectatic (n = 2), or mixed (n = 7). The specimens were obtained from the primary site in 40 patients and from the metastatic site in eight patients. The locations of the tumors were distal femur (n = 21), proximal tibia (n = 9), proximal humerus (n = 5), pelvis (n = 6), foot (n = 1), head (n = 3), and other locations (n = 3). At the time of diagnosis, distant metastases were present in 11 patients (23%) and absent in 37 patients (77%). All patients or their guardians provided written informed consent for participation in this biology study, which was approved by the Memorial Hospital Institutional Review Board.
Table 1.
Clinical characteristics of the osteosarcoma patients
| Characteristic | Number of patients |
|---|---|
| Gender | |
| Male | 22 (46%) |
| Female | 26 (54%) |
| Histologic subtype | |
| Osteoblastic | 25 (52%) |
| Chondroblastic | 22 (23%) |
| Fibroblastic | 2 (4%) |
| Giant cell | 1 (2%) |
| Telangiectatic | 2 (4%) |
| Mixed | 7 (14%) |
| Type of specimen | |
| Primary | 40 (83%) |
| Metastasis | 8 (17%) |
| Primary site | |
| Distal femur | 21 (44%) |
| Proximal tibia | 9 (19%) |
| Proximal humerus | 5 (10%) |
| Pelvis | 6 (12%) |
| Foot | 1 (2%) |
| Head | 3 (6%) |
| Other | 3 (6%) |
| Metastasis at diagnosis | |
| Present | 11 (23%) |
| Absent | 37 (77%) |
| Huvos grade | |
| I | 20 (42%) |
| II | 10 (21%) |
| III | 11 (23%) |
| IV | 7 (15%) |
Patients received therapy according to CCG 7921, Regimen A. Induction chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate with leucovorin rescue preceded definitive surgical resection. After recovery from definitive surgery, patients received maintenance chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate with leucovorin rescue. We assessed chemotherapy efficacy histologically by the Huvos grading system [36]. In this system, four grades have been described: Grade I, little or no evidence of necrosis; Grade II, tumor necrosis of 50% to 90%; Grade III, necrosis between 90% to 99%; and Grade IV, 100% of necrosis. The chemotherapy responses were classified as Grade I (n = 20, 42%), Grade II (n = 10, 21%), Grade III (n = 11, 23%), and Grade IV (n = 7, 15%). Tumor specimens were reviewed by a pathologist (AH) to ensure there was less than 30% contamination with normal stromal cells.
We studied protein expression of HIF-1α, VEGF, Factor VIII, and Ki-67 by immunohistochemistry of 6-μm sections using an avidin-biotin immunoperoxidase assay on OCT-embedded frozen blocks. Sections were incubated with primary antibody against HIF-1α (1:100 dilution; Novus Biologicals, Littleton, CO), VEGF (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), Factor VIII (1:100 dilution; NeoMarkers, Fremont, CA), and Ki-67 (MIB-1; dilution 1:100; Immunotech, Berkeley, CA). Subsequently, the sections were incubated with biotinylated secondary antibodies, followed by avidin-peroxidase, which formed a complex. Diaminobenzidine (0.06%) was used as the final chromogen, and hematoxylin was used as the nuclear counterstain. The intensity of immunostaining was scored as (–) negative, (+1) positive staining, and (+2) strong by a pathologist (AH) blinded to patient identity and clinical information. The vascularization was evaluated by one of the authors (HM) in three different random areas of tumor by vessel count on a ×200 field (×20 objective and ×10 ocular, 0.74 mm2 per field), and the average was recorded. The cell proliferative activity (labeling index) was calculated as the percentage of the Ki-67 proliferating cell antigen-positive cells to the total number of tumor cells in a microscopic field (×200). At least 500 tumor cells per sample were counted by one of the authors (HM). For each parameter, the immunostaining in patient samples was compared with normal bone tissue as a control.
The association of HIF-1α and VEGF protein expression, neovascularization, and cell proliferation with clinical data was investigated by the chi square test. Followup data was too limited to allow analysis of the relationship with survival. Differences between immunohistochemical staining results and clinical parameters were compared using the chi square test. An arbitrary p value of 0.05 or less was used to determine significance. In assessing the microvascular density of the specimens, receiver operating characteristic (ROC) curves were generated to divide the data into separate groups to allow for comparison to clinical data. In brief, ROC curves were generated using continuous data to maximize the sensitivity and specificity of a given cutoff value.
