Abstract
Recombinant vaccinia viruses containing the 22-kilodalton protein (matrixlike or 22K protein) or phosphoprotein gene from respiratory syncytial virus were constructed. These recombinant viruses expressed proteins which were immunoprecipitated by appropriate respiratory syncytial virus antibodies and comigrated with authentic proteins produced by respiratory syncytial virus infection. The new recombinant viruses (and others previously described containing the attachment glycoprotein, fusion, or nucleoprotein genes of respiratory syncytial virus) were used to infect target cells for cultured polyclonal cytotoxic T lymphocytes generated from the spleens of BALB/c or DBA/2 mice primed by intranasal infection with respiratory syncytial virus. Respiratory syncytial virus-specific cytotoxic T lymphocytes (CTL) showed strong Kd (but not Dd)-restricted recognition of the 22K protein. As previously reported, the fusion protein and nucleoprotein were both seen by CTL, but recognition of these proteins was comparatively weak. There was no detectable recognition of other respiratory syncytial virus proteins tested (including phosphoprotein). 22K protein-specific splenic memory CTL persisted for at least 11 months after infection of BALB/c mice. Priming BALB/c mice with recombinant vaccinia virus containing the 22K protein gene induced respiratory syncytial virus-specific memory CTL at lower levels than that previously reported following infection with a similar recombinant containing the fusion protein gene. These data identify the 22K protein as a major target antigen for respiratory syncytial virus-specific CTL from H-2d mice primed by respiratory syncytial virus infection.
Full text
PDF

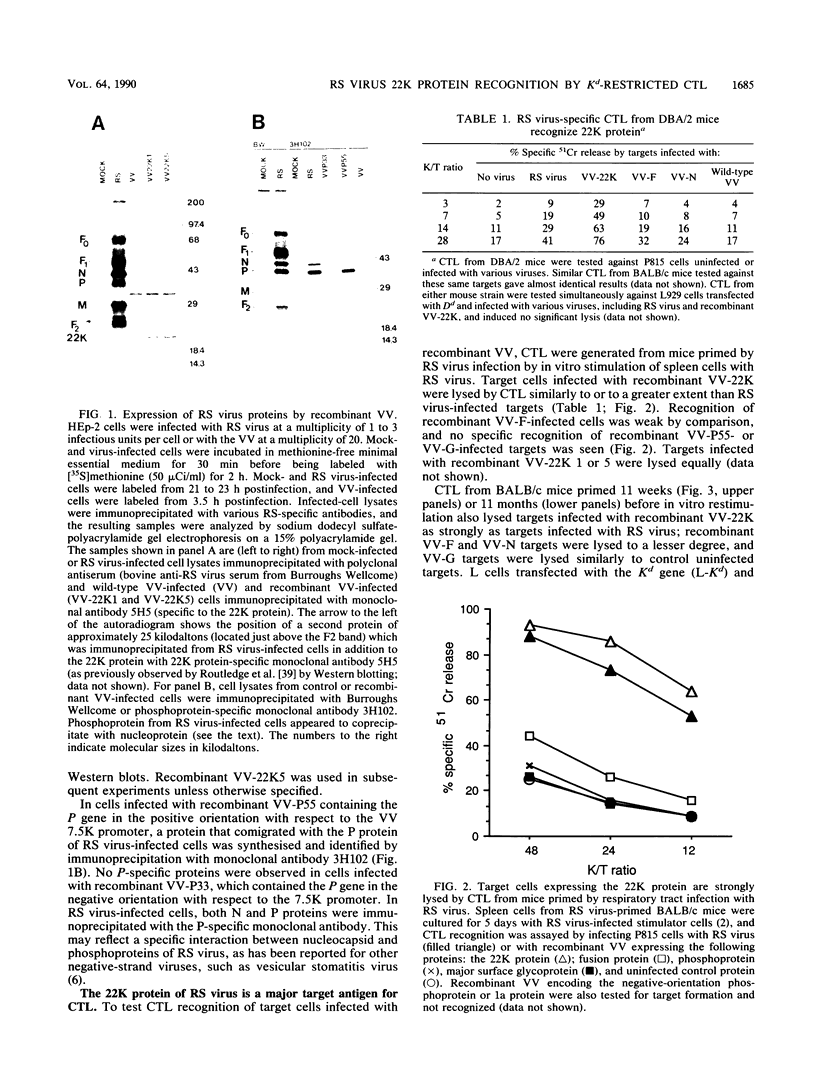
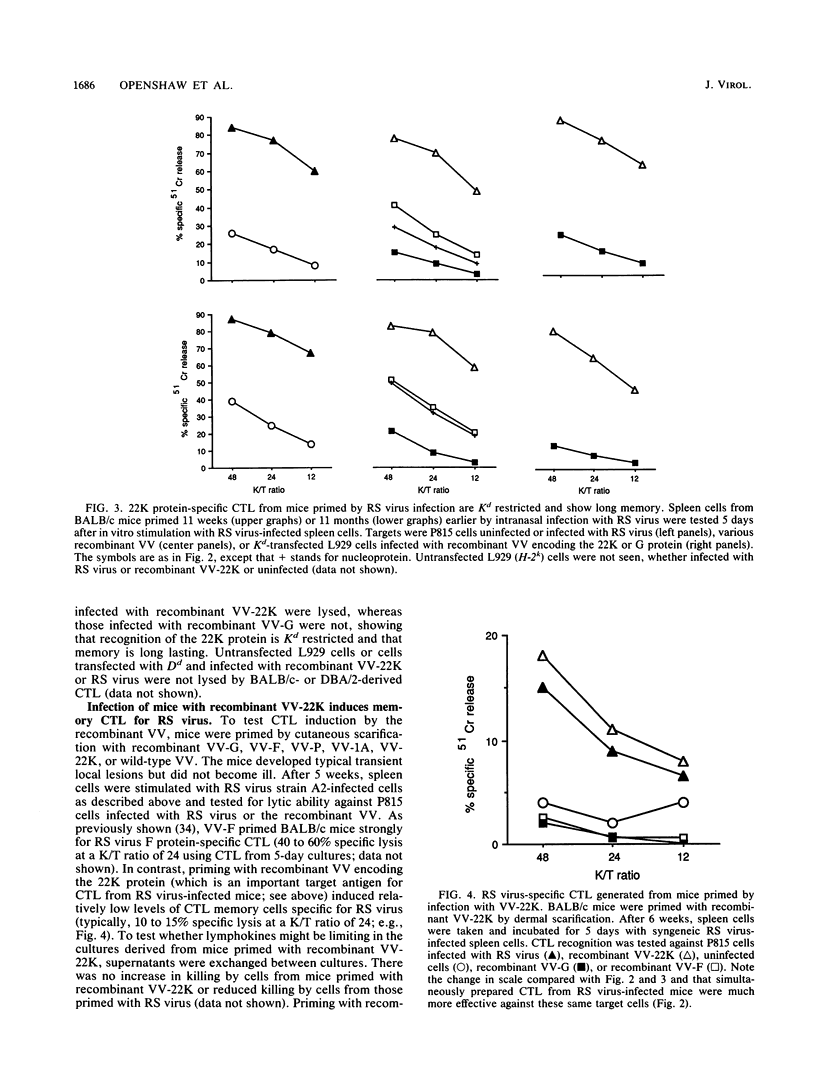



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham C. R., Askonas B. A. Murine cytotoxic T cells specific to respiratory syncytial virus recognize different antigenic subtypes of the virus. J Gen Virol. 1986 Apr;67(Pt 4):623–629. doi: 10.1099/0022-1317-67-4-623. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Bastin J., Rothbard J., Davey J., Jones I., Townsend A. Use of synthetic peptides of influenza nucleoprotein to define epitopes recognized by class I-restricted cytotoxic T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1508–1523. doi: 10.1084/jem.165.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt H. N., Pringle C. R. Molecular cloning and sequencing of the F and 22K membrane protein genes of the RSS-2 strain of respiratory syncytial virus. J Gen Virol. 1987 Nov;68(Pt 11):2789–2796. doi: 10.1099/0022-1317-68-11-2789. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Brown E. G., Takayesu D., Prevec L. Protein kinase activity associated with immunoprecipitates of the vesicular stomatitis virus phosphoprotein NS. Virology. 1984 Jan 30;132(2):229–238. doi: 10.1016/0042-6822(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Gerhard W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature. 1982 Mar 4;296(5852):75–76. doi: 10.1038/296075a0. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987 Apr;61(4):1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Bangham C. R. Recognition of respiratory syncytial virus fusion protein by mouse cytotoxic T cell clones and a human cytotoxic T cell line. J Gen Virol. 1989 Jan;70(Pt 1):79–87. doi: 10.1099/0022-1317-70-1-79. [DOI] [PubMed] [Google Scholar]
