Abstract
Most of the previous work on the sphingolipid ceramide has been devoted to its function as an apoptosis inducer. Recent studies, however, have shown that in stem cells, ceramide has additional nonapoptotic functions. In this article, ceramide signaling will be reviewed in light of ‘systems interface biology’: as an interconnection of sphingolipid metabolism, membrane biophysics and cell signaling. The focus will be on the metabolic interconversion of ceramide and sphingomyelin or sphingosine-1-phosphate. Lipid rafts and sphingolipid-induced protein scaffolds will be discussed as a membrane interface for lipid-controlled cell signaling. Ceramide/sphingomyelin and ceramide/sphingosine-1-phosphate-interdependent cell-signaling pathways are significant for the regulation of cell polarity, apoptosis and/or proliferation, and as novel pharmacologic targets in cancer and stem cells.
Keywords: apoptosis, cancer, cell polarity, ceramide, ES cells, lipid rafts, sphingomyelin, sphingosine-1-phosphate
Ceramide & its derivatives: from metabolism to cell signaling
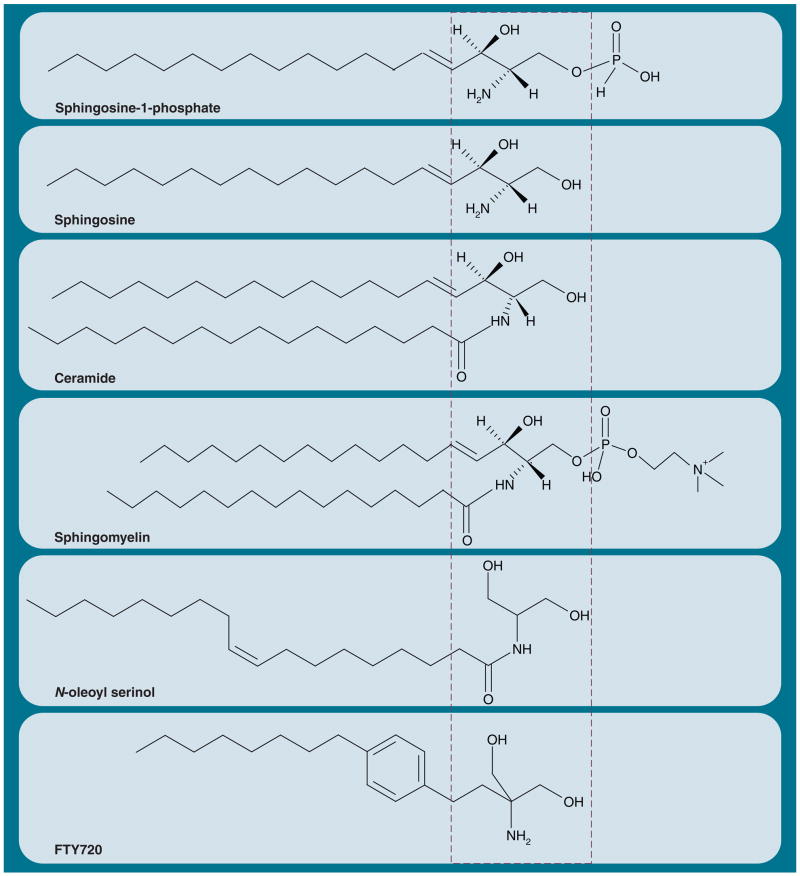
The structural core of ceramide is the sphingoid base sphingosine (Figure 1), a lipid that is synthesized from the amino acid serine and the activated fatty acid palmitoyl-CoA (Figure 2). Sphingosine (or sphingenine) was first isolated and characterized by the German pathologist and ‘father of neurochemistry’ Johann Ludwig Wilhelm Thudichum (1829–1901) [1,2]. He coined the term sphingolipids, which is derived from ‘sphingos’, the genitive of Sphinx. In Greek, ‘sphingo’ means ‘to strangle’. According to the legend, a fate of death was meant for everyone who could not solve the riddles posed by the Sphinx. Quite ironically, this is how many sphingolipid researchers may feel when faced with analyzing the function of ceramide. The riddle of ceramide, its function and how it acts as signaling lipid, is far from being solved. To ease our fears when confronted with the Sphinx in sphingolipid biochemistry, a related Greek word, ‘sphingein’, meaning ‘to bind tight’, alludes to the possibility of solving the enigma of ceramide by identifying its binding proteins.
Figure 1. Ceramide and its derivatives.
The sphingoid base (sphingosine portion) of all of the sphingolipids is derived from the condensation reaction of serine with palmitoyl-CoA. The minimal structural motif of many active sphingolipid analogues is composed of two hydroxyl groups β-positioned to an amino group or an imino group linked to a hydrocarbon chain (dashed box, S18 and FTY720 are closely related and derivatives of 2-amino 1,3-propanediol [serinol]).
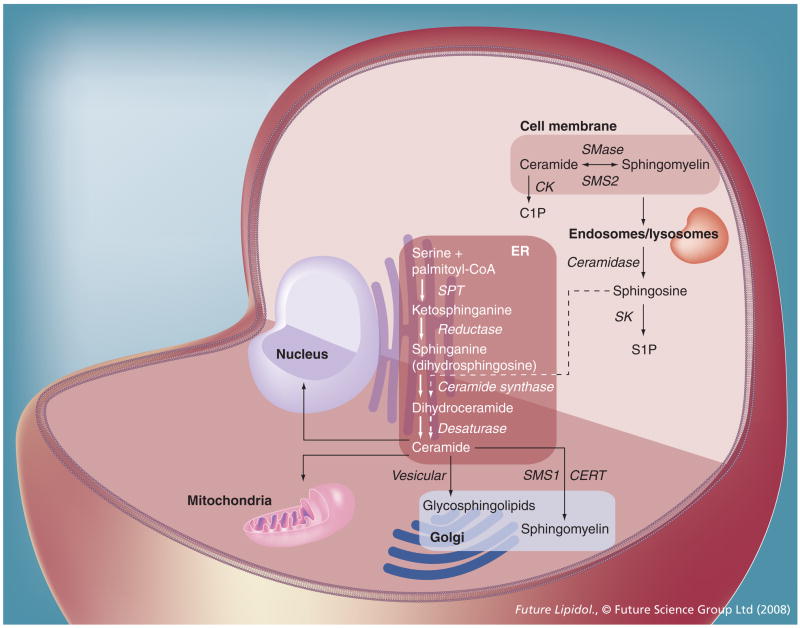
Figure 2. Metabolism and function of sphingolipids.
De novo biosynthesis of sphingolipids is initiated in the ER, followed by transport of ceramide to the Golgi (vesicular or via CERT). In the Golgi, ceramide is used as precursor substrate for glycosphingolipid and sphingomyelin biosynthesis. Sphingomyelin is then transported to the cell membrane, where it can be hydrolyzed to ceramide (sphingomyelin cycle). In endosomes, ceramide can be hydrolyzed to sphingosine and is then recycled in the ER, which is mediated by the ‘salvage pathway’ (dashed arrows). Ceramide generated in the ER can be transported to the nucleus (via perinuclear ER cisternae) and the mitochondria (via mitochondrial-associated ER membrane). Ceramide can also be converted to C1P or S1P.
C1P: Ceramide-1-phosphate; CERT: Ceramide transport protein; CK: Ceramide kinase; ER: Endoplasmic reticulum; S1P: Sphingosine-1-phosphate; SK: Sphingosine kinase; SMase: Sphingomyelinase; SMS: Sphingomyelin synthase; SPT: Serine palmitoyltransferase.
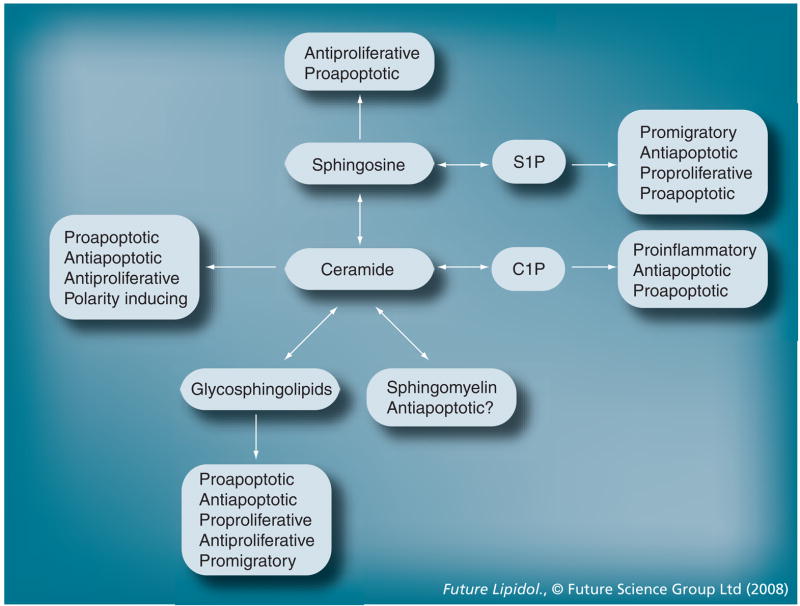
In addition to ceramide, more than a hundred different sphingolipids are known. The most comprehensive collection of sphingolipids, with respect to their characteristics and structures, can be found at [401], a Webpage supported by the National Institutes of General Medical Sciences [3,4]. Sphingolipids are essential components of cellular membranes and have been implicated in a variety of biological functions (Figure 3) [5–27]. Among these, their roles as pro-or anti-apoptotic and pro- or anti-proliferative signaling lipids are the most important. Most recently, our group has found that establishment of cell polarity during embryonic development is another biological process regulated by sphingolipids [28]. To define a profile of functions for individual sphingolipids is difficult because of their rapid metabolic interconversion. To add to this predicament, several derivatives of ceramide have functions similar to ceramide itself. Hence, novel techniques are needed that will help to unravel the function of individual sphingolipids.
Figure 3. Metabolism and function of sphingolipids.
Ceramide and its derivatives have similar as well as opposite cell-signaling functions.
C1P: Ceramide-1-phosphate; S1P: Sphingosine-1-phosphate.
In the ‘protein world’, the function of a protein is defined by its interaction with another protein and the biological effect of this interaction. Because of this, knockout mice of two interacting proteins often show similar phenotypes, which is of tremendous help to achieve an ‘educated guess’ on candidate proteins to test for interaction. The characterization of protein interaction has become standard procedure: two-hybrid assays identify all associated proteins, ectopic expression and coimmunoprecipitation/pull-down assays confirm the interaction, and in vitro mutagenesis narrows down binding domains. Perhaps at the end, a pharmacologic approach to weaken or strengthen the interaction is tested to foster a translational goal. The scenario looks quite different in the ‘lipid world’. Certainly, it is still the interaction of a lipid with another lipid or a protein that defines its biological activity. However, there is not yet a protein–lipid two-hybrid assay, coimmunoprecipitation assays are difficult because of a lack of efficient antilipid antibodies, and mutagenesis of a lipid demands for rather complicated synthetic chemistry. Nevertheless, in order to define a cell-signaling function of a lipid, it is inevitable to identify the interface to the protein world – the initially interacting protein that induces a signaling cascade potentially regulated by the lipid.
A major drawback in identifying the interfacing protein between lipid and protein-dependent cell signaling is that the proteins with which they interact are known only for a few sphingolipids [29]. However, there have been successful studies to determine binding proteins and even specific binding domains for many other signaling lipids. For example, phosphatidylinositolphosphates (PIPs) bind to the pleckstrin homology (PH) domain and structurally related domains [30,31]. Diacylglycerol (DAG), an important pro-proliferative lipid, interacts with the C1 domain of classical PKC (PKCα) and PKD [31–35]. Within the family of sphingolipids, binding partners have only been specified for ceramide [29,36–46], ceramide-1-phosphate [47–49], sphingosine-1-phosphate (S1P) [19,50–52] and some gangliosides (e.g., GM1).
Ceramide-binding proteins: from interaction to action
Starting 20 years ago, landmark studies from Hannun’s and Kolesnick’s laboratories described the effect of ceramide on ceramide-activated protein phosphatase(s) [36,37,53–56] and kinase(s) [46,57–59], respectively. The activating effect of ceramide on protein phosphatase (PP)1 and PP2a is now well established. Recent studies have shown that ceramide activation of PP1 alters splicing of Bcl-x and caspase 9 from anti-to pro-apoptotic proteins [60,61]. Most recently, ceramide activation of PP1cγ in a confluent cell culture has been shown to lead to dephosphorylation of β-catenin [62]. It has been known for a long time that ceramide-activated PP2a is involved in dephosphorylation of a variety of key factors in cell-signaling pathways regulating proliferation, apoptosis and differentiation [36,54,63,64]. Ceramide-induced repression of telomerase has been found to arrest cells in the G1 phase, an elegant way to explain the anti-proliferative effect of ceramide [65,66]. New studies suggest that this is caused by activation (possibly mediated by PP2a) of histone deacetylase by C18 ceramide, which deacetylates and inactivates SP3, a transcription factor that upregulates telomerase expression [67,68].
In addition to phosphatases, protein kinases have been suggested to be regulated by ceramide. Within the group of protein kinases, a ceramide-activated protein kinase, which was later characterized as kinase suppressor of Ras (KSR) was the first protein kinase found to be activated by ceramide [46,57]. It has been shown that dephosphorylation of serine 136 in the proapoptotic protein Bad is a mechanism by which ceramide activation of KSR triggers apoptosis via inactivation of the Ras–Raf1/MEK1 pathway [69]. Interestingly, ceramide-mediated suppression of a phosphorylation pathway (here from Ras to Bad) together with ceramide activation of phosphatases may complement each other in cell signaling, a principle on which we elaborate in one of the following sections.
Several laboratories have independently shown that the PKC family is regulated by ceramide and other sphingolipids [28,38–44,70–76]. Classical PKCα was the first PKC isoform found to be regulated by sphingolipids [70,73,75–77]. From early on, it was recognized that sphingosine could be an inhibitor of PKC because serine is phosphorylated by PKC and serine is a structural component in sphingosine (Figure 1). Indeed, in vitro data suggested that positively charged sphingosine inhibits PKCα by simple displacement and charge neutralization [77]. From there on, many studies used sphingosine as a specific inhibitor of PKCα, although the physiological function of this interaction is still unclear. Soon after these initial studies, PKCα was also found to be inhibited by ceramide in cells, although this inhibition appears to be mediated by ceramide activation of PP1-catalyzed dephosphorylation of PKCα [75,78].
In 1994, Moscat’s group described for the first time that PKCζ is activated by ceramide, which was found to inhibit nuclear factor (NF)-κB, a major cell-survival transcription factor in neural and other cell types [38]. Soon thereafter, these results were confirmed by Pfizenmaier’s group, who proposed that ceramide may activate or inhibit PKCζ depending on its concentration [39]. From there on, ceramide-dependent regulation of PKCζ has been found by several other laboratories, including our group, which has specialized on the biological effect of this interaction in cancer and stem cells [28,40–43,72,79–82]. PKCζ belongs to the group of atypical PKCs (aPKCs), a family of three highly similar isoenzymes termed PKCζ, λ and τ [83–90]. Although identical in their activity in vitro, knockout mice for PKCζ and λ have clearly demonstrated that these two isoforms have redundant as well as distinct functions. Loss of PKCλ is embryonic lethal, while PKCζ deficiency does not disrupt embryonic development, but affects NF-κB and insulin signaling and results in impaired glucose transport [91–94]. The function of aPKC for embryonic development and the potential involvement of ceramide in its regulation will be discussed in one of the following sections.