Results
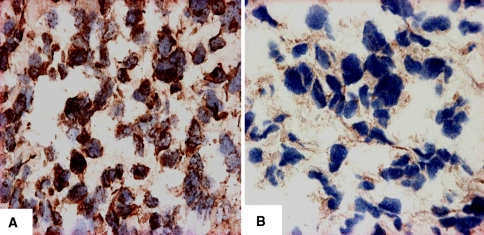
HIF-1α staining was positive in 18 of 48 patients (37.5%). Positivity was predominantly nuclear, except in three cases, in which the immunoreactivity was located in the cytoplasm. In nine of 18 cases, it was detected as strong staining, and in nine of 18, it was scored as weak. In 30 of 48 patients (62.5%), the protein expression could not be detected and was considered negative (Fig. 1A-B). We observed an association between HIF-1α and metastatic disease. Presence of metastases was related to HIF-1α protein expression. There were more (p = 0.019) metastases in HIF-1α-positive patients as compared to HIF-1α-negative patients (11 of 18 [61%] versus eight of 30 [27%], respectively) (Table 2). Similarly, 11 of 19 of metastases were HIF-1α-positive. HIF-1α expression was associated with a 4.3-fold greater risk for having metastatic disease as compared to HIF-1α-negative patients. HIF-1α protein expression was not associated with chemotherapy response according to the Huvos grading system (data not shown).
Fig. 1A–B.
Photomicrographs show HIF-1α immunostaining of osteosarcoma samples (original magnification, ×400). (A) In 18 of 48 patients, the immunostaining for HIF-1α was positive. The staining was predominantly observed within the nucleus. (B) In 30 of 48 patients, the immunostaining was considered negative.
Table 2.
Hypoxia-induced transcription factor (HIF-1α) expression and metastatic disease
| Metastatic disease | Number of patients | |
|---|---|---|
| HIF-1α (+) | HIF-1α (–) | |
| Yes | 11 | 8 |
| No | 7 | 22 |
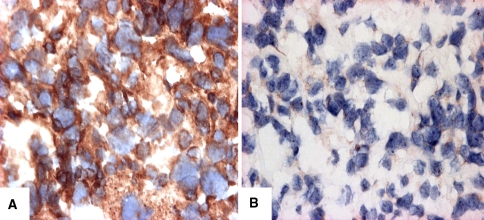
VEGF protein expression was detected in 35 of 48 patients (73%) and not detected in 13 of 48 patients (27%) (Fig. 2A–B). VEGF expression was associated with gender (chi square = 9.42, p = 0.002). Twelve of 23 male patients (52%) demonstrated VEGF expression, whereas 23 of 25 female patients (92%) were VEGF positive (Table 3). We noted an insignificant (p = 0.08) trend for a higher frequency of VEGF expression to be present in the high-grade as compared to low-grade osteosarcomas. Thirty-one of 35 VEGF-positive patients (88.5%) had high-grade osteosarcomas, whereas only four of these 35 patients (11.5%) had low-grade osteosarcomas (Table 4). We observed no other association between the clinical data and VEGF expression (data not shown).
Fig. 2A–B.
Photomicrographs show VEGF immunostaining of osteosarcoma samples (original magnification, ×400). (A) Thirty-five of 48 patients were considered positive for VEGF cytoplasmic staining. (B) Thirteen of 48 cases were considered negative.
Table 3.
Vascular endothelial growth factor (VEGF) expression and gender
| Gender | Number of patients | |
|---|---|---|
| VEGF (+) | VEGF (–) | |
| Male | 12 | 11 |
| Female | 23 | 2 |
Table 4.
Vascular endothelial growth factor (VEGF) expression and tumor grade
| Tumor grade | Number of patients | |
|---|---|---|
| VEGF (+) | VEGF (–) | |
| Low | 4 | 4 |
| High | 31 | 9 |
The number of vessels per field ranged between three and 55 (mean ± SD, 16 ± 13). Using ROC curves, we determined the value of 16 vessels per field as a cutoff for classifying the cases into high-vessel-density versus low-vessel-density specimens. This cutoff point was the point that maximally discriminated the samples by the primary clinical variables – Huvos grade and the presence or absence of metastases. Despite this statistical optimization, we found no association between the number of vessels and clinical parameters (data not shown).