- Cannon M. J. Microplaque immunoperoxidase detection of infectious respiratory syncytial virus in the lungs of infected mice. J Virol Methods. 1987 Jul;16(4):293–301. doi: 10.1016/0166-0934(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. The envelope-associated 22K protein of human respiratory syncytial virus: nucleotide sequence of the mRNA and a related polytranscript. J Virol. 1985 Apr;54(1):65–71. doi: 10.1128/jvi.54.1.65-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Both G. W., Boyle D. B. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur J Immunol. 1986 Dec;16(12):1479–1487. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J. Analysis of proteins synthesized in respiratory syncytial virus-infected cells. J Virol. 1982 May;42(2):372–378. doi: 10.1128/jvi.42.2.372-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Z., Sridhar P., Pacha R. F., Condit R. C. Efficient targeted insertion of an unselected marker into the vaccinia virus genome. Virology. 1986 Nov;155(1):97–105. doi: 10.1016/0042-6822(86)90171-6. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gomez A., Bourgault I., Gomard E., Picard F., Levy J. P. Role of different lymphocyte subsets in human anti-viral T cell cultures. Cell Immunol. 1989 Feb;118(2):312–327. doi: 10.1016/0008-8749(89)90380-8. [DOI] [PubMed] [Google Scholar]
- Gotch F., McMichael A., Smith G., Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987 Feb 1;165(2):408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiner M., Kempe T., Tjian R. A novel strategy for constructing clustered point mutations. Nucleic Acids Res. 1985 Feb 11;13(3):1015–1025. doi: 10.1093/nar/13.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Collins P. L., Wertz G. W. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 1985 Mar;2(2):157–173. doi: 10.1016/0168-1702(85)90246-1. [DOI] [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- King A. M., Stott E. J., Langer S. J., Young K. K., Ball L. A., Wertz G. W. Recombinant vaccinia viruses carrying the N gene of human respiratory syncytial virus: studies of gene expression in cell culture and immune response in mice. J Virol. 1987 Sep;61(9):2885–2890. doi: 10.1128/jvi.61.9.2885-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Jacobson S. Pathways of viral antigen processing and presentation to CTL: defined by the mode of virus entry? Immunol Today. 1989 Feb;10(2):45–48. doi: 10.1016/0167-5699(89)90303-4. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. D., Taylor P. M., Askonas B. A. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology. 1989 Jul;67(3):375–381. [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986 Nov 1;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J., Pemberton R. M., Ball L. A., Wertz G. W., Askonas B. A. Helper T cell recognition of respiratory syncytial virus in mice. J Gen Virol. 1988 Feb;69(Pt 2):305–312. doi: 10.1099/0022-1317-69-2-305. [DOI] [PubMed] [Google Scholar]