Considering the convincing evidence that ceramide activates aPKC, it is logical to speculate that there is a potential binding site for ceramide. aPKC contains three major protein domains that are common to all of the members of the PKC family, as shown in Figure 4 [84]. The N-terminal pseudosubstrate domain folds back onto the active site of the C-terminal catalytic domain. This ‘self inactivates’ aPKC, unless binding of an activating factor or phosphorylation ‘opens up’ the protein conformation and primes the enzyme for further activation. In PKCα, two factors are known to activate the enzyme: calcium and DAG [95]. Calcium binds to the C2 domain (absent in aPKC), which is part of a polypartite regulatory protein moiety located between the pseudosubstrate and catalytic domain. A second motif found in all of the members of the PKC family, KSR and DAG kinase, is the C1 domain, which in PKCα mediates activation of the enzyme by DAG and phorbol ester [33,95]. Since DAG is structurally similar to ceramide, it was suggested that a relative of the C1 domain, the modified phorbolester/diacylglycerol binding (C1B) domain, may bind to ceramide in aPKC [32,96,97]. In addition to aPKC, the C1B domain is present in PKCδ, which has led to several studies determining the function of ceramide for the regulation of this enzyme [71,98]. It should be noted that in addition to ceramide-activated phosphatases or kinases, several other proteins have been found to bind to ceramide [29]. Apart from enzymes using ceramide as a substrate, cathepsin D and ceramide transport protein (CERT) have been shown to associate with ceramide. Ceramide binding of endosomal cathepsin D is involved in the induction of apoptosis by acid sphingomyelinase (ASMase), an enzyme that will be discussed in one of the following sections [99,100]. In this section, we will also elaborate on the function of CERT. Among the ceramide-binding proteins, only ceramide-activated phosphatases and kinases have been found to participate in major regulatory cell-signaling pathways. Therefore, we have focused on the effect of ceramide on aPKC based on its involvement in embryonic development and stem cell differentiation [42,43].
Figure 4. Structure and binding domains of atypical PKC.
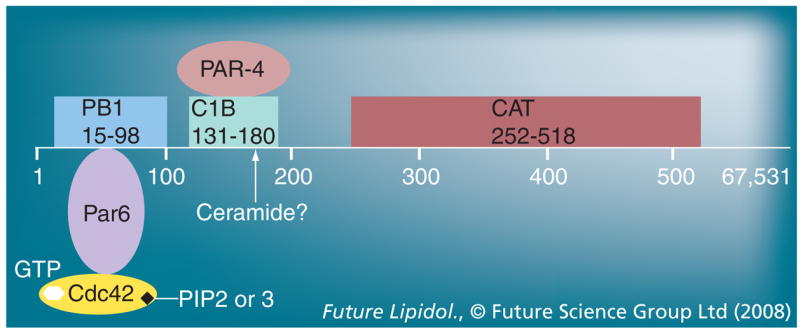
Atypical PKC (aPKC; figure shows PKCζ) consists of an N-terminal (regulatory) and C-terminal (catalytic) moiety connected by a hinge region. The N-terminal moiety contains a pseudosubstrate (PS) motif and a PB1 domain. The PB1 domain associates with the polarity protein Par6 that itself binds to Cdc42. The hinge region contains a C1B domain that has been suggested to associate with ceramide, and a putative binding site for PAR-4. The C-terminal moiety contains the catalytic domain and several sites, the phosphorylation of which stably activates the enzyme. In the inactive state of aPKC, the N-terminal PS motif ‘folds back’ onto the C-terminal catalytic domain and blocks its access to protein substrates. Binding of effectors to the N-terminal moiety ‘opens up’ aPKC and primes its phosphorylation and activation. In our model, ceramide binding, followed by the association with Par6 and Cdc42, and phosphorylation of the catalytic moiety are consecutive steps of robust aPKC activation. PIP2 or 3 may participate in aPKC activation and subcellular translocation by binding to active (GTP-associated) Cdc42 in this complex.
C1B: Modified phorbolester/diacylglycerol binding; PAR-4: Prostate apoptosis response-4; PB: Protein binding; PIP: Phosphatidylinositolphosphate.
It was not until recently that evidence for the direct binding of aPKC to ceramide was found [38,41–43,101]. Ceramide, and probably many ceramide analogues, form structurally organized lipid microdomains in the cell membrane [40,99,102–107]. These microdomains, also referred to as lipid rafts (note discussion on the nature of ceramide microdomains in the following section), may allow for the repeated or multiple binding of ceramide-associated proteins, such as aPKC, thereby enhancing the formation of protein complexes with cell-signaling functions. Therefore, it is tempting to speculate that ceramide-induced rafts and raft-associated proteins form an initial platform for growth factor or cytokine-dependent cell-signaling pathways.
The rising of the raft: interfacing membrane biophysics & cell signaling
In chemistry, an interface is defined as the surface boundary between two phases (e.g., solid and liquid). An interface is also the interconnection point between two signaling networks. In our concept of systems interface biology, the plasma membrane is viewed as a surface interface that converts extracellular cues into the activation of intracellular cell-signaling cascades. Lipids in the membrane support this process by generating a signaling platform that brings together receptors and other signaling molecules. Therefore, oligomerization and avidity (multiple binding) of receptors and other signaling molecules is likely to be catalyzed by lipid rafts, which, in principle, is based on a process of lipid aggregation and coalescence forming specific lipid microdomains in the cell membrane. Rafts are defined as lipid microdomains with ordered (Lo) structure in the liquid phase of the membrane [108–120]. The terms lipid microdomains, rafts and detergent-insoluble membranes are often used interchangeably. However, the characterization of detergent-insoluble membranes containing specific phospho- and sphingolipids occurred earlier than the concept of the lipid raft. It dates back to several studies from 1972 to 1974 showing that alkaline (carbonate) or cold Triton X-100™-insoluble (Sigma Chemical Co., MO, USA) membrane fractions were enriched in cholesterol and sphingolipids, in particular sphingomyelin and glycosphingolipids [121,122]. 10 years later, Hakomori’s group found that detergent-insoluble membranes that contain glycosphingolipids (i.e., the ganglioside GM3) sustain cell attachment [123]. In the following decade, detergent-insoluble membranes were linked to many processes of cell signaling and were suggested to represent the equivalent of lipid rafts or microdomains. In principle, all of the cell-signaling pathways known to be affected by sphingolipid and cholesterol metabolism or modification, such as Ras, TNF-α, IL-1, TGFβ, Fas/CD95, Sonic hedgehog, Wnt and insulin, are potentially regulated by lipid rafts [124–130].
Unfortunately, it has turned out to be difficult to directly visualize lipid rafts, which is important for demonstrating their biological significance. One of the main reasons for this shortcoming was the unavailability of raft-specific probes that can be used for fluorescence microscopy. While proteins known to segregate to detergent-insoluble membranes can be expressed as fluorescent protein (e.g., green fluorescent protein [GFP])-tagged probes, the lipid portion is more difficult to label. Nitrobenzoxadiazole (NBD) and borondipyrromethene (Bodipy)-labeled cholesterol or sphingolipids often behave distinctly from their nonconjugated counterparts [131,132]. Antibodies against raft lipids or binding proteins such as cholera toxin subunit B (for GM1 binding) are not always specific and can only be used in fixed tissues because they change the distribution of their associated lipid by binding to it. In the last 5 years, 6-dodecanoyl-2-dimethylaminonaphthalene (laurdan) has emerged as a raft-specific probe that shows a blue shift of fluorescence emission when packed in microdomains of living cells [133–137]. Another promising development to visualize lipid rafts in living cells and tissues is the generation of nonlytic, fluorescently labeled lysenin, an earthworm-derived protein that binds specifically to sphingomyelin microdomains [138]. However, a concern similar to that with lipid-specific antibodies arises with respect to the prolonged effect of binding proteins to the distribution of their associated membrane lipids. On the protein side, glycosylphosphatidylinositol-conjugated proteins (e.g., Thy-1, CD59 and neural cell adhesion molecule [NCAM]), caveolin and flotillin are generally used as raft markers [139–141]. However, regardless of the method of visualization, it is recommended to confirm the colocalization or codistribution of potential raft lipids and proteins using both fluorescence microscopy and density-gradient centrifugation of detergent- or alkaline-insoluble membranes.
Lipid microdomains have been found to be specific for individual sphingolipids. Gangliosides, such as GM3 and GM1, form glycosphingolipid rafts that are important for the activation of B cells via so-called ‘glycosynapses’ [142,143]. Other lipid microdomains are involved in caveolin-mediated internalization of endocytotic vesicles [125,143,144]. These caveolae are distinct from glycosphingolipid rafts [143].
Recently, evidence has amounted suggesting that ceramide forms specific microdomains termed ceramide rafts [145–147]. These rafts are segregated from sphingomyelin-containing microdomains, although they can be derived from them [103,104,127,148]. The raft nature and significance of ceramide microdomains is still a matter of debate. The ‘classical’ sphingomyelin/cholesterol-rich raft is commonly accepted as being in the Lo state with low membrane fluidity. By contrast, the biophysics of ceramide rafts is less clearly defined. In some studies, ceramide rafts are described as another, highly ordered state or, alternatively, as lipid microdomains that rather perturb sphingomyelin-rich rafts leading to increased membrane fluidity [102,103,149]. It is clear that these two models are distinct from each other. While highly ordered ceramide rafts may organize their own specific cell-signaling platforms, ceramide as a fluidizer inactivates pre-existing, raft-induced cell-signaling pathways. The final decision on which model is biologically significant has not yet been found because of the notorious intricacies involved in the visualization and detection of lipid rafts in living cells. However, it may very well be that both models are significant in different contexts. The variety of biological functions of ceramide, ranging from apoptosis to cell polarity, is unlikely to rely on a single biophysical process. Instead, the biophysical effect of ceramide, as a raft organizer or perturbator, may be regulated by the fatty acid species within ceramide (e.g., variation in chain length and degree of saturation), the overall lipid composition of the membrane or localized areas of it (e.g., proportions of ceramide, sphingomyelin and cholesterol) and the proteins associated with rafts. Remarkably, the two models for ceramide microdomains have in common that ceramide and sphingomyelin microdomains are segregated, but can still be metabolically converted into each other.
Sphingolipid biosynthesis in the endoplasmic reticulum: enzymology & pharmacology
Sphingolipid metabolism is maintained in different subcellular compartments [9,15,17,150–155]. It all starts in the endoplasmic reticulum (ER), where condensation of serine with palmitoyl-CoA generates 3-ketodihydrosphingosine (ketosphinganine) (Figure 2). This reaction is catalyzed by serine palmitoyltransferase (SPT), a membrane-resident enzyme consisting of two subunits, SPT1 (SPTLC-1) and SPT2 (SPTLC-2) [156–162]. Most recently, a third subunit, SPTLC-3, has been described and suggested to form a high molecular mass complex with the other two subunits [163]. Conventional knockout mice with SPTLC-1 or -2 deficiency are embryonic lethal well before gestational day E14 [161]. Since embryos could not be recovered from these knockout mice at any developmental stage, the exact time point of embryonic lethality is unknown. It may be well before neural tube closure or gastrulation, suggesting an essential role of sphingolipid biosynthesis for embryo development. This significance appears to be evolutionarily well conserved, since SPT deficiency in Saccharomyces, Aspergillus, Arabidopsis and Drosophila leads to developmental abnormalities [164–169]. In humans, the only well-characterized congenital disease associated with mutant SPT is hereditary sensory and autonomic neuropathy type I, an autosomal-dominant disorder of the peripheral nervous system caused by mutations of the gene coding for SPTLC-1 on chromosome 9q22.1–22.3 [170–174]. Another disease recently found to be associated with abnormal SPT activity is psoriasis, an inflammatory skin overgrowth, which may result from perturbed ceramide levels in the skin [175,176]. However, many other diseases, ranging from atherosclerosis to cancer, appear to be linked to dysregulated sphingolipid biosynthesis, underlining once more the biological significance of ceramide and its derivatives [11,25,63,146,161,177–182].
Several pharmacologic inhibitors of sphingolipid biosynthesis are available. Myriocin, or ISP-1, was first isolated from thermophilic fungi such as Mycelia sterilia, and it is now commonly used in cell culture for loss-of-function studies by inhibiting de novo sphingolipid biosynthesis [183–187]. It is complemented by fumonisin B1 (FB1), an inhibitor of ceramide synthase, a later enzymatic step in the de novo and salvage pathways of sphingolipid biosynthesis (Figure 2) [188–190]. To clarify the definition of these two processes; de novo biosynthesis includes the initial generation of the sphingoid base (ketosphinganine) by SPT, while the salvage pathway reacylates the sphingoid base (sphingosine) from degraded sphingolipids, which is catalyzed by ceramide synthase (Figure 2). Its inhibitor, FB1, is a teratogen that causes neural tube defects in animals and humans who consume Fusarium mold-contaminated food [190–194]. Currently, several novel SPT and other enzyme inhibitors for sphingolipid biosynthesis are being tested for their pharmacologic relevance [185,195–198]. These inhibitors are likely to be effective in diseases accompanied by degenerative or inflammatory processes. For example, myriocin has been found to prevent ceramide-induced apoptosis in a lung emphysema mouse model [199,200], and it has also shown ameliorative effects in genetic mouse models for atherosclerosis [201]. Unfortunately, myriocin and FB1 are too toxic (mainly for liver and kidney) to find broad systemic application in humans. However, a structural analogue of myriocin, FTY720 (Figure 1), is of pharmacologic use in clinical trials, which will be discussed in the final section.
How about activators of sphingolipid biosynthesis that could induce apoptosis by ceramide elevation in cancer cells? A very effective activator of ceramide generation is UV and γ-irradiation, which is clinically relevant but does not necessarily rely on sphingolipid biosynthesis. The reactions underlying (ir)radiation-induced ceramide generation will be discussed in one of the following sections. Several pharmacologic anticancer compounds have been shown to elevate sphingolipid biosynthesis by upregulating the gene expression or activity of the respective enzymes. Prominent examples are daunorubicin, etoposide and tamoxifen, which appear, at least in part, to act via ceramide-induced apoptosis [65,181,202–206]. Further promising agents to treat cancer are inhibitors for enzymes that metabolize ceramide, such as ceramidase or glucosyl-transferase (Figure 2) [207–211]. Ceramide itself is rather difficult to use as a drug because of its poor water solubility and rapid physiological degradation. However, recent attempts to deliver ceramide in liposomes or nanovesicles may allow for new therapeutic strategies [212,213]. In addition, water-soluble analogues of ceramide may play an important role in eliminating cancer cells or replacing ceramide when it is needed [79,214]. Our laboratory has, for the first time, synthesized novel ceramide analogues on the serinol basis (N-acylated 2-amino-1,3-propanediol) (Figure 1), the application of which will be discussed in a later section [43,79].
Approximately 40 years ago, it was thought that sphingosine (Figure 1) was directly derived from sphinganine and that sphingosine was acylated by ceramide synthase to generate ceramide [215,216]. This view has changed more than 10 years after the original labeling experiments with radioactive serine and palmitoyl-CoA [217,218]. The commonly accepted pathway for de novo biosynthesis of ceramide is now based on the generation of dihydroceramide as the first step followed by reduction to ceramide by dihydroceramide desaturase (Figure 2). Sphingosine is derived from ceramide by hydrolysis of the amide bond, which is catalyzed by ceramidase.
In addition to sphinganine, ceramide synthase accepts sphingosine as a substrate for the acylation reaction, which is an essential step in the previously mentioned salvage pathway of ceramide biosynthesis (Figure 2). Recently, this pathway has gained attention since many processes involving ceramide as an apoptosis inducer appear to be related to ceramide in a perinuclear, possibly endosomal, compartment [219]. The salvage pathway is also interesting with respect to the fatty acid specificity of ceramide synthase. To date, six ceramide synthase isoenzymes have been described, which are encoded by the longevity assurance (lass) genes 1–6 [220–223]. Each isoenzyme shows group specificity for particular fatty acids, leading to the lass gene-controlled biosynthesis of ceramide and ceramide derivatives with a specific fatty acid composition. This composition may very well determine the biophysical properties of ceramide, as discussed in the previous section.
C16 ceramide (Figure 1) is a prevalent physiological ceramide species, the biosynthesis of which is catalyzed by the gene products of lass 5 and 6. We have found that in hippocampal tissue derived from a mouse model of early-onset Alzheimer’s disease (PS1M146V presenilin 1-knockin mouse), the gene expression of lass 2 and 4 is upregulated [224]. The tissue consiquently contains four- or 8.5-fold more long chain ceramide of the C20 and C24 type, respectively. This ceramide species induces apoptosis in PS1 (PSEN1) mouse-derived astrocytes, which may contribute to the etiology of Alzheimer’s disease. The salvage pathway could rapidly convert various ceramide species and derivatives to proapoptotic ceramide, thereby enhancing degenerative processes.