The number of cells per field immunopositive for Ki-67 ranged between 9.5 and 53.5 (mean ± SD, 32 ± 12). In the case of cell proliferation, a value of 32 was used as the cutoff value for classifying the cases as it optimized discrimination of clinical parameters. We found no association between labeling index and clinical data.
Discussion
Neoplastic cells growing under hypoxic conditions exhibit a more aggressive phenotype by activating a cascade of molecular events partly mediated by hypoxia-inducible transcription factor (HIF-1α) and vascular endothelial growth factor (VEGF). We therefore hypothesized markers of hypoxia would correlate with the prognosis of osteosarcoma in part through defining chemotherapy response and in part through defining the metastatic phenotype. Thus, we determined the frequency of HIF-1α expression, VEGF expression, degree of vascularization, and cell proliferation in osteosarcoma patients as markers of hypoxia and determined preliminarily if these measures predicted the clinical outcome through correlation with chemotherapy response and metastases.
The major limitation of our study is that it is an immunohistochemical analysis of an existing patient cohort. Although this group represents all material available from a single large institution, in any analysis of this type, the results should be considered exploratory. Any associations identified may not necessarily be causal. One must be cautious in interpreting data. In particular, our primary clinical parameters being assessed were Huvos grade and metastases (outcome), and the relationship of VEGF positivity with gender was an unexpected finding. Although potentially justifiable scientifically as discussed later, confirmation in a subsequent study will be crucial. Finally, limited sample size severely curtails the power of the study. Negative results cannot be interpreted as a nonexistent association, and the ability to analyze subgroups was limited. Despite these limitations, interesting results were obtained. Validation of these results in a subsequent prospective patient cohort has the potential to identify predictive and prognostic factors supporting the utility of reporting these results.
Our results expand upon previous work regarding hypoxia markers and osteosarcoma, and demonstrate an association between HIF-1α expression and metastatic disease. In 61% of HIF-1α-positive patients, metastases were present, representing a 4.3-fold greater risk for having metastatic disease in patients with HIF-1α expression compared to that of HIF-1α-negative patients. In addition, increased VEGF protein expression in osteosarcoma samples from female patients versus male patients was seen. Finally, we noted an insignificant trend for higher frequency of VEGF expression to be present in the high-grade as compared to low-grade osteosarcoma. Further analysis of larger cohorts of low-grade osteosarcoma samples would be worthwhile in order to confirm or refute the trend.
Hypoxia induces the expression of a number of hypoxia-regulated genes. Induction of HIF-1α protein appears to be a critical step in the hypoxic response and occurs via increased mRNA expression, protein stabilization, nuclear translocation, and augmented activity of its transcriptional activation domains [37]. Zhong et al. [50] reported most normal human tissues showed no HIF-1α immunoreactivity (153 of 174 clinical specimens [88%]). Furthermore, they reported overexpression of HIF-1α protein in 69 of 131 primary malignant tumors (53%), representing 13 of 19 tumor types screened [50]. Recent studies using the human osteosarcoma cell line SaOS-2 demonstrated a relationship between hypoxia and increased HIF-1α protein expression and subsequent VEGF expression [47]. Our data suggest this increased HIF-1α expression is an important marker for metastases in osteosarcoma patients. Metastases were present in 61% of the HIF-1α-positive patients, and 73% of HIF-1α-negative patients showed no evidence of metastases. These results for osteosarcoma correlate with studies reporting a higher incidence of overexpression of HIF-1α in metastases [4]. We found no other association between HIF-1α protein expression and clinical data.