- Pala P., Askonas B. A. Low responder MHC alleles for Tc recognition of influenza nucleoprotein. Immunogenetics. 1986;23(6):379–384. doi: 10.1007/BF00372670. [DOI] [PubMed] [Google Scholar]
- Pemberton R. M., Cannon M. J., Openshaw P. J., Ball L. A., Wertz G. W., Askonas B. A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987 Aug;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Berndt J., Suffin S. C., Chanock R. M. Respiratory syncytial virus infection in inbred mice. Infect Immun. 1979 Nov;26(2):764–766. doi: 10.1128/iai.26.2.764-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Routledge E. G., Willcocks M. M., Morgan L., Samson A. C., Scott R., Toms G. L. Expression of the respiratory syncytial virus 22K protein on the surface of infected HeLa cells. J Gen Virol. 1987 Apr;68(Pt 4):1217–1222. doi: 10.1099/0022-1317-68-4-1217. [DOI] [PubMed] [Google Scholar]
- Routledge E. G., Willcocks M. M., Morgan L., Samson A. C., Scott R., Toms G. L. Heterogeneity of the respiratory syncytial virus 22K protein revealed by Western blotting with monoclonal antibodies. J Gen Virol. 1987 Apr;68(Pt 4):1209–1215. doi: 10.1099/0022-1317-68-4-1209. [DOI] [PubMed] [Google Scholar]
- Satake M., Elango N., Venkatesan S. Sequence analysis of the respiratory syncytial virus phosphoprotein gene. J Virol. 1984 Dec;52(3):991–994. doi: 10.1128/jvi.52.3.991-994.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G., Ball L. A., Anderson K., Young K. K., King A. M., Wertz G. W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987 Dec;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hayle A. J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985 Dec;66(Pt 12):2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hughes M., Collins A. P. Respiratory syncytial virus infection in mice. Infect Immun. 1984 Feb;43(2):649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. A., Lambden P. R., Ogilvie M. M., Watt P. J. Antibodies to respiratory syncytial virus polypeptides and their significance in human infection. J Gen Virol. 1983 Sep;64(Pt 9):1867–1876. doi: 10.1099/0022-1317-64-9-1867. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Stott E. J., Young K. K., Anderson K., Ball L. A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987 Feb;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Gebhard J. R., Lewicki H., Tishon A., Oldstone M. B. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988 Mar;62(3):687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Southern P. J., Oldstone M. B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988 Feb;162(2):321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]