Sphingolipid metabolism in the Golgi: ceramide transport & exit to the cell membrane
Before the salvage pathway can utilize ceramide, it has to be converted into its derivatives. To achieve this, ceramide is transported from the ER to the Golgi apparatus following two distinct transport routes (Figure 2). Until recently, ceramide was thought to be transported exclusively via lipid vesicles. However, 5 years ago, Hanada showed that a cytosolic protein, namely Good-pasture antigen-binding protein, picks up ceramide from the ER and delivers it to the Golgi [225,226]. From there on, Goodpasture antigen-binding protein has been termed CERT. The two important binding domains of CERT are the pleckstrin homology (PH) domain, which anchors the protein to specific sites within the Golgi, and the steroidogenic acute regulatory protein (STAR)-related lipid transfer (START) domain, a conserved lipid-binding domain that associates with sterols and ceramide [227]. It is now thought that ceramide transported by CERT is used for sphingomyelin biosynthesis while that converted to glycosphingolipids follows the classical vesicular route [225,226]. At this point, it should be noted that ceramide may also distribute throughout specialized ER compartments, such as the perinuclear membrane and the mitochondrial-associated ER membrane (MAM). In the ER, ceramide is known to affect calcium hemostasis, which has been suggested to induce ER (stress)-dependent apoptosis [228–230].
Using ceramide-specific antibodies, we have shown that ceramide is present in ER-associated membranes, such as the perinuclear membrane and MAMs [42,231]. The nucleus and mitochondria themselves contain ceramide; although it is not entirely clear whether ceramide is synthesized in these organelles or transported from the ER as indicated in Figure 2 [232–236]. Recent studies suggest that ceramide is involved in the initiation of apoptosis by forming pores in the outer mitochondrial membrane [237].
After ceramide has been transported to the Golgi, the polar head group flips from the outer (cytosolic) to the inner (luminal) leaflet before sphingomyelin is synthesized. This transbilayer movement appears to occur at a rapid rate, although it may be regulated and will then become a rate-limiting step [27]. In the Golgi lumen, sphingomyelin (Figure 1) is synthesized by sphingomyelin synthase (SMS)1 and then transported to the cell membrane (Figure 2) [238–240]. SMS1 is distinct from SMS2, which regenerates sphingomyelin from ceramide in the cell membrane [241,242].
Ceramide & sphingomyelin: interfacing sphingolipid, phospholipids & C1 metabolism
Sphingomyelin synthase 1 and 2 utilize phosphatidylcholine (PC) and ceramide as substrates to generate sphingomyelin and DAG in a phospho(ryl)choline transfer reaction. The SMS reaction links major metabolic pathways with cell-signaling function (Figure 5). We have discussed that DAG is an activator of PKCα. It can be used to regenerate PC from CDP-choline (CDP-choline or Kennedy pathway), which can be regenerated from phospho(ryl)choline (via CTP:phosphocholine cytidylyltransferase), a product of sphingomyelin hydrolysis by sphingomyelinase (SMase) (Figure 5A) [243–248].
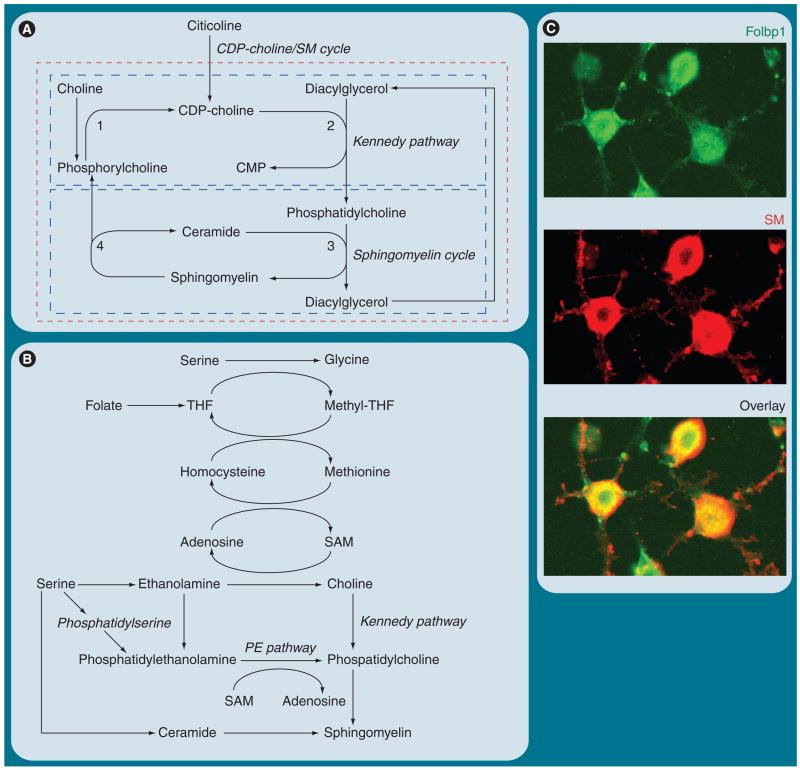
Figure 5. Interconnection of phospholipid, C1 (one carbon unit) and sphingolipid metabolism.
(A) The ‘CDP-choline/sphingomyelin (SM) cycle’ is a potential interconnection of the Kennedy (CDP-choline) pathway for phosphatidylcholine biosynthesis and the SM cycle. This cycle is sustained by supply with choline or CDP-choline (citicoline), which converts ceramide into SM. 1: CTP:phosphocholine cytidylyltransferase. 2: CDP-choline:DAG cholinephosphotransferase. 3: SM synthase. 4: Sphingomyelinase. (B) The biosynthesis of choline is mediated by C1 metabolism, which depends on the supply of folate as cofactor for methylation reactions. Folate deficiency or perturbation of C1 metabolism may impair SM biosynthesis via the proposed CDP-choline/SM cycle. (C) Embryonic stem cell-derived neural progenitors express Folbp1 concurrent with SM. The immonocytochemistry was performed with anti-Folbp1 antibody and lysenin/anti-lysenin antibody [Bieberich, Unpublished Data]. Lysenin is an earthworm protein that binds specifically to sphingomyelin microdomains.
CMP: Cytidine monophosphate; Folbp1: Folate-binding protein 1; PE: Phosphatidyl ethanolamine; SAM: S-adenosyl methionine; THF: Tetrahydrofolate.
Interestingly, de novo synthesis of PC as a substrate for sphingomyelin biosynthesis is immediately linked to methylation reactions of C1 (one carbon unit) metabolism (Figure 5B). One would not expect a shortage of PC since it is abundant in our nutrition and is also synthesized by the liver. However, studies on primary-cultured oligodendrocytes have shown that a major portion of PC is de novo synthesized from phosphatidylserine by decarboxylation and methylation reactions (Figure 5B) [249,250]. Therefore, a supply of methyl group donors appears to be essential for sphingomyelin biosynthesis and could explain why deficiency of folate, an essential cofactor for the generation of the methyl group donor S-adenosyl methionine (Figure 5B) contributes to neural tube defects. In fact, studies in our laboratory [Bieberich, Unpublished Data] show that embryonic stem (ES) cell-derived neural progenitor cells that express folate-binding protein 1 are also enriched for sphingomyelin (Figure 5C). These results are consistent with studies from our [Bieberich, Unpublished Data] and other groups demonstrating that perturbation of C1 and choline metabolism reduces the level of PC and sphingomyelin in stem cells and mouse embryos, and leads to neural tube defects [251,252]. In summary, concurrent PC and sphingomyelin biosynthesis sustained by C1 metabolism and the postulated CDP-choline/sphingomyelin cycle appear to be important pathways to regenerate sphingomyelin and to ‘detoxify’ and recycle pro-apoptotic ceramide. Perturbation of these pathways may lead to aberrant stem cell differentiation and embryo malformation. CDP-choline or citicoline is currently in clinical trials to treat stroke and cognitive impairment, which may be based on its function as a precursor substrate for sphingomyelin biosynthesis [253].
Another interesting area pursued in our laboratory is the regulation of sphingomyelin biosynthesis by cholesterol derivatives such as oxysterols and bile acids. We found that sphingomyelin biosynthesis in human breast cancer cells is elevated by incubation with 25-hydroxy-cholesterol, concurrent with increased migration of these cells [254]. These results were consistent with previous studies reporting coregulation of cholesterol and sphingolipid transport and metabolism [255]. In a follow-up study, cell survival and migration of human breast cancer (MDA-MB-231) cells was also found to be enhanced by the secondary bile acid deoxycholate at physiological plasma concentrations (5–10 μM) [256]. This was very surprising since a wealth of literature describes induction of apoptosis in cancer cells by deoxycholate, probably by activation of SMase and elevation of ceramide [257,258]. However, these studies used deoxycholate at concentrations that were more than tenfold higher than the concentration used in our study. It is quite possible that depending on its concentration, deoxycholate activates or inhibits cell survival in breast cancer cells, which may be mediated by the regulation of ceramide and sphingomyelin biosynthesis.
Previously, ceramide and sphingomyelin have been described as part of the sphingomyelin cycle, however, without invoking CDP-choline and DAG in the Kennedy pathway for PC biosynthesis as intermediate substrates (Figure 5A) [56]. The sphingomyelin cycle links growth factor and cytokine-induced activation of SMase to the interconversion of sphingomyelin and ceramide microdomains/rafts and vice versa [259,260]. There are two main groups of SMases, depending on their enzyme activity at neutral or acidic pH [261]. ASMases are localized in lysosomes or secreted via the Golgi secretory pathway [147]. Deficiency of lysosomal ASMase causes Niemann–Pick disease, a lysosomal storage disease. The secreted form of ASMase associates with the outer leaflet of the cell membrane and is activated by proapoptotic cytokines, such as TNF-α or Fas ligand [105,147]. In addition, ASMase is activated by UV or γ-irradiation, probably via the radiation-induced generation of reactive oxygen species [146,262,263]. Neutral SMases (NSMases) can also be activated by TNF-α; however, these enzymes are located at the inner leaflet. Any activation of NSMase will require flipping the polar head group of sphingomyelin toward the inner leaflet before hydrolysis to ceramide can occur. Currently, it is not completely understood how this transfer of the polar head group is achieved. It is thought that a phospholipid-binding protein, a ‘scramblase’ or ‘flippase’, transfers sphingomyelin to the inner membrane leaflet, while at the same time externalizing phosphatidylserine [264–266]. This model would be consistent with phosphatidylserine externalization being an early event in the onset of apoptosis induced by ceramide.
The activation of NSMase has found recent attention owing to its potential link to the binding of NGF to the low affinity neurotrophin receptor p75NTR [259,267–269]. In contrast to the cytokine-activated receptors (e.g., Fas/CD95 and TNF receptor), the p75NTR cell-signaling pathway is not a priori proapoptotic. p75NTR is expressed by many neural cell types and induces axonal outgrowth in the peripheral nervous system when stimulated with NGF [267,270–273]. However, evidence has amounted that p75NTR-induced apoptosis is a major factor in neuro-degenerative diseases such as Alzheimer’s disease [269,274–281]. This apparently paradoxical, dual function of p75NTR, proapoptotic or pro-outgrowth, has been explained by a model suggesting that the effect of p75NTR activation depends on heterodimerization with other neurotrophin receptors [282–292]. If p75NTR forms heterodimers with tropomyosin-related kinase A, a tyrosine kinase receptor, activation of the chimeric receptor induces axonal outgrowth. However, if p75NTR forms homodimers, binding of NGF to p75NTR activates NSMase, which then generates ceramide and may induce apoptosis.
Most recently, our group has found that p75NTR is highly expressed in the hippocampus of the PS1M146V (early-onset Alzheimer’s disease model) mouse. p75NTR has been discussed as a substrate for the γ-secretase subunit of the PS1 or 2 protein complex [293,294]. A deficiency of this secretase could lead to accumulation of p75NTR and, in turn, increased activation of NSMase. In conjunction with elevation of the lass 2 or 4 gene products, this would be consistent with elevation of long-chain fatty acid ceramide from sphingomyelin and ceramide-induced apoptosis of astrocytes as discussed in the preceding section [224]. Dysregulated receptor turnover may lead to conversion of sphingomyelin to ceramide rafts and, in turn, induce apoptosis in a neurodegenerative disease process. In the following section, further examples for the cell-signaling function of ceramide will be discussed as well as how the generation of a ceramide microdomain is translated into a protein-driven cell-signaling cascade.
Rafts-to-SLIPS hypothesis: nucleating the lipid–protein interactome
The translation of lipid formation into the activation of a particular cell-signaling pathway demands for a mechanism that engages raft-associated lipids into a complex with proteins, the lipid–protein interactome. We introduce the term nucleation to describe a two-step mechanism that is initiated by the association of a lipid with a protein (step 1), which induces a protein–protein interaction to form a larger lipid–protein complex, or interactome (step 2). The working models discussed in this section are based on ideas and studies that originated in our laboratory; however, they should be considered hypothetical until substantiated by further experiments. To date, there are two processes known by which membrane lipids nucleate this interactome resulting from the initial association of a lipid with a protein. Proteins themselves can be covalently conjugated to lipids such as the glycosylphosphatidylinositol-anchor and cholesteryl, geranyl, farnesyl, myristyl or palmitoyl residues [125,139]. Prominent examples relevant to stem cell differentiation and cancer cell proliferation are the morphogen Sonic hedgehog, which is covalently linked to cholesterol and palmitic acid, and the small GTPase Ras, which is modified by farnesylation [125,295]. We and other groups have shown that Ras codistributes with glycosphingolipid rafts that contain GM1 and cholesterol [254]. Blocking farnesylation with farnesyltransferase inhibitors or statins that inhibit hydroxymethyl glutaryl CoA reductase, the enzyme essential for the biosynthesis of farnesyl and cholesterol precursors, will prevent raft localization of Ras [254]. We have shown that these pharmacologic inhibitors reduce migration of breast cancer cells, which indicates that farnesyltransferase inhibitors as well as statins are potential anticancer drugs. Indeed, farnesyltransferase inhibitors as well as statins are currently being tested in clinical Phase I, II and III trials to treat various types of cancer, including lung and mammary carcinoma, lymphoma and melanoma [296–300].
Nucleation of a lipid–protein interactome by lipid modification and raft association of Ras is one way of activating client cell-signaling pathways that induce cell proliferation and migration. The second mechanism relies on nucleation by noncovalent interaction of a protein with a lipid. Approximately 10 years ago, the PH domain was described as the first protein domain specifically binding to PIPs, in particular PIP2 and 3 [301,302]. Currently, a variety of additional PIP-binding domains have been characterized and found to be a part of essential signaling factors, such as small GTPases, Akt/PKB and many others [303–305]. Consistent with the function of these factors, PIPs have been known for a long time to activate cell-signaling pathways for cell polarity, migration and proliferation. However, in contrast to bona fide raft lipids such as sphingomyelin, which contain only saturated or monounsaturated fatty acid residues, PIPs are mostly endowed with polyunsaturated fatty acids [306]. These fatty acid chain ‘kinks’ prevent the proper alignment and clustering of PIPs in rafts. To date, it is still not clear whether raft or nonraft association of PIPs is important for their function.
One of the proteins regulated by PIPs is aPKC, the PKC isoform that is also associated with ceramide as discussed in one of the preceding sections. The N terminus of aPKC contains a protein-binding (PB1) domain that interacts with the polarity protein Par6, which associates with the Rho type GTPase Cdc42 (Figure 4). Recently, conditional knockout of Cdc42 in murine neural progenitor cells has shown that it is indispensable for cell polarity in the neuroepithelium and normal brain development [307]. Cdc42 is endowed with a PH domain, which allows for binding to PIP and recruitment of the Par6–aPKC complex (Figure 4) [308–313]. Conditional knockout of aPKC in neural progenitor cells leads to a phenotype that is similar to that of the Cdc42 knockout, suggesting that cell polarity is dependent on a Cdc42–Par6–aPKC polarity complex, the formation of which is regulated by PIPs [91].