Wu et al. [47] reported evidence of increased VEGF expression in a human osteosarcoma cell line. We observed VEGF protein expression in 73% of patients. More specifically, an association between VEGF expression and female gender was demonstrated. The mechanism for this relationship is not entirely clear. A relationship between estrogen and bone tissue is well-known, mainly through clinical studies where 17β-estradiol (E2) deficiency in postmenopausal or ovariectomized women caused a rapid loss of trabecular bone. Furthermore, in both men and women, estrogen deficiency is associated with an age-related sustained bone loss that can lead to osteoporosis [25, 26, 33, 34]. Although the association between gender and VEGF expression has not been clearly determined, in classic hormone-dependent tissues such as the endometrium, VEGF gene transcription is increased under the influence of estrogen [27]. Koos et al. [21] observed estrogen induces rapid, transient binding of HIF-1 to the promoter, which subsequently mediates VEGF transcription in response to hypoxia. One study, however, reported no relationship between VEGF expression and gender [51], suggesting differential VEGF expression in various cancers.
Correlations between VEGF expression and increased vascularization have already been demonstrated in osteosarcoma. Using biopsies from 30 osteosarcoma patients, Lee et al. [24] detected VEGF mRNA transcripts in all primary osteosarcoma samples and nine xenograft specimens examined and showed the number of vessels in VEGF-positive tumors was greater than in VEGF-negative tumors. Kaya et al. [18] studied 27 primary osteosarcoma samples and found VEGF was positive in 63% of samples. Microvascular density was higher in VEGF-positive tumors than in VEGF-negative tumors [18]. Other investigators have suggested VEGF expression does not always relate to increased tumor neoangiogenesis. In non-small-cell lung cancer, about ½ of the cases expressing VEGF had poor vascular density, which suggested VEGF-induced angiogenesis may be dependent on other regulators [10]. We observed no relationship between VEGF expression and the microvascular density in our patient samples.
Malignant progression is another hallmark of the poor outcome of solid tumors. In giant cell tumor of bone, overexpression of VEGF is associated with the biologic behavior of the tumor as determined by clinical parameters. Stage III giant cell tumors display relatively higher levels of VEGF gene expression than Stage I/II tumors [49].
The final marker we investigated on osteosarcomas was the related cell proliferation utilizing a Ki-67 index. Ki-67 is a nuclear antigen that is only expressed in proliferating cells and is often used as an estimate of tumor growth. Studies on breast cancer have demonstrated high concentrations of HIF-1α were associated with increased proliferation as shown by positive correlations with Ki-67 [2]. Analysis of 25 head and neck osteosarcomas demonstrated 88% were positive for Ki-67 [17]. In a different study, the positive rate of Ki-67 in recurrent osteosarcoma was 81.8% but only 35.5% in the primary tumor [30]. In addition, Ki-67 positivity is believed one of the risk factors for recurrence in meningiomas. However we observed no association between the Ki-67 labeling index and clinical data. The high proliferative potential of tumor cells as a prognostic factor has given variable results. Some studies described a more favorable outcome for tumors with higher labeling indices [22, 28]. However, proliferation indices were not correlated with overall outcome for patients with rectal or cervical cancers [5, 41, 45].
We examined hypoxia markers in osteosarcoma. HIF-1α is overexpressed in a substantial proportion of patients with metastases, confirming previous work showing this relationship in several other cancers. Previous studies have suggested HIF-1 mediates resistance to chemotherapy and radiation [15]. Targeting the HIF-1 pathway, therefore, has become an important area for cancer therapy research. Tan et al. [39] demonstrated inhibition of HIF-1α protein synthesis in several cancer lines including human breast cancer, prostate cancer, and glioblastoma prevented the activation of HIF-1 target genes, such as VEGF and glucose transporter-1. Using small hairpin RNAs to target the expression of the HIF-1α gene in human osteosarcoma cell line SaOS-2, Wu et al. [48] blocked the hypoxia transduction pathway efficiently and inhibited the growth of osteosarcoma cells; expression of the HIF-1α gene was inhibited by 90%. This direction of research shows promise for the development of even more efficient cancer therapies for osteosarcoma. We also observed an overexpression of VEGF protein in female patients, which may support previous work postulating VEGF gene transcription is increased under the influence of estrogen. Studies investigating breast cancer are currently considering cyclooxygenase-2 (COX-2) inhibitors as a possible mechanism for cancer therapy. Substantial evidence exists linking COX-2 to the development of cancer [38]. Furthermore, COX-2 expression in ductal carcinoma in situ has been correlated with VEGF and HER-2/neu [31]. Although COX-2 has no relationship to estrogen receptors [7], further investigation should reveal the role estrogen plays in the development of cancer. Finally, we also observed a trend describing a relationship between VEGF expression and tumor grade, an area that needs further examination to prove its importance in osteosarcoma and patient prognosis. The data provides novel findings regarding hypoxic markers in human osteosarcoma and introduces new areas for further investigation in osteosarcoma research.