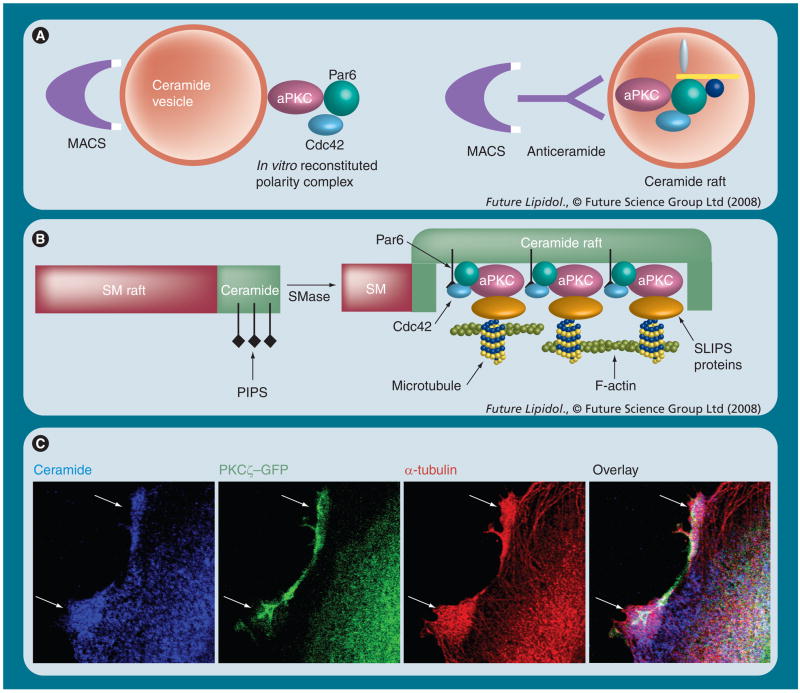
Based on the fundamental significance of cell polarity for development, the Cdc42–Par6–aPKC complex should already be initiated when cell polarity is established in the first primitive germ layer, the primitive ectoderm. Recently, we found that this polarity complex is critical for primitive ectoderm formation and depends on ceramide [28]. Inhibition of de novo sphingolipid biosynthesis with myriocin prevented primitive ectoderm formation, while incubation with C16 ceramide or the novel ceramide analogue S18 restored this process. In the absence of ceramide, aPKC and Cdc42 were no longer translocated to the membrane and they did not form a protein complex. These results suggested that ceramide is critical for the formation of the aPKC and Cdc42-containing polarity complex. We then performed an in vitro binding assay using ceramide vesicles and purified proteins (Figure 6A, left panel). Ceramide vesicles bound aPKC, Par6, and Cdc42 when Cdc42 was activated with GTPγS, but not when it was inactivated with GDP [28]. A similar assay is currently used in our group to identify additional ceramide raft-binding proteins (Figure 6A, right panel). These results confirmed the importance of ceramide for the formation of a polarity complex, which is depicted in Figures 4 & 6B. These figures show our proposed model involving PIPs and ceramide in activation of Cdc42 and recruitment of the polarity complex.
Figure 6. Isolation, metabolic integration and visualization of sphingolipid rafts and SLIPS.
(A) The lipid vesicle-mediated affinity chromatography (LIMAC) technique was developed in our laboratory to isolate ceramide-associated protein complexes. Originally, the lipid vesicles were made of ceramide and phosphatidylserine (PS), which allowed for MACS using magnetic particle-conjugated annexin V (highly affine to PS) to isolate the vesicles and their associated proteins. We have used the LIMAC technique for the in vitro reconstitution of a ceramide-associated polarity complex (left panel) and the isolation of ceramide-binding proteins from cell lysates. LIMAC can be modified using other interaction partners for the isolation of the vesicles (e.g., [glycol]lipid-specifc lectins or antibodies; right panel). (B) Hypothesis: activation of SMase converts SM into ceramide, which segregates into lipid microdomains with higher membrane fluidity. PIP2 or 3 embedded in the ceramide microdomain may bind to Cdc42 (via PH domain), while ceramide-associated aPKC binds to Par6. The formation of a SLIPS then regulates the dynamics of the cytoskeleton via additional proteins such as glycogen synthase kinase-3β or adenomatous polyposis coli (Figure 8). This may sustain ceramide-rich platforms (e.g., in the apical membrane of primitive ectoderm cells) or initiate processes (sphingopodia) in neural stem and progenitor cells. (C) Imaging of ceramide, aPKC and associated microtubules using the respective antibodies for confocal immunuofluorescence microscopy with fixed neural progenitor cells [Wang, Krishnamurthy, Bieberich, Unpublished Data]. Ceramide-rich processes have been termed ‘sphingopodia’ and are proposed to be initiated by SLIPS.
aPKC: Atypical PKC; GFP: Green fluorescent protein; MACS: Magnetic-activated cell sorting; PIP: Phosphatidylinositolphosphate; SLIPS: Sphingolipd (here ceramide)-induced protein scaffold; SM: Sphingomyelin; SMase: Sphingomyelinase.
A polarity complex, the nucleation of which is initiated by ceramide, is the role model for a sphingolipid-induced protein scaffold (SLIPS) (Figure 6B), a term we introduced several years ago to explain how ceramide may regulate the cytoskeleton [25]. A SLIPS is an interface linking the ‘lipid world’ (ceramide microdomain) to the ‘protein world’ (cytoskeleton or cell-signaling cascades). Although this is still a matter of our ongoing research, the ceramide-associated Cdc42–Par6–aPKC complex converting ceramide raft formation into the stabilization/regulation of F-actin or microtubules is one of the proposed functions of SLIPS. The immunocytochemistry for ceramide and α-tubulin (detected with anti-ceramide and antitubulin antibodies) in PKCζ–GFP-expressing neural progenitor cells is shown in Figure 6C. There is significant codistribution of ceramide and tubulin (arrows), which confirms previously published data that suggest ceramide/aPKC-dependent regulation of microtubule formation [25]. Other functions of SLIPS depend on the ceramide/aPKC interactome, a group of aPKC-associated proteins, the interaction of which is potentially regulated by binding of ceramide to aPKC. A selection of proteins that are mainly involved in the regulation of cell polarity, migration and adhesion is depicted in Figure 7A. Additional factors associated with or phosphorylated by aPKC are involved in vesicular transport (e.g., p62), alternative splicing of mRNA (e.g., caspase 2) and receptor activation (e.g., ZIP3) [72,83,314–316]. Some of these functions may very well be regulated by the association of aPKC with ceramide. Our group has focused on the effect of ceramide on aPKC-dependent regulation of cell polarity and apoptosis in stem and cancer cells [25,28,42,43,79–82]. The proapoptotic effect is mainly based on the ceramide-induced association of aPKC with prostate apoptosis response (PAR)-4 and Akt, two signaling factors that will be discussed in the following section.
Figure 7. Ceramide–atypical PKC interactome and the flipside model of ceramide (inter)action.
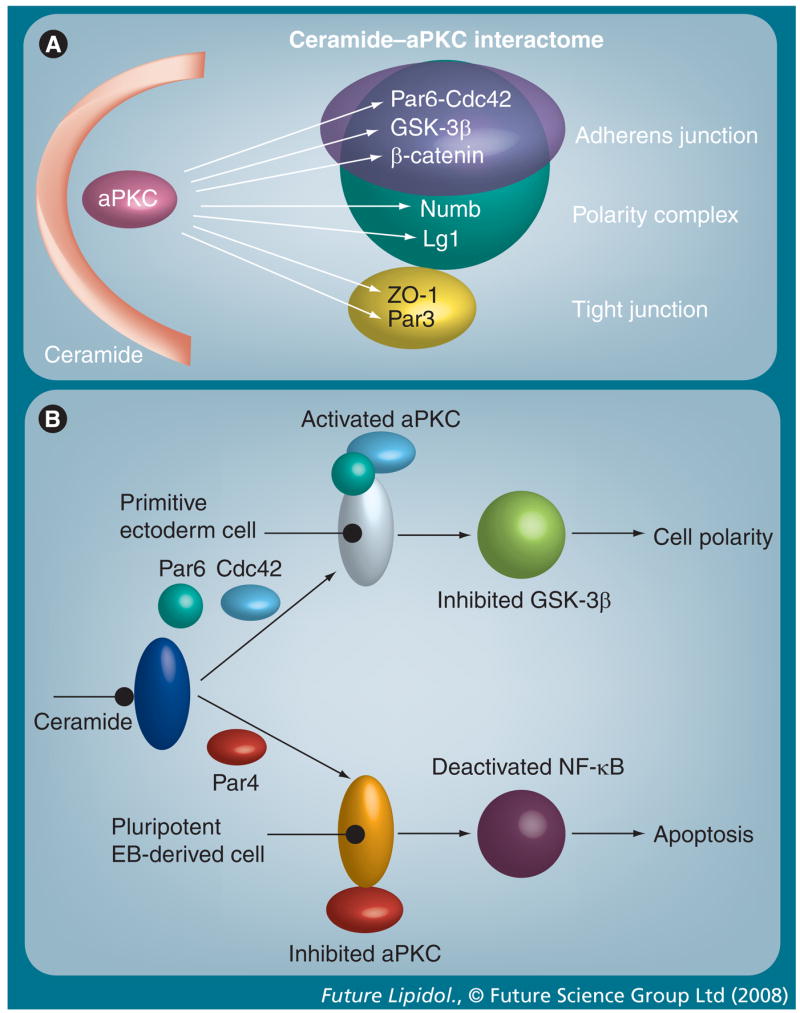
(A) Ceramide-associated aPKC binds and/or phosphorylates a variety of junctional or polarity proteins, which is suggested to regulate cytoskeletal dynamics and cell adhesion. (B) ‘Flipside’ model of ceramide-induced aPKC activation or inhibition. If prostate apoptosis response (PAR)-4 is not expressed, ceramide-associated aPKC forms a polarity complex with Par6 and Cdc42 (upper panel). This may inhibit GSK-3β and control cell polarity or process formation as described in the legend of Figure 8 (top panel). In the presence of PAR-4, ceramide-associated aPKC binds to PAR-4, which inhibits its activity and induces apoptosis (bottom panel).
aPKC: Atypical PKC; EB: Embryoid body; GSK-3β: Glycogen synthase kinase 3β; NF-κB: Nuclear factor-κB.
Ceramide & S1P: close relatives, but conditional antagonists
From the previous sections, it is clear that the function of ceramide is modified by the cell signaling context. The main theme and hypothesis originally developed in our group is that the outcome of ceramide elevation depends on the protein, the association of which with aPKC is induced or strengthened by ceramide. The lipid vesicle-binding assays with purified or cell lysate-derived proteins show that ceramide-associated aPKC will bind to Par6–Cdc42 or PAR-4, most likely depending on their expression level, activation state or accessibility [28,42]. We termed the mechanistic model describing the outcome of ceramide-induced association of aPKC with either Par6–Cdc42 or PAR-4 the ‘flipside model of ceramide/aPKC interaction’ (Figure 7B). Binding to Par6–Cdc42 will initiate the polarized stabilization of actin filaments or microtubules and sustain cell polarity or cell migration, while association with PAR-4 will lead to cell death. This opposite effect is not surprising since Cdc42 is known to activate aPKC, while PAR-4 inhibits the enzyme. Hence, even ceramide-induced pre-activation of aPKC may eventually lead to its inhibition if PAR-4 is expressed (Figure 7B). As with SLIPS, this model is considered to be hypothetical until further experimental evidence has been achieved.
Our model is consistent with studies from our laboratory showing that the nonapoptotic function(s) of ceramide depend on a low expression level of PAR-4 [28,42,80,82]. PAR-4 is a leucine zipper protein with several functions. It was discovered by differential hybridization assays to identify proapoptotic genes expressed in androgen-dependent prostate cells [317]. Using two-hybrid assays it was soon found to be an inhibitor of aPKC and a transcriptional corepressor of Wilms’ tumor suppressor 1 [318,319]. PAR-4 has recently gained attention owing to its multifaceted function in neural cells. It has been suggested to contribute to neurodegeneration in Alzheimer’s and Parkinson’s diseases, and to the etiology of amyotrophic lateral sclerosis and stroke [291,320–324]. We have found that there are three stages during ES cell differentiation at which expression of PAR-4 is absent or low: undifferentiated ES cells, ES cell-derived suspension embryoid bodies (EBs) and EB-derived neural progenitor cells [28,42,80–82]. In these differentiation stages of stem cells, ceramide or ceramide analogues are nonapoptotic (undifferentiated ES cells), sustain cell polarity and primitive ectoderm formation (suspension EBs), or induce sphingopodia and process formation (neural progenitor cells). In other differentiation stages, such as early pluripotent EB-derived cells, PAR-4 is highly expressed and conveys sensitivity to ceramide or its analogues. In PAR-4(−) suspension EBs, ceramide is indispensable for normal development. Ceramide depletion prevents membrane association of aPKC, disrupts the interaction between aPKC and Cdc42 and results in decreased phosphorylation of glycogen synthase kinase (GSK)-3β [28]. The novel ceramide analogue S18 restores primitive ectoderm formation, suggesting that it is ceramide and not one of its derivatives that regulates cell polarity. Although this implies a regulatory effect of ceramide on the noncanonical Wnt or cell-polarity pathway, further studies are needed to clarify the significance of ceramide for cell polarity in a wider spectrum of cells.
We propose the working model to explain the function of ceramide for cell polarity in neural progenitor cells and other cell types as shown in Figure 6B. Based on our observation that ceramide microdomains codistribute or even associate with microtubules (Figure 6C) [25,231], we focus on the effect of ceramide-associated aPKC on GSK-3β. GSK-3β phosphorylates many proteins that regulate cell adhesion or formation of microtubules. Key factors are β-catenin, adenomatous polyposis coli (APC) and tau protein (Figure 8A) [309,325–332]. Phosphorylation of β-catenin by GSK-3β renders it susceptible to proteolytic degradation [309], while hyperphosphorylation of tau causes its aggregation in tauopathy, a neurodegenerative disorder that is also involved in the etiology of Alzheimer’s disease [325,329,330]. Phosphorylation of APC by GSK-3β disrupts its function in stabilizing the plus end of microtubules [309]. According to our model, ceramide-induced activation of aPKC will result in phosphorylation and/or inactivation of GSK-3β (Figure 8A). Hence, ceramide and S18 should stabilize microtubules, while ceramide depletion will destabilize them.
Figure 8. Interdependence of ceramide and S1P metabolism and cell-signaling pathways (hypothesis).
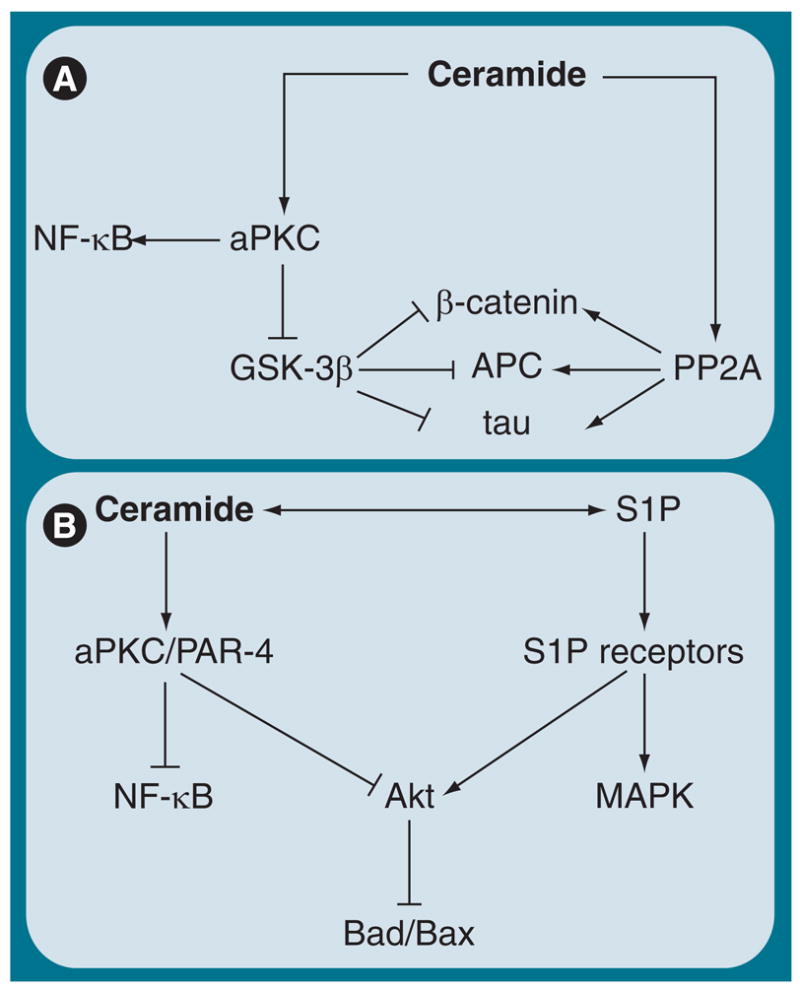
(A) In the absence of PAR-4, ceramide activates aPKC, which may inactivate GSK-3β and stabilize β-catenin, increase binding of APC to the plus ends of microtubules and prevent aggregation of tau. Ceramide-activated PP2a may complement this effect of ceramide on aPKC. (B) In PAR-4-expressing cells, ceramide induces inhibition of aPKC by PAR-4, which may inactivate PI3K/Akt and NF-κB, two major cell-signaling pathways for cell survival in cancer and stem cells. Inhibition of Akt prevents inactivation of Bad/Bax and induces apoptosis. This is antagonized by S1P-mediated activation of Akt.
aPKC: Atypical PKC; APC: Adenomatous polyposis coli; GSK-3β: Glycogen synthase kinase 3β; NF-κB: Nuclear factor-κB; PAR: Prostate apoptosis response; PP2a: Protein phosphatase 2a; S1P: Sphingosine-1-phosphate.
Among the factors that regulate adherens junctions and microtubules, ceramide-activated aPKC and PP2a may complement each other (Figure 8A). It is known that PP2a dephosphorylates β-catenin, APC and tau [326,330]. Hence, loss of phosphorylation by ceramide-mediated inactivation of GSK-3β and enhanced dephosphorylation by ceramide-activated PP2a should act synergistically on promoting the stability of microtubules. Interestingly, GSK-3β can also phosphorylate and inactivate PP2a [333]. Therefore, ceramide can activate PP2a in two ways: by direct binding to PP2a and by inactivating GSK-3β (via aPKC). By contrast, ceramide-activated PP2a may also dephosphorylate GSK-3β, thereby antagonizing phosphorylation of GSK-3β [334]. Clearly, more studies on the ceramide-dependent interplay of aPKC, GSK-3β and PP2a are needed to unravel these intricate regulatory cell-signaling pathways.
When the proapoptotic form of PAR-4 is expressed, the nonapoptotic effect of ceramide changes fundamentally. Using lipid vesicles consisting of ceramide and phospholipids as described before (the lipid vesicle-mediated affinity chromatography [LIMAC] technique, Figure 6A) we have found that association of aPKC with ceramide will enhance the affinity of aPKC to its inhibitor PAR-4 [42]. In the presence of PAR-4, ceramide will not activate aPKC, but on the contrary, enhance its inhibition by PAR-4 (Figures 4 & 8B). Because of this, an initial non-apoptotic or even prosurvival function of ceramide can rapidly turn into the induction of apoptosis. Most recently, our group has found that in astrocytes from the PS1M146V mouse, the expression of PAR-4 was almost tenfold higher than in wild-type astrocytes [224]. These cells showed tremendously enhanced apoptosis (>tenfold) when incubated with C20 ceramide, which was elevated in the hippocampus of the early-onset Alzheimer’s disease mouse model, as discussed previously. Wild-type astrocytes (and neurons) were not affected, clearly showing that the elevated expression of PAR-4 sensitizes neural cells toward ceramide-induced apoptosis. These data are consistent with previous reports demonstrating the involvement of PAR-4 in the etiology of Alzheimer’s disease [335]. However, our data extended the earlier studies, in that we demonstrated that aberrant expression of PAR-4 contributes to ceramide-induced glial cell death in a mouse model for Alzheimer’s disease.
Our data are also consistent with a recent study reporting that transgenic expression of an active fragment of PAR-4 (SAC domain) protects mice from induced and spontaneous tumors as a result of apoptosis-mediated elimination of cancer cells [336]. We have found that the expression of PAR-4 not only sensitizes stem cells and astrocytes toward ceramide-induced apoptosis, but also particular cancer cells. Interestingly, the aPKC isoform PKCλ/τ has been identified as an oncogene in multiple forms of human cancer [86]. The striking difference between PKCλ/τ and PKCζ is that the first one does not contain the caspase 3 cleavage site that converts PKCζ into the catalytically active 50 kDa form. Therefore, it is possible that PKCλ/τ can be inhibited by ceramide-induced apoptosis when the expression of PAR-4 is elevated. Hence, concurrent elevation of ceramide and expression of PAR-4 may be a potential strategy to treat cancer by inducing apoptosis.
In addition to its immediate cell-signaling function, ceramide serves as a metabolic precursor for another important cell-signaling lipid, S1P. Ceramide is hydrolyzed by ceramidase to sphingosine, which is then phosphorylated by sphingosine kinase (SK)1 or 2 to S1P (Figures 1 & 2) [13,337–343]. S1P is a soluble ligand that can bind and activate five isoforms of the S1P receptor [29,50–52,109,344–346]. Knockout mice for SK1 and 2 or S1P receptors have clearly demonstrated the essential function of S1P for vascular and neural development [339]. Recent studies indicate that one of the functions of S1P is to counterbalance ceramide-induced apoptosis [341,347,348]. S1P is known to increase phosphorylation of p42/44-MAPK and Akt/PKB, two important protein kinases that inactivate the proapoptotic proteins Bad and Bax (Figure 8B) [25,69,81,344,349–354]. Unlike S1P, ceramide has been shown to reduce the activity of p42/44-MAPK and Akt [40,81,354,355]. Hence, it is tempting to speculate that ceramide and S1P counter-regulate the phosphorylation of Bax and Bad, thereby controlling apoptosis and cell survival (Figure 8B). This may be of importance for the pharmacologic interference with neurodegenerative diseases linked to ceramide-induced apoptosis and for treatment of cancer. In the first case, FTY720 or S1P-receptor agonists may counteract ceramide-induced neurodegeneration, while in the second case, cancer cells may be sensitized to ceramide-induced apoptosis by concurrent inhibition of S1P biosynthesis or S1P-receptor antagonists. Indeed, FTY720 has been shown to promote oligodendrocyte differentiation, and it is currently being tested in Phase III clinical trials to treat multiple sclerosis [356,357]. Although this effect has been attributed to the immunosuppressive activity of the drug, neuro-protection may be another beneficial outcome of FTY720 treatment.
This hypothesis is consistent with data from our laboratory showing that S1P and FTY720 protect a small population (≤5%) of neural precursor cells (NPCs), termed NPC2, from ceramide-induced apoptosis [358]. We routinely incubate EB-derived cells with S18 to eliminate residual pluripotent stem cells that will form stem cell-derived tumors if they contaminate the stem cell graft [82]. This method has been recognized as a significant step toward safer stem cell therapy [359–362]. In contrast to the major population of nestin(+)/PAR-4(−) NPC1 cells, NPC2 cells maintain the expression of PAR-4 and are eliminated by incubation with S18 [358]. These cells are protected by adding S1P or FTY720, which underlines the importance of S1P as a metabolically derived antagonist of ceramide (Figure 8B). We are currently investigating the potential of NPC2 cells for neural differentiation after grafting.
While FTY720 may be useful to boost the survival and differentiation of transplanted or endogenous stem and progenitor cells to treat neurodegenerative diseases, inhibition of SK will be a critical step toward cancer treatment by inhibiting cell S1P-dependent survival pathways (Figure 8B) [363–365]. The classical SK inhibitor dimethylsphingosine has already been shown to reduce tumor growth in mouse models, and novel SK inhibitors are currently being developed and tested [366–368]. However, while SK1 generates antiapoptotic S1P, SK2 has been found to generate S1P that is converted to proapoptotic ceramide. Therefore, cancer therapy by inhibition of S1P biosynthesis should specifically target SK1. In conjunction with drugs that elevate ceramide, SK1 inhibitors are promising agents for future cancer therapy. Taken together, the examples discussed in this article clearly demonstrate that ceramide and its derivatives have taken center stage in cell signaling and will certainly surprise us with novel therapeutic interventions in stem cell and cancer therapies.
Conclusion
The discussion of various aspects of ceramide and sphingolipid biology has demonstrated that research in this field is indeed an example of systems interface biology, a genuine multi-disciplinary approach that attempts to explain cell signaling by sphingolipid metabolism, membrane biophysics, lipid–protein binding and downstream effects on cell polarity, proliferation and apoptosis. Ceramide as a structural interface has been recognized to generate cell-signaling platforms in the plasma membrane (and other subcellular compartments) that translate cues from diffusible factors (e.g., growth factors and cytokines) into the localized and organized activation of intracellular cell-signaling cascades. Ceramide as a metabolic interface has been suggested to interconnect C1, cholesterol, phospholipid and sphingolipid metabolism. Finally, ceramide has been shown to be a signaling interface in that its immediate derivatives are co- or counter-players in regulating cell survival, polarity and differentiation versus apoptosis.
Executive summary
Ceramide & its derivatives: from metabolism to cell signaling
Many metabolic derivatives of ceramide have similar cell-signaling functions. To identify a cell-signaling pathway that is specifically regulated by ceramide, it is indispensable to define the signaling proteins with which ceramide interacts.
Ceramide-binding proteins: from interaction to action
Among ceramide-binding proteins, ceramide-activated protein phosphatases and kinases are the most important. Specific protein phosphatases and kinases have been shown to be activated by ceramide, which includes atypical PKC (aPKC)-dependent cell-signaling pathways for cancer and stem cell polarity and apoptosis.
The rising of the raft: interfacing membrane biophysics & cell signaling
Lipid microdomains or rafts have been proposed to constitute cell-signaling platforms, although their function in living cells is still debated. Ceramide microdomains have been suggested to be segregated from sphingomyelin (SM) rafts, although they can be derived from them. Ceramide microdomain-induced cell-signaling pathways are implicated in the induction of apoptosis.
Sphingolipid biosynthesis in the endoplasmic reticulum: enzymology & pharmacology
Following biosynthesis by the de novo or salvage pathway in the endoplasmic reticulum, ceramide is converted to SM in the Golgi. Sphingolipid biosynthesis can be blocked by two pharmacologic inhibitors, myriocin and fumonisin B1. Ceramide analogues can restore the function of ceramide.
Sphingolipid metabolism in the Golgi: ceramide transport & exit to the cell membrane
SM is transported to the cell membrane and serves as a substrate for the generation of ceramide microdomains, which is regulated by the SM cycle. This cycle can be interconnected with major pathways for lipid metabolism.
Ceramide & sphingomyelin: interfacing sphingolipid, phospholipid & C1 metabolism
The interconnection of the SM cycle with lipid metabolism involves the biosynthesis of CDP-choline and phosphatidylcholine (Kennedy pathway). The proposed ‘CDP-choline/SM cycle’ uses methyl donor groups provided by the C1 (one carbon unit) metabolism and regulates phospholipid and sphingolipid biosynthesis, which is important for the generation of lipid rafts.
Rafts-to-SLIPS hypothesis: nucleating the lipid–protein interactome
When generated by hydrolysis of SM, a ceramide raft-signaling platform will still need to interface with intracellular signaling pathways. Our group has proposed, for the first time, the term sphingolipid-induced protein scaffolds (SLIPS) that link ceramide microdomains via aPKC (or other ceramide-associated proteins) to polarity protein complexes and regulation of the cytoskeleton.
Ceramide & sphingosine-1-phosphate: close relatives, but conditional antagonists
Sphingosine-1-phosphate (S1P) is another derivative of ceramide with pro-proliferative and antiapoptotic function. It may antagonize ceramide by preventing inactivation of cell-survival pathways such as Akt/PKB. This antagonism can be used to prevent apoptotic, degenerative processes (using S1P analogues and neutralizing ceramide) or to eliminate cancer cells (enhancing ceramide and inhibiting S1P) in future combination therapies.
Future perspective
Translational research has always been important but has gained tremendous attention in the past few years. The purpose of translational research is to translate the results of basic research into clinical application. Ceramide and other sphingolipids are ideally suited for translational research in that they are small, lipophilic molecules regulating basic cell biology. Once the function of a sphingolipid has been determined, it is an immediate structural lead for drug development. In comparison, finding a pharmacological effector for a specific protein–protein interaction can be a daunting task. Ceramide is already known to regulate protein–protein interactions. Therefore, lipidology of ceramide and other sphingolipids is rewarding and will be full of surprises. To be of translational relevance, future research will also focus on the in vivo function of ceramide. Most of our knowledge on ceramide was obtained from cell culture experiments. However, genetic models, such as knockout mice for enzymes in sphingolipid metabolism, will be significant to define novel functions and pharmacologic targets in ceramide biology. From the examples discussed in this article it is clear that understanding sphingolipids will be an important avenue for future therapeutics in cancer and stem cell biology.
Acknowledgments
This work is supported by NIH grant R01NS046835 and the Susan Komen Breast Cancer Research Foundation grant BCTR0600658. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The author thanks Dr Rhea-Beth Markowitz for critically reading and editing this manuscript.