Acknowledgments
We thank the late Andrew Huvos, MD, Department of Pathology, and Alexander Chou, Department of Pediatrics, Memorial Sloan-Kettering Cancer Center, for their contributions to this study.
Footnotes
One or more of the authors (RG) have received funding from the National Cancer Institute (Grant Number R01 CA 83132) and the Yurman Limb Preservation Fund.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bolger BS, Symonds RP, Stanton PD, MacLean AB, Burnett R, Kelly P, Cooke TG. Prediction of radiotherapy response of cervical carcinoma through measurement of proliferation rate. Br J Cancer. 1996;74:1223–1226. [DOI] [PMC free article] [PubMed]
- 2.Bos R, van Diest PJ, van der Groep P, Shvarts A, Greijer AE, van der Wall E. Expression of hypoxia-inducible factor-1alpha and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res. 2004;6:R450–R459. [DOI] [PMC free article] [PubMed]
- 3.Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E, Workman P, Ashcroft M. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1α induction in response to hypoxic stress and growth factors. Cancer Res. 2005;65:4918–4928. [DOI] [PubMed]
- 4.Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35:207–213. [DOI] [PubMed]
- 5.Cole DJ, Brown DC, Crossley E, Alcock CJ, Gatter KC. Carcinoma of the cervix uteri: an assessment of the relationship of tumour proliferation to prognosis. Br J Cancer. 1992;65:783–785. [DOI] [PMC free article] [PubMed]
- 6.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed]
- 7.Ferrandina G, Ranelletti FO, Gallotta V, Martinelli E, Zannoni GF, Gessi M, Scambia G. Expression of cyclooxygenase-2 (COX-2), receptors for estrogen (ER), and progesterone (PR), p53, ki67, and neu protein in endometrial cancer. Gynecol Oncol. 2005;98:383–389. [DOI] [PubMed]
- 8.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. [DOI] [PubMed]
- 9.Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, Chapman W, Levin W, Manchul L, Keane TJ, Hill RP. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–156. [DOI] [PubMed]
- 10.Giatromanolaki A, Koukourakis MI, Kakolyris S, Turley H, O’Byrne K, Scott PA, Pezzella F, Georgoulias V, Harris AL, Gatter KC. Vascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small lung cancer. Clin Cancer Res. 1997;3:2485–2492. [PubMed]
- 11.Graeber TG, Osmanian C, Jacks T, Housman DE, Kock CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. [DOI] [PubMed]
- 12.Grau C, Overgaard J. Effect of etoposide, carmustine, vincristine, 5–fluorouracil, or methotrexate on radiobiologically oxic and hypoxic cells in a C3H mouse mammary carcinoma in situ. Cancer Chemother Pharmacol. 1992;30:277–280. [DOI] [PubMed]
- 13.Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. [DOI] [PubMed]
- 14.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed]
- 15.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. [DOI] [PubMed]
- 16.Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1α gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. [DOI] [PubMed]
- 17.Junior AT, de Abreu Alves F, Pinto CA, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 2003;39:521–530. [DOI] [PubMed]
- 18.Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F, Ishii S. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–577. [PubMed]
- 19.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. [DOI] [PubMed]
- 20.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–887. [PubMed]
- 21.Koos RD, Kazi AA, Roberson MS, Jones JM. New insight into the transcriptional regulation of vascular endothelial growth factor expression in the endometrium by estrogen and relaxin. Ann N Y Acad Sci. 2005;1041:233–247. [DOI] [PubMed]
- 22.Lagrange JL, Courdi A, Chauvel P, Gioanni J, Ettore F, Bongain A, Duforestel T, Gillet JY. The labelling index in carcinoma of the uterine cervix: its correlation with tumour sterilization. Br J Radiol. 1992;65:63–65. [DOI] [PubMed]
- 23.Lee AH, Dublin EA, Bobrow LG, Poulsom R. Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol. 1998;185:394–401. [DOI] [PubMed]