Footnotes
Websites
401. Lipid Metabolites and Pathways Strategy www.lipidmaps.org
Financial & competing interests disclosure
Bibliography
- 1.Sourkes TL. Thudichum’s Successors. Neurochem Res. 2007;32:1808–1812. doi: 10.1007/s11064-006-9182-z. [DOI] [PubMed] [Google Scholar]
- 2.Merrill AH, Jr, Schmelz EM, Dillehay DL, et al. Sphingolipids – the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 3.Sullards MC, Allegood JC, Kelly S, et al. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography–tandem mass spectrometry: ‘inside-out’ sphingolipidomics. Methods Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 4.Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35:W606–W612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoetzl S, Sprong H, van Meer G. The way we view cellular (glyco)sphingolipids. J Neurochem. 2007;103(Suppl 1):3–13. doi: 10.1111/j.1471-4159.2007.04721.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Brocklyn JR. Sphingolipid signaling pathways as potential therapeutic targets in gliomas. Mini Rev Med Chem. 2007;7:984–990. doi: 10.2174/138955707782110123. [DOI] [PubMed] [Google Scholar]
- 7.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 8.Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, Kollmeyer J, Symolon H, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 11.Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, Levade T. Sphingolipids as modulators of cancer cell death: potential therapeutic targets. Biochim Biophys Acta. 2006;1758:2104–2120. doi: 10.1016/j.bbamem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 14.Milstien S, Gude D, Spiegel S. Sphingosine 1-phosphate in neural signalling and function. Acta Paediatr Suppl. 2007;96:40–43. doi: 10.1111/j.1651-2227.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 15.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dbaibo GS, Hannun YA. Signal transduction and the regulation of apoptosis: roles of ceramide. Apoptosis. 1998;3:317–334. doi: 10.1023/a:1009668802718. [DOI] [PubMed] [Google Scholar]
- 17.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 18.Perry DK, Hannun YA. The role of ceramide in cell signaling. Biochim Biophys Acta. 1998;1436:233–243. doi: 10.1016/s0005-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 20.Merrill AH, Jr, Sullards MC, Wang E, Voss KA, Riley RT. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ Health Perspect. 2001;109(Suppl 2):283–289. doi: 10.1289/ehp.01109s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buccoliero R, Futerman AH. The roles of ceramide and complex sphingolipids in neuronal cell function. Pharmacol Res. 2003;47:409–419. doi: 10.1016/s1043-6618(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 22.Venkataraman K, Futerman AH. Ceramide as a second messenger: sticky solutions to sticky problems. Trends Cell Biol. 2000;10:408–412. doi: 10.1016/s0962-8924(00)01830-4. [DOI] [PubMed] [Google Scholar]
- 23.Futerman AH. Distinct roles for sphingolipids and glycosphingolipids at different stages of neuronal development. Acta Biochim Pol. 1998;45:469–478. [PubMed] [Google Scholar]
- 24.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 25.Bieberich E. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J. 2004;21:315–327. doi: 10.1023/B:GLYC.0000046274.35732.47. [DOI] [PubMed] [Google Scholar]
- 26.Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:R57–R74. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- 27.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy K, Wang G, Silva J, Condie BG, Bieberich E. Ceramide regulates atypical PKCζ/λ-mediated cell polarity in primitive ectoderm cells: a novel function of sphingolipids in morphogenesis. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 29.Snook CF, Jones JA, Hannun YA. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Kubiseski TJ, Chook YM, Parris WE, Rozakis-Adcock M, Pawson T. High affinity binding of the pleckstrin homology domain of mSos1 to phosphatidylinositol (4,5)-bisphosphate. J Biol Chem. 1997;272:1799–1804. doi: 10.1074/jbc.272.3.1799. [DOI] [PubMed] [Google Scholar]
- 31.Wang QJ, Fang TW, Yang D, et al. Ligand structure-activity requirements and phospholipid dependence for the binding of phorbol esters to protein kinase D. Mol Pharmacol. 2003;64:1342–1348. doi: 10.1124/mol.64.6.1342. [DOI] [PubMed] [Google Scholar]
- 32.Houssa B, van Blitterswijk WJ. Specificity of cysteine-rich domains in diacylglycerol kinases and protein kinases C. Biochem J. 1998;331(Pt 2):677–679. doi: 10.1042/bj3310677u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim Biophys Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Giorgione J, Hysell M, Harvey DF, Newton AC. Contribution of the C1A and C1B domains to the membrane interaction of protein kinase C. Biochemistry. 2003;42:11194–11202. doi: 10.1021/bi0350046. [DOI] [PubMed] [Google Scholar]
- 35.Feng H, Ren M, Chen L, Rubin CS. Properties, regulation and in vivo functions of a novel protein kinase D: C. elegans DKF-2 links diacylglycerol second messenger to the regulation of stress responses and lifespan. J Biol Chem. 2007;282:31273–31288. doi: 10.1074/jbc.M701532200. [DOI] [PubMed] [Google Scholar]
- 36.Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine–threonine protein phosphatases. J Lipid Res. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- 38.Lozano J, Berra E, Municio MM, et al. Protein kinase C ζ isoform is critical for κ B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 39.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC ζ is a molecular switch in signal transduction of TNF-α, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox TE, Houck KL, O’Neill SM, et al. Ceramide recruits and activates protein kinase Cζ (PKCζ) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 41.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase Cζ to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Cζ before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 43.Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. doi: 10.1074/jbc.275.1.177. [DOI] [PubMed] [Google Scholar]
- 44.Wang YM, Seibenhener ML, Vandenplas ML, Wooten MW. Atypical PKCζ is activated by ceramide, resulting in coactivation of NF-κB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. doi: 10.1002/(SICI)1097-4547(19990201)55:3<293::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Mathias S, Yang Z, Kolesnick RN. Renaturation and tumor necrosis factor-α stimulation of a 97-kDa ceramide-activated protein kinase. J Biol Chem. 1994;269:3047–3052. [PubMed] [Google Scholar]
- 46.Mathias S, Dressler KA, Kolesnick RN. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor α. Proc Natl Acad Sci USA. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiura M, Kono K, Liu H, et al. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 48.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J Biol Chem. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 49.Pettus BJ, Bielawska A, Subramanian P, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 50.Takuwa Y, Okamoto H, Takuwa N, Gonda K, Sugimoto N, Sakurada S. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol Cell Endocrinol. 2001;177:3–11. doi: 10.1016/s0303-7207(01)00441-5. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 52.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 54.Galadari S, Kishikawa K, Kamibayashi C, Mumby MC, Hannun YA. Purification and characterization of ceramide-activated protein phosphatases. Biochemistry. 1998;37:11232–11238. doi: 10.1021/bi980911+. [DOI] [PubMed] [Google Scholar]
- 55.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 56.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 57.Zhang Y, Yao B, Delikat S, et al. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 58.Kolesnick RN, Clegg S. 1,2-diacylglycerols, but not phorbol esters, activate a potential inhibitory pathway for protein kinase C in GH3 pituitary cells. Evidence for involvement of a sphingomyelinase. J Biol Chem. 1988;263:6534–6537. [PubMed] [Google Scholar]
- 59.Kolesnick RN, Hemer MR. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. J Biol Chem. 1990;265:18803–18808. [PubMed] [Google Scholar]
- 60.Chalfant CE, Rathman K, Pinkerman RL, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh N, Patel N, Jiang K, et al. Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, serine/arginine-rich protein 40, and ribonucleic acid splicing in L6 skeletal muscle cells. Endocrinology. 2007;148:1359–1366. doi: 10.1210/en.2006-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchesini N, Jones JA, Hannun YA. Confluence induced threonine41/serine45 phospho-β-catenin dephosphorylation via ceramide-mediated activation of PP1cγ. Biochim Biophys Acta. 2007;1771:1418–1428. doi: 10.1016/j.bbalip.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383–392. doi: 10.1016/s1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 65.Ogretmen B, Schady D, Usta J, et al. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem. 2001;276:24901–24910. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- 66.Kraveka JM, Li L, Bielawski J, Obeid LM, Ogretmen B. Involvement of endogenous ceramide in the inhibition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch Biochem Biophys. 2003;419:110–119. doi: 10.1016/j.abb.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 67.Wooten LG, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–28876. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- 68.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 69.Basu S, Bayoumy S, Zhang Y, Lozano J, Kolesnick R. BAD enables ceramide to signal apoptosis via Ras and Raf-1. J Biol Chem. 1998;273:30419–30426. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- 70.Hannun YA, Bell RM. Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin Chim Acta. 1989;185:333–345. doi: 10.1016/0009-8981(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 71.Kajimoto T, Shirai Y, Sakai N, et al. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cδ in the Golgi complex. J Biol Chem. 2004;279:12668–12676. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez P, De Carcer G, Sandoval IV, Moscat J, Diaz-Meco MT. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia XJ, Gu XB, Sartorelli AC, Yu RK. Effects of inducers of differentiation on protein kinase C and CMP-N-acetylneuraminic acid:lactosylceramide sialyltransferase activities of HL-60 leukemia cells. J Lipid Res. 1989;30:181–188. [PubMed] [Google Scholar]
- 74.Kanda T, Ariga T, Yamawaki M, Yu RK. GM3 regulates protein kinase systems in cultured brain microvascular endothelial cells. J Neurochem. 1993;61:1969–1972. doi: 10.1111/j.1471-4159.1993.tb09842.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Cα. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- 76.Vaccarino F, Guidotti A, Costa E. Ganglioside inhibition of glutamate-mediated protein kinase C translocation in primary cultures of cerebellar neurons. Proc Natl Acad Sci USA. 1987;84:8707–8711. doi: 10.1073/pnas.84.23.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bazzi MD, Nelsestuen GL. Mechanism of protein kinase C inhibition by sphingosine. Biochem Biophys Res Commun. 1987;146:203–207. doi: 10.1016/0006-291x(87)90711-x. [DOI] [PubMed] [Google Scholar]
- 78.Kitatani K, Idkowiak-Baldys J, Hannun YA. Mechanism of inhibition of sequestration of protein kinase C α/βII by ceramide. Roles of ceramide-activated protein phosphatases and phosphorylation/dephosphorylation of protein kinase C α/βII on threonine 638/641. J Biol Chem. 2007;282:20647–20656. doi: 10.1074/jbc.M609162200. [DOI] [PubMed] [Google Scholar]
- 79.Bieberich E, Hu B, Silva J, et al. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 2002;181:55–64. doi: 10.1016/s0304-3835(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 80.Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol. 2003;162:469–479. doi: 10.1083/jcb.200212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and B-series complex gangliosides. J Biol Chem. 2001;276:44396–44404. doi: 10.1074/jbc.M107239200. [DOI] [PubMed] [Google Scholar]
- 82.Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–734. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirai T, Chida K. Protein kinase Cζ (PKCζ): activation mechanisms and cellular functions. J Biochem (Tokyo) 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki A, Akimoto K, Ohno S. Protein kinase Cλ/τ (PKCλ/τ): a PKC isotype essential for the development of multicellular organisms. J Biochem (Tokyo) 2003;133:9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- 86.Fields AP, Regala RP. Protein kinase τ C: human oncogene, prognostic marker and therapeutic target. Pharmacol Res. 2007;55:487–497. doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu XJ, He AB, Chang YS, Fang FD. Atypical protein kinase C in glucose metabolism. Cell Signal. 2006;18:2071–2076. doi: 10.1016/j.cellsig.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Moscat J, Rennert P, Diaz-Meco MT. PKCζ at the crossroad of NF-κB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2006;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- 89.Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans. 2005;33:350–353. doi: 10.1042/BST0330350. [DOI] [PubMed] [Google Scholar]
- 90.Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab. 2002;283:E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- 91.Imai F, Hirai S, Akimoto K, et al. Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 92.Leitges M, Sanz L, Martin P, et al. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 93.Martin P, Duran A, Minguet S, et al. Role of ζ PKC in B-cell signaling and function. EMBO J. 2002;21:4049–4057. doi: 10.1093/emboj/cdf407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farese RV, Sajan MP, Yang H, et al. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 96.van Blitterswijk WJ. Hypothesis: ceramide conditionally activates atypical protein kinases C, Raf-1 and KSR through binding to their cysteine-rich domains. Biochem J. 1998;331(Pt 2):679–680. [PMC free article] [PubMed] [Google Scholar]
- 97.Kashiwagi K, Shirai Y, Kuriyama M, Sakai N, Saito N. Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J Biol Chem. 2002;277:18037–18045. doi: 10.1074/jbc.M111761200. [DOI] [PubMed] [Google Scholar]
- 98.Sawai H, Okazaki T, Takeda Y, et al. Ceramide-induced translocation of protein kinase C-δ and -ε to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 99.Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta. 2002;1585:139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- 100.Heinrich M, Wickel M, Schneider-Brachert W, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fox TE, Houck KL, O’Neill SM, et al. Ceramide recruits and activates PKCζ within structured membrane microdomains. J Biol Chem. 2007;282(17):12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 102.Megha Sawatzki P, Kolter T, Bittman R, London E. Effect of ceramide N-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts) Biochim Biophys Acta. 2007;1768:2205–2212. doi: 10.1016/j.bbamem.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chiantia S, Kahya N, Schwille P. Raft domain reorganization driven by short- and long-chain ceramide: a combined AFM and FCS study. Langmuir. 2007;23:7659–7665. doi: 10.1021/la7010919. [DOI] [PubMed] [Google Scholar]
- 104.Johnston I, Johnston LJ. Ceramide promotes restructuring of model raft membranes. Langmuir. 2006;22:11284–11289. doi: 10.1021/la061636s. [DOI] [PubMed] [Google Scholar]
- 105.Cremesti A, Paris F, Grassme H, et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 106.Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 107.Gulbins E, Dreschers S, Wilker B, Grassme H. Ceramide, membrane rafts and infections. J Mol Med. 2004;82:357–363. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- 108.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 109.Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci. 2001;58:2053–2068. doi: 10.1007/PL00000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barenholz Y. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell Biochem. 2004;37:167–215. doi: 10.1007/978-1-4757-5806-1_5. [DOI] [PubMed] [Google Scholar]
- 111.Gombos I, Steinbach G, Pomozi I, et al. Some new faces of membrane microdomains: a complex confocal fluorescence, differential polarization, and FCS imaging study on live immune cells. Cytometry A. 2008;73:220–229. doi: 10.1002/cyto.a.20516. [DOI] [PubMed] [Google Scholar]
- 112.Garner AE, Smith DA, Hooper NM. Visualization of detergent solubilization of membranes: implications for the isolation of rafts. Biophys J. 2008;94:1326–1340. doi: 10.1529/biophysj.107.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polozov IV, Gawrisch K. Characterization of the liquid-ordered state by proton MAS NMR. Biophys J. 2006;90:2051–2061. doi: 10.1529/biophysj.105.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mishra S, Joshi PG. Lipid raft heterogeneity: an enigma. J Neurochem. 2007;103(Suppl 1):135–142. doi: 10.1111/j.1471-4159.2007.04720.x. [DOI] [PubMed] [Google Scholar]
- 115.Sengupta P, Baird B, Holowka D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin Cell Dev Biol. 2007;18:583–590. doi: 10.1016/j.semcdb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 117.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 118.Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5:231–240. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 119.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 121.Butters TD, Hughes RC. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. Biochem J. 1974;140:469–478. doi: 10.1042/bj1400469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gurd JW, Evans WH, Perkins HR. Chemical characterization of the proteins and glycoproteins of mouse liver plasma membranes solubilized by sequential extraction with aqueous and organic solvents. Biochem J. 1972;126:459–466. doi: 10.1042/bj1260459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okada Y, Mugnai G, Bremer EG, Hakomori S. Glycosphingolipids in detergent-insoluble substrate attachment matrix (DISAM) prepared from substrate attachment material (SAM). Their possible role in regulating cell adhesion. Exp Cell Res. 1984;155:448–456. doi: 10.1016/0014-4827(84)90205-2. [DOI] [PubMed] [Google Scholar]
- 124.Coulombe J, Traiffort E, Loulier K, Faure H, Ruat M. Hedgehog interacting protein in the mature brain: membrane-associated and soluble forms. Mol Cell Neurosci. 2004;25:323–333. doi: 10.1016/j.mcn.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 125.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 126.Yamazaki S, Iwama A, Morita Y, Eto K, Ema H, Nakauchi H. Cytokine signaling, lipid raft clustering, and HSC hibernation. Ann NY Acad Sci. 2007;1106:54–63. doi: 10.1196/annals.1392.017. [DOI] [PubMed] [Google Scholar]