- 24.Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, Fukuda H, Nakano H, Abe S, Tateishi A, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y, Nakamura M. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer. 1999;35:1089–1093. [DOI] [PubMed]
- 25.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. [DOI] [PubMed]
- 26.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. [DOI] [PubMed]
- 27.Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. [DOI] [PMC free article] [PubMed]
- 28.Nakano T, Oka K, Arai T. Histological and immunohistochemical prediction for local control of cervical squamous cell carcinoma treated with radiotherapy alone. Int J Radiation Oncology Biol Phys. 1990;19:1011–1019. [DOI] [PubMed]
- 29.Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of alpha-helices beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. [DOI] [PMC free article] [PubMed]
- 30.Peng TS, Qiu JS, Wu HX, Liang HZ, Luo CQ. Expressions of CD44s, MMP-9, and Ki-67: possible association with invasion, metastasis, and recurrence of osteosarcoma. Ai Zheng. 2002;21:745–750. [PubMed]
- 31.Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A, Tonini G, Rabitti C. COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology. 2005;46:561–568. [DOI] [PubMed]
- 32.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. [DOI] [PubMed]
- 33.Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. [DOI] [PubMed]
- 34.Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. [DOI] [PubMed]
- 35.Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999;80:169–707. [DOI] [PMC free article] [PubMed]
- 36.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 1982;49:1221–1230. [DOI] [PubMed]
- 37.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. [DOI] [PubMed]
- 38.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. [DOI] [PubMed]
- 39.Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005; 65:605–612. [PubMed]
- 40.Tsang RW, Fyles AW, Kirkbride P, Levin W, Manchul LA, Milosevic MF, Rawlings GA, Banerjee D, Pintilie M, Wilson GD. Proliferation measurements with flow cytometry Tpot in cancer of the uterine cervix: correlation between two laboratories and preliminary clinical results. Int J Radiat Oncol Biol Phys. 1995;32:1319–1329. [DOI] [PubMed]
- 41.Tsang RW, Fyles AW, Milosevic M, Syed A, Pintilie M, Levin W, Manchul LA. Interrelationship of proliferation and hypoxia in carcinoma of the cervix. Int J Radiation Oncology Biol Phys. 2000;46:95–99. [DOI] [PubMed]
- 42.Tsang RW, Wong CS, Fyles AW, Levin W, Manchul LA, Milosevic M, Chapman W, Li YQ, Pintilie M. Tumour proliferation, apoptosis in human uterine cervix carcinoma II: correlations with clinical outcome. Radiother Oncol. 1999;50:93–101. [DOI] [PubMed]
- 43.Vaupel P, Kallinowski F, Okuniell P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed]
- 44.Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. [DOI] [PubMed]
- 45.Willet CG, Warland G, Hagan MP, Daly WJ, Coen J, Shellito PC, Compton CC. Tumor proliferation in rectal cancer following preoperative irradiation. J Clin Oncol. 1995;13:1417–1424. [DOI] [PubMed]
- 46.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–550. [DOI] [PubMed]
- 47.Wu Q, Yang SH, Wang RY, Ye SN, Xia T, Ma DZ. Effect of silencing HIF-1alpha by RNA interference on expression of vascular endothelial growth factor in osteosarcoma cell line SaOS-2 under hypoxia. Ai Zheng. 2005;24:531–535. [PubMed]
- 48.Wu Q, Yang SH, Ye SN, Wang RY. Therapeutic effects of RNA interference targeting HIF-1 alpha gene on human osteosarcoma. Zhonghua Yi Xue Za Zhi. 2005;85:409–413. [PubMed]
- 49.Zheng MH, Xu J, Robbins P, Pavlos N, Wysocki S, Kumta SM, Wood DJ, Papadimitriou JM. Gene expression of vascular endothelial growth factor in giant cell tumors of bone. Hum Pathol 2000;31:804–812. [DOI] [PubMed]
- 50.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed]
- 51.Zhu ZH, Rong TH, Zeng CG, Wu QL, Ma Y, Huang XP, Li BJ, Zhang PY, Zhao JM, Hu W, Zhang SY, Yu H, Ma GW, Zhang LJ, Wen ZS, Fu JH, Long H. Vascular endothelial growth factor expression and microvessel density in Stage I–II non-small cell lung cancer and their prognostic significances. Ai Zheng. 2005;24:865–869. [PubMed]