- 127.Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 128.Ikonen E, Vainio S. Lipid microdomains and insulin resistance: is there a connection? Sci STKE 2005. 2005;268:pe3. doi: 10.1126/stke.2682005pe3. [DOI] [PubMed] [Google Scholar]
- 129.Golub T, Wacha S, Caroni P. Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol. 2004;14:542–550. doi: 10.1016/j.conb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 130.Karpen HE, Bukowski JT, Hughes T, Gratton JP, Sessa WC, Gailani MR. The sonic hedgehog receptor patched associates with caveolin-1 in cholesterol-rich microdomains of the plasma membrane. J Biol Chem. 2001;276:19503–19511. doi: 10.1074/jbc.M010832200. [DOI] [PubMed] [Google Scholar]
- 131.Wang TY, Silvius JR. Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys J. 2000;79:1478–1489. doi: 10.1016/S0006-3495(00)76399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaw JE, Epand RF, Epand RM, Li Z, Bittman R, Yip CM. Correlated fluorescence-atomic force microscopy of membrane domains: structure of fluorescence probes determines lipid localization. Biophys J. 2006;90:2170–2178. doi: 10.1529/biophysj.105.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dietrich C, Bagatolli LA, Volovyk ZN, et al. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hinkovska-Galcheva V, Boxer LA, Kindzelskii A, et al. Ceramide 1-phosphate, a mediator of phagocytosis. J Biol Chem. 2005;280:26612–26621. doi: 10.1074/jbc.M501359200. [DOI] [PubMed] [Google Scholar]
- 135.Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gaus K, Zech T, Harder T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol Membr Biol. 2006;23:41–48. doi: 10.1080/09687860500466857. [DOI] [PubMed] [Google Scholar]
- 137.Kim HM, Choo HJ, Jung SY, et al. A two-photon fluorescent probe for lipid raft imaging: C-laurdan. Chembiochem. 2007;8:553–559. doi: 10.1002/cbic.200700003. [DOI] [PubMed] [Google Scholar]
- 138.Hullin-Matsuda F, Kobayashi T. Monitoring the distribution and dynamics of signaling microdomains in living cells with lipid-specific probes. Cell Mol Life Sci. 2007;64:2492–2504. doi: 10.1007/s00018-007-7281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baumann CA, Ribon V, Kanzaki M, et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 141.Dermine JF, Duclos S, Garin J, et al. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- 142.Hakomori SI. Inaugural article: the glycosynapse. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hakomori S. Glycosynapses: microdomains controlling carbohydrate-dependent cell adhesion and signaling. An Acad Bras Cienc. 2004;76:553–572. doi: 10.1590/s0001-37652004000300010. [DOI] [PubMed] [Google Scholar]
- 144.Taieb N, Yahi N, Fantini J. Rafts and related glycosphingolipid-enriched microdomains in the intestinal epithelium: bacterial targets linked to nutrient absorption. Adv Drug Deliv Rev. 2004;56:779–794. doi: 10.1016/j.addr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Kilkus J, Goswami R, Testai FD, Dawson G. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J Neurosci Res. 2003;72:65–75. doi: 10.1002/jnr.10549. [DOI] [PubMed] [Google Scholar]
- 146.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 147.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem. 2005;280:26425–26434. doi: 10.1074/jbc.M414569200. [DOI] [PubMed] [Google Scholar]
- 148.Wang TY, Silvius JR. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys J. 2003;84:367–378. doi: 10.1016/S0006-3495(03)74857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Silva LC, de Almeida RF, Castro BM, Fedorov A, Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Merrill AH, Jr, Wang E. Enzymes of ceramide biosynthesis. Methods Enzymol. 1992;209:427–437. doi: 10.1016/0076-6879(92)09053-6. [DOI] [PubMed] [Google Scholar]
- 151.van Echten-Deckert G, Herget T. Sphingolipid metabolism in neural cells. Biochim Biophys Acta. 2006;1758:1978–1994. doi: 10.1016/j.bbamem.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 152.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 153.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 154.Sandhoff K, Kolter T. Biosynthesis and degradation of mammalian glycosphingolipids. Philos Trans R Soc Lond B Biol Sci. 2003;358:847–861. doi: 10.1098/rstb.2003.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Linn SC, Kim HS, Keane EM, Andras LM, Wang E, Merrill AH., Jr Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans. 2001;29:831–815. doi: 10.1042/0300-5127:0290831. [DOI] [PubMed] [Google Scholar]
- 156.Yasuda S, Nishijima M, Hanada K. Localization, topology, and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. J Biol Chem. 2003;278:4176–4183. doi: 10.1074/jbc.M209602200. [DOI] [PubMed] [Google Scholar]
- 157.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 158.Batheja AD, Uhlinger DJ, Carton JM, Ho G, D’Andrea MR. Characterization of serine palmitoyltransferase in normal human tissues. J Histochem Cytochem. 2003;51:687–696. doi: 10.1177/002215540305100514. [DOI] [PubMed] [Google Scholar]
- 159.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 160.Yard BA, Carter LG, Johnson KA, et al. The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis. J Mol Biol. 2007;370:870–886. doi: 10.1016/j.jmb.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 161.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 162.Hanada K, Nishijima M. Purification of mammalian serine palmitoyltransferase, a hetero-subunit enzyme for sphingolipid biosynthesis, by affinity-peptide chromatography. Methods Mol Biol. 2003;228:163–174. doi: 10.1385/1-59259-400-X:163. [DOI] [PubMed] [Google Scholar]
- 163.Hornemann T, Wei Y, von Eckardstein A. Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? Biochem J. 2007;405:157–164. doi: 10.1042/BJ20070025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fyrst H, Herr DR, Harris GL, Saba JD. Characterization of free endogenous C14 and C16 sphingoid bases from Drosophila melanogaster. J Lipid Res. 2004;45:54–62. doi: 10.1194/jlr.M300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 165.Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J Biol Chem. 1997;272:32566–32572. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]
- 166.Adachi-Yamada T, Gotoh T, Sugimura I, et al. De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Mol Cell Biol. 1999;19:7276–7286. doi: 10.1128/mcb.19.10.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Acharya U, Acharya JK. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci. 2005;62:128–142. doi: 10.1007/s00018-004-4254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB. The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell. 2006;18:3576–3593. doi: 10.1105/tpc.105.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheng J, Park TS, Fischl AS, Ye XS. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol Cell Biol. 2001;21:6198–6209. doi: 10.1128/MCB.21.18.6198-6209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bejaoui K, Wu C, Scheffler MD, et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet. 2001;27:261–262. doi: 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- 171.Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- 172.McCampbell A, Truong D, Broom DC, et al. Mutant SPTLC1 dominantly inhibits serine palmitoyltransferase activity in vivo and confers an age-dependent neuropathy. Hum Mol Genet. 2005;14:3507–3521. doi: 10.1093/hmg/ddi380. [DOI] [PubMed] [Google Scholar]
- 173.Houlden H, King R, Blake J, et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I) Brain. 2006;129:411–425. doi: 10.1093/brain/awh712. [DOI] [PubMed] [Google Scholar]
- 174.Bi H, Gao Y, Yao S, Dong M, Headley AP, Yuan Y. Hereditary sensory and autonomic neuropathy type I in a Chinese family: British C133W mutation exists in the Chinese. Neuropathology. 2007;27:429–433. doi: 10.1111/j.1440-1789.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 175.Hong KK, Cho HR, Ju WC, Cho Y, Kim NI. A study on altered expression of serine palmitoyltransferase and ceramidase in psoriatic skin lesion. J Korean Med Sci. 2007;22:862–867. doi: 10.3346/jkms.2007.22.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 177.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang J, Yu Y, Sun S, Duerksen-Hughes PJ. Ceramide and other sphingolipids in cellular responses. Cell Biochem Biophys. 2004;40:323–350. doi: 10.1385/CBB:40:3:323. [DOI] [PubMed] [Google Scholar]
- 179.Schenck M, Carpinteiro A, Grassme H, Lang F, Gulbins E. Ceramide: physiological and pathophysiological aspects. Arch Biochem Biophys. 2007;462:171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 180.Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 2006;5:200–208. doi: 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- 181.Jaffrezou JP, Laurent G. Ceramide: a new target in anticancer research? Bull Cancer. 2004;91:E133–E161. [PubMed] [Google Scholar]
- 182.Brady RO. Disorders of lipid metabolism. Biochem Soc Symp. 1972;35:113–127. [PubMed] [Google Scholar]
- 183.Fujita T, Inoue K, Yamamoto S, et al. Fungal metabolites. Part 11 A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 184.He Q, Johnson VJ, Osuchowski MF, Sharma RP. Inhibition of serine palmitoyltransferase by myriocin, a natural mycotoxin, causes induction of c-Myc in mouse liver. Mycopathologia. 2004;157:339–347. doi: 10.1023/b:myco.0000024182.04140.95. [DOI] [PubMed] [Google Scholar]
- 185.Hanada K, Nishijima M, Fujita T, Kobayashi S. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem Pharmacol. 2000;59:1211–1216. doi: 10.1016/s0006-2952(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 186.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 187.Kluepfel D, Bagli J, Baker H, Charest MP, Kudelski A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo) 1972;25:109–115. doi: 10.7164/antibiotics.25.109. [DOI] [PubMed] [Google Scholar]
- 188.Desai K, Sullards MC, Allegood J, et al. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim Biophys Acta. 2002;1585:188–192. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- 189.Riley RT, Wang E, Schroeder JJ, et al. Evidence for disruption of sphingolipid metabolism as a contributing factor in the toxicity and carcinogenicity of fumonisins. Nat Toxins. 1996;4:3–15. doi: 10.1002/19960401nt2. [DOI] [PubMed] [Google Scholar]
- 190.Riley RT, Hinton DM, Chamberlain WJ, et al. Dietary fumonisin B1 induces disruption of sphingolipid metabolism in Sprague-Dawley rats: a new mechanism of nephrotoxicity. J Nutr. 1994;124:594–603. doi: 10.1093/jn/124.4.594. [DOI] [PubMed] [Google Scholar]
- 191.Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002;66:169–176. doi: 10.1002/tera.10089. [DOI] [PubMed] [Google Scholar]
- 192.Flynn TJ, Stack ME, Troy AL, Chirtel SJ. Assessment of the embryotoxic potential of the total hydrolysis product of fumonisin B1 using cultured organogenesis-staged rat embryos. Food Chem Toxicol. 1997;35:1135–1141. doi: 10.1016/s0278-6915(97)85466-x. [DOI] [PubMed] [Google Scholar]
- 193.Gelineau-van Waes J, Starr L, Maddox J, et al. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol. 2005;73:487–497. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- 194.Marasas WF, Riley RT, Hendricks KA, et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 195.Yamaji-Hasegawa A, Takahashi A, Tetsuka Y, Senoh Y, Kobayashi T. Fungal metabolite sulfamisterin suppresses sphingolipid synthesis through inhibition of serine palmitoyltransferase. Biochemistry. 2005;44:268–277. doi: 10.1021/bi048605l. [DOI] [PubMed] [Google Scholar]
- 196.Medlock KA, Merrill AH., Jr Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by β-chloroalanine. Biochemistry. 1988;27:7079–7084. doi: 10.1021/bi00418a061. [DOI] [PubMed] [Google Scholar]
- 197.Kraveka JM, Li L, Szulc ZM, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bedia C, Triola G, Casas J, Llebaria A, Fabrias G. Analogs of the dihydroceramide desaturase inhibitor GT11 modified at the amide function: synthesis and biological activities. Org Biomol Chem. 2005;3:3707–3712. doi: 10.1039/b510198k. [DOI] [PubMed] [Google Scholar]
- 199.Medler TR, Petrusca DN, Lee PJ, et al. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0274OC. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Petrache I, Natarajan V, Zhen L, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Glaros EN, Kim WS, Wu BJ, et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol. 2007;73:1340–1346. doi: 10.1016/j.bcp.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 202.Toman RE, Movsesyan V, Murthy SK, Milstien S, Spiegel S, Faden AI. Ceramide-induced cell death in primary neuronal cultures: upregulation of ceramide levels during neuronal apoptosis. J Neurosci Res. 2002;68:323–330. doi: 10.1002/jnr.10190. [DOI] [PubMed] [Google Scholar]
- 203.Wang H, Giuliano AE, Cabot MC. Enhanced de novo ceramide generation through activation of serine palmitoyltransferase by the P-glycoprotein antagonist SDZ PSC 833 in breast cancer cells. Mol Cancer Ther. 2002;1:719–726. [PubMed] [Google Scholar]
- 204.Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 205.Jarvis WD, Grant S, Kolesnick RN. Ceramide and the induction of apoptosis. Clin Cancer Res. 1996;2:1–6. [PubMed] [Google Scholar]
- 206.Wang H, Charles AG, Frankel AJ, Cabot MC. Increasing intracellular ceramide: an approach that enhances the cytotoxic response in prostate cancer cells. Urology. 2003;61:1047–1052. doi: 10.1016/s0090-4295(02)02511-6. [DOI] [PubMed] [Google Scholar]
- 207.Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 208.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585:172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 209.Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63:150–159. doi: 10.1016/j.critrevonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 210.Holman DH, Turner LS, El-Zawahry A, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61:231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 211.Selzner M, Bielawska A, Morse MA, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- 212.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 213.Stover TC, Kim YS, Lowe TL, Kester M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials. 2008;29:359–369. doi: 10.1016/j.biomaterials.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 214.Senkal CE, Ponnusamy S, Rossi MJ, et al. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- 215.Stoffel W, Assmann G, Bister K. Metabolism of sphingosine bases. XVII Stereospecificities in the introduction of the 4t-double bond into sphinganine yielding 4t-sphingenine (sphingosine) Hoppe Seylers Z Physiol Chem. 1971;352:1531–1544. doi: 10.1515/bchm2.1971.352.2.1531. [DOI] [PubMed] [Google Scholar]
- 216.Ong DE, Brady RN. In vivo studies on the introduction of the 4-t-double bond of the sphingenine moiety of rat brain ceramides. J Biol Chem. 1973;248:3884–3888. [PubMed] [Google Scholar]
- 217.Stoffel W, Melzner I. Studies in vitro on the biosynthesis of ceramide and sphingomyelin. A reevaluation of proposed pathways. Hoppe Seylers Z Physiol Chem. 1980;361:755–771. doi: 10.1515/bchm2.1980.361.1.755. [DOI] [PubMed] [Google Scholar]
- 218.Rother J, van Echten G, Schwarzmann G, Sandhoff K. Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells. Biochem Biophys Res Commun. 1992;189:14–20. doi: 10.1016/0006-291x(92)91518-u. [DOI] [PubMed] [Google Scholar]
- 219.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2007 doi: 10.1016/j.cellsig.2007.12.006. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? : insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 222.Lahiri S, Futerman AH. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J Biol Chem. 2005;280:33735–33738. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- 223.Riebeling C, Allegood JC, Wang E, Merrill AH, Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- 224.Wang G, Silva J, Dasgupta S, Bieberich E. Long-chain ceramide is elevated in presenilin 1 (PS1M146V) mouse brain and induces apoptosis in PS1 astrocytes. Glia. 2008;56:449–456. doi: 10.1002/glia.20626. [DOI] [PubMed] [Google Scholar]
- 225.Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 226.Hanada K, Kumagai K, Yasuda S, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 227.Kudo N, Kumagai K, Tomishige N, et al. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci USA. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to pro-apoptotic proteins. J Lipid Res. 2007;49:625–634. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 229.Rizzuto R, Pinton P, Ferrari D, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 230.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J Lipid Res. 2007;48:968–975. doi: 10.1194/jlr.D600043-JLR200. [DOI] [PubMed] [Google Scholar]
- 232.Ledeen RW, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim Biophys Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 233.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008 doi: 10.1194/jlr.R800009-JLR200. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Albi E, Lazzarini R, Viola Magni M. Phosphatidylcholine/sphingomyelin metabolism crosstalk inside the nucleus. Biochem J. 2008;410:381–389. doi: 10.1042/BJ20070758. [DOI] [PubMed] [Google Scholar]
- 235.Albi E, Cataldi S, Bartoccini E, et al. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J Cell Physiol. 2006;206:189–195. doi: 10.1002/jcp.20448. [DOI] [PubMed] [Google Scholar]
- 236.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. J Biol Chem. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 239.Ding T, Li Z, Hailemariam T, et al. SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J Lipid Res. 2008;49:376–385. doi: 10.1194/jlr.M700401-JLR200. [DOI] [PubMed] [Google Scholar]
- 240.Guillen N, Navarro MA, Surra JC, et al. Cloning, characterization, expression and comparative analysis of pig Golgi membrane sphingomyelin synthase 1. Gene. 2007;388:117–124. doi: 10.1016/j.gene.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 241.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vos JP, Giudici ML, van der Bijl P, et al. Sphingomyelin is synthesized at the plasma membrane of oligodendrocytes and by purified myelin membranes: a study with fluorescent- and radio-labelled ceramide analogues. FEBS Lett. 1995;368:393–396. doi: 10.1016/0014-5793(95)00695-6. [DOI] [PubMed] [Google Scholar]
- 243.Tsao FH, Zachman RD. Phosphatidylcholine-lysophosphatidylcholine cycle pathway enzymes in rabbit lung. II Marked differences in the effect of gestational age on activity compared to the CDP-choline pathway. Pediatr Res. 1977;11:858–861. doi: 10.1203/00006450-197707000-00016. [DOI] [PubMed] [Google Scholar]
- 244.Marggraf WD, Anderer FA, Kanfer JN. The formation of sphingomyelin from phosphatidylcholine in plasma membrane preparations from mouse fibroblasts. Biochim Biophys Acta. 1981;664:61–73. doi: 10.1016/0005-2760(81)90028-x. [DOI] [PubMed] [Google Scholar]
- 245.Zimmermann LJ, Hogan M, Carlson KS, Smith BT, Post M. Regulation of phosphatidylcholine synthesis in fetal type II cells by CTP:phosphocholine cytidylyltransferase. Am J Physiol. 1993;264:L575–L580. doi: 10.1152/ajplung.1993.264.6.L575. [DOI] [PubMed] [Google Scholar]
- 246.Vance JE, Pan D, Campenot RB, Bussiere M, Vance DE. Evidence that the major membrane lipids, except cholesterol, are made in axons of cultured rat sympathetic neurons. J Neurochem. 1994;62:329–337. doi: 10.1046/j.1471-4159.1994.62010329.x. [DOI] [PubMed] [Google Scholar]
- 247.Kent C. CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1997;1348:79–90. doi: 10.1016/s0005-2760(97)00112-4. [DOI] [PubMed] [Google Scholar]
- 248.Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidol. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vos JP, de Haas CG, van Golde LM, Lopes-Cardozo M. Relationships between phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin metabolism in cultured oligodendrocytes. J Neurochem. 1997;68:1252–1260. doi: 10.1046/j.1471-4159.1997.68031252.x. [DOI] [PubMed] [Google Scholar]
- 250.Vos JP, Giudici ML, van der Bijl P, Lopes-Cardozo M. Synthesis of sphingomyelin by oligodendrocytes – how and where? J Lipid Mediat Cell Signal. 1996;14:313–319. doi: 10.1016/0929-7855(96)00540-8. [DOI] [PubMed] [Google Scholar]
- 251.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 252.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–122. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- 253.Adibhatla RM, Hatcher JF. Cytidine 5′-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30:15–23. doi: 10.1007/s11064-004-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Silva J, Beckedorf A, Bieberich E. Osteoblast-derived oxysterol is a migration-inducing factor for human breast cancer cells. J Biol Chem. 2003;278:25376–25385. doi: 10.1074/jbc.M301233200. [DOI] [PubMed] [Google Scholar]
- 255.Perry RJ, Ridgway ND. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta. 2005;1734:220–234. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 256.Silva J, Dasgupta S, Wang G, Krishnamurthy K, Ritter E, Bieberich E. Lipids isolated from bone induce the migration of human breast cancer cells. J Lipid Res. 2006;47:724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- 257.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281:14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 258.Becker S, Reinehr R, Grether-Beck S, Eberle A, Haussinger D. Hydrophobic bile salts trigger ceramide formation through endosomal acidification. Biol Chem. 2007;388:185–196. doi: 10.1515/BC.2007.021. [DOI] [PubMed] [Google Scholar]
- 259.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 260.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 261.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 262.Dumitru CA, Zhang Y, Li X, Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid Redox Signal. 2007;9:1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- 263.Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Exp Cell Res. 2007;313:2680–2686. doi: 10.1016/j.yexcr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 264.Sillence DJ. Apoptosis and signalling in acid sphingomyelinase deficient cells. BMC Cell Biol. 2001;2:24. doi: 10.1186/1471-2121-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Romsicki Y, Sharom FJ. Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry. 2001;40:6937–6947. doi: 10.1021/bi0024456. [DOI] [PubMed] [Google Scholar]
- 266.Tepper AD, Ruurs P, Wiedmer T, Sims PJ, Borst J, van Blitterswijk WJ. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol. 2000;150:155–164. doi: 10.1083/jcb.150.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brann AB, Scott R, Neuberger Y, et al. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Brann AB, Tcherpakov M, Williams IM, Futerman AH, Fainzilber M. Nerve growth factor-induced p75-mediated death of cultured hippocampal neurons is age-dependent and transduced through ceramide generated by neutral sphingomyelinase. J Biol Chem. 2002;277:9812–9818. doi: 10.1074/jbc.M109862200. [DOI] [PubMed] [Google Scholar]
- 269.Costantini C, Weindruch R, Della Valle G, Puglielli L. A TrkA-to-p75NTR molecular switch activates amyloid β-peptide generation during aging. Biochem J. 2005;391:59–67. doi: 10.1042/BJ20050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Barker PA. p75NTR: a study in contrasts. Cell Death Differ. 1998;5:346–356. doi: 10.1038/sj.cdd.4400375. [DOI] [PubMed] [Google Scholar]
- 271.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 272.Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 273.Barker PA. High affinity not in the vicinity? Neuron. 2007;53:1–4. doi: 10.1016/j.neuron.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 274.Costantini C, Scrable H, Puglielli L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid β-peptide generation. EMBO J. 2006;25:1997–2006. doi: 10.1038/sj.emboj.7601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yaar M, Zhai S, Pilch PF, et al. Binding of β-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kuner P, Hertel C. NGF induces apoptosis in a human neuroblastoma cell line expressing the neurotrophin receptor p75NTR. J Neurosci Res. 1998;54:465–474. doi: 10.1002/(SICI)1097-4547(19981115)54:4<465::AID-JNR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 277.Naumann T, Casademunt E, Hollerbach E, et al. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 279.Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–272. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- 280.Coulson EJ. Does the p75 neurotrophin receptor mediate Aβ-induced toxicity in Alzheimer’s disease? J Neurochem. 2006;98:654–660. doi: 10.1111/j.1471-4159.2006.03905.x. [DOI] [PubMed] [Google Scholar]
- 281.Hatchett CS, Tyler S, Armstrong D, Dawbarn D, Allen SJ. Familial Alzheimer’s disease presenilin 1 mutation M146V increases γ secretase cutting of p75NTR in vitro. Brain Res. 2007;1147:248–255. doi: 10.1016/j.brainres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 282.Niederhauser O, Mangold M, Schubenel R, Kusznir EA, Schmidt D, Hertel C. NGF ligand alters NGF signaling via p75(NTR) and TrkA. J Neurosci Res. 2000;61:263–272. doi: 10.1002/1097-4547(20000801)61:3<263::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 283.Diolaiti D, Bernardoni R, Trazzi S, et al. Functional cooperation between TrkA and p75(NTR) accelerates neuronal differentiation by increased transcription of GAP-43 and p21(CIP/WAF) genes via ERK1/2 and AP-1 activities. Exp Cell Res. 2007;313:2980–2992. doi: 10.1016/j.yexcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 284.Micera A, Lambiase A, Stampachiacchiere B, Bonini S, Bonini S, Levi-Schaffer F. Nerve growth factor and tissue repair remodeling: TrkA(NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18:245–256. doi: 10.1016/j.cytogfr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 285.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 286.Verdi JM, Birren SJ, Ibanez CF, et al. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 287.Heumann R. Neurotrophin signalling. Curr Opin Neurobiol. 1994;4:668–679. doi: 10.1016/0959-4388(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 288.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 289.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 290.Greene LA, Kaplan DR. Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 291.Culmsee C, Gerling N, Lehmann M, et al. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 292.Plo I, Bono F, Bezombes C, Alam A, Bruno A, Laurent G. Nerve growth factor-induced protein kinase C stimulation contributes to TrkA-dependent inhibition of p75 neurotrophin receptor sphingolipid signaling. J Neurosci Res. 2004;77:465–474. doi: 10.1002/jnr.20189. [DOI] [PubMed] [Google Scholar]
- 293.Gowrishankar K, Zeidler MG, Vincenz C. Release of a membrane-bound death domain by γ-secretase processing of the p75NTR homolog NRADD. J Cell Sci. 2004;117:4099–4111. doi: 10.1242/jcs.01263. [DOI] [PubMed] [Google Scholar]
- 294.Ito Y, Ishii A, Passmore AP, McIlroy SP. Analysis of alteration of p75NTR processing and signalling by PS2 mutation and γ-secretase inhibition. Neurobiol Dis. 2007;27:258–264. doi: 10.1016/j.nbd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 295.Pepinsky RB, Zeng C, Wen D, et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 296.Kieran MW, Packer RJ, Onar A, et al. Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: a Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2007;25:3137–3143. doi: 10.1200/JCO.2006.09.4243. [DOI] [PubMed] [Google Scholar]
- 297.Mesa RA, Camoriano JK, Geyer SM, et al. A Phase II trial of tipifarnib in myelofibrosis: primary, post-polycythemia vera and post-essential thrombocythemia. Leukemia. 2007;21:1964–1970. doi: 10.1038/sj.leu.2404816. [DOI] [PubMed] [Google Scholar]
- 298.Harousseau JL, Lancet JE, Reiffers J, et al. A Phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109:5151–5156. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 299.Glynn SA, O’Sullivan D, Eustace AJ, Clynes M, O’Donovan N. The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells. BMC Cancer. 2008;8:9. doi: 10.1186/1471-2407-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 301.Shirai T, Tanaka K, Terada Y, et al. Specific detection of phosphatidylinositol 3,4,5-trisphosphate binding proteins by the PIP3 analogue beads: an application for rapid purification of the PIP3 binding proteins. Biochim Biophys Acta. 1998;1402:292–302. doi: 10.1016/s0167-4889(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 302.Van Keymeulen A, Wong K, Knight ZA, et al. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007;74:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The domain PH: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 305.Barnett SF, Bilodeau MT, Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr Top Med Chem. 2005;5:109–125. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- 306.Tong J, Nguyen L, Vidal A, Simon SA, Skene JH, McIntosh TJ. Role of GAP-43 in sequestering phosphatidylinositol 4,5-bisphosphate to raft bilayers. Biophys J. 2008;94:125–133. doi: 10.1529/biophysj.107.110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chen L, Liao G, Yang L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci USA. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128:239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 309.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 310.Kim SK. Cell polarity: new PARtners for Cdc42 and Rac. Nat Cell Biol. 2000;2:E143–E145. doi: 10.1038/35019620. [DOI] [PubMed] [Google Scholar]
- 311.Wang HR, Zhang Y, Ozdamar B, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 312.Welchman DP, Mathies LD, Ahringer J. Similar requirements for CDC-42 and the PAR-3/PAR-6/PKC-3 complex in diverse cell types. Dev Biol. 2007;305:347–357. doi: 10.1016/j.ydbio.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Solier S, De Cian MC, Bettaieb A, Desoche L, Solary E, Corcos L. PKC ζ controls DNA topoisomerase-dependent human caspase-2 pre-mRNA splicing. FEBS Lett. 2008;582:372–378. doi: 10.1016/j.febslet.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 315.Croci C, Brandstatter JH, Enz R. ZIP3, a new splice variant of the PKC-ζ-interacting protein family, binds to GABAreceptors C, PKC-ζ, and Kv β 2. J Biol Chem. 2003;278:6128–6135. doi: 10.1074/jbc.M205162200. [DOI] [PubMed] [Google Scholar]
- 316.Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sells SF, Wood DP, Jr, Joshi-Barve SS, et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 318.Diaz-Meco MT, Municio MM, Frutos S, et al. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 319.Johnstone RW, Wang J, Tommerup N, Vissing H, Roberts T, Shi Y. Ciao 1 is a novel WD40 protein that interacts with the tumor suppressor protein WT1. J Biol Chem. 1998;273:10880–10887. doi: 10.1074/jbc.273.18.10880. [DOI] [PubMed] [Google Scholar]
- 320.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 321.Duan W, Rangnekar VM, Mattson MP. Prostate apoptosis response-4 production in synaptic compartments following apoptotic and excitotoxic insults: evidence for a pivotal role in mitochondrial dysfunction and neuronal degeneration. J Neurochem. 1999;72:2312–2322. doi: 10.1046/j.1471-4159.1999.0722312.x. [DOI] [PubMed] [Google Scholar]
- 322.Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor κB in neuronal survival and plasticity. J Neurochem. 2000;74:443–456. doi: 10.1046/j.1471-4159.2000.740443.x. [DOI] [PubMed] [Google Scholar]
- 323.Mattson MP, Meffert MK. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 324.Mattson MP, Duan W, Chan SL, Camandola S. Par-4: an emerging pivotal player in neuronal apoptosis and neurodegenerative disorders. J Mol Neurosci. 1999;13:17–30. doi: 10.1385/JMN:13:1-2:17. [DOI] [PubMed] [Google Scholar]
- 325.Rankin CA, Sun Q, Gamblin TC. Tau phosphorylation by GSK-3β promotes tangle-like filament morphology. Mol Neurodegener. 2007;2:12. doi: 10.1186/1750-1326-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3β-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by β-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 327.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 328.Gartner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3β) independently of Akt/PKB serine phosphorylation. J Cell Sci. 2006;119:3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- 329.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 330.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Leclerc S, Garnier M, Hoessel R, et al. Indirubins inhibit glycogen synthase kinase-3 β and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 332.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liu GP, Zhang Y, Yao XQ, et al. Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.012. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 334.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3β acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 335.Xie J, Chang X, Zhang X, Guo Q. Aberrant induction of Par-4 is involved in apoptosis of hippocampal neurons in presenilin-1 M146V mutant knock-in mice. Brain Res. 2001;915:1–10. doi: 10.1016/s0006-8993(01)02803-7. [DOI] [PubMed] [Google Scholar]
- 336.Zhao Y, Burikhanov R, Qiu S, et al. Cancer resistance in transgenic mice expressing the SAC module of Par-4. Cancer Res. 2007;67:9276–9285. doi: 10.1158/0008-5472.CAN-07-2124. [DOI] [PubMed] [Google Scholar]
- 337.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 338.Spiegel S, Cuvillier O, Edsall LC, et al. Sphingosine-1-phosphate in cell growth and cell death. Ann NY Acad Sci. 1998;845:11–18. doi: 10.1111/j.1749-6632.1998.tb09658.x. [DOI] [PubMed] [Google Scholar]
- 339.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 341.Huwiler A, Pfeilschifter J. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des. 2006;12:4625–4635. doi: 10.2174/138161206779010422. [DOI] [PubMed] [Google Scholar]
- 342.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 343.Maceyka M, Sankala H, Hait NC, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 344.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 345.Hla T, Lee MJ, Ancellin N, et al. Sphingosine-1-phosphate signaling via the EDG-1 family of G-protein-coupled receptors. Ann NY Acad Sci. 2000;905:16–24. doi: 10.1111/j.1749-6632.2000.tb06534.x. [DOI] [PubMed] [Google Scholar]
- 346.Lee MJ, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- 347.Colombaioni L, Garcia-Gil M. Sphingolipid metabolites in neural signalling and function. Brain Res Brain Res Rev. 2004;46:328–355. doi: 10.1016/j.brainresrev.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 348.Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rakhit S, Conway AM, Tate R, Bower T, Pyne NJ, Pyne S. Sphingosine 1-phosphate stimulation of the p42/p44 mitogen-activated protein kinase pathway in airway smooth muscle. Role of endothelial differentiation gene 1, c-Src tyrosine kinase and phosphoinositide 3-kinase. Biochem J. 1999;338(Pt 3):643–649. [PMC free article] [PubMed] [Google Scholar]
- 350.Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol. 2006;207:757–766. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- 351.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88:1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 352.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of S1P1 by differential chemical perturbation and proteomics. J Biol Chem. 2007 doi: 10.1074/jbc.M610581200. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 353.Oh JE, So KS, Lim SJ, Kim MY. Induction of apoptotic cell death by a ceramide analog in PC-3 prostate cancer cells. Arch Pharm Res. 2006;29:1140–1146. [PubMed] [Google Scholar]
- 354.Stoica BA, Movsesyan VA, Lea PM, 4th, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3β, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 355.Jarvis WD, Fornari FA, Jr, Auer KL, et al. Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol Pharmacol. 1997;52:935–947. doi: 10.1124/mol.52.6.935. [DOI] [PubMed] [Google Scholar]
- 356.Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323:626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- 357.Dev KK, Mullershausen F, Mattes H, et al. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 358.Bieberich E. Smart drugs for smarter stem cells: making SENSe (sphingolipid-enhanced neural stem cells) of ceramide. Neurosignals. 2008;16:124–139. doi: 10.1159/000111558. [DOI] [PubMed] [Google Scholar]
- 359.Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 360.Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 361.Gardner RL. Stem cells and regenerative medicine: principles, prospects and problems. C R Biol. 2007;330:465–473. doi: 10.1016/j.crvi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 362.Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007;25:24–32. doi: 10.1016/j.tibtech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 363.Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 364.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 365.Sarkar S, Maceyka M, Hait NC, et al. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 366.Shirahama T, Sweeney EA, Sakakura C, et al. In vitro and in vivo induction of apoptosis by sphingosine and N, N-dimethylsphingosine in human epidermoid carcinoma KB-3–1 and its multidrug-resistant cells. Clin Cancer Res. 1997;3:257–264. [PubMed] [Google Scholar]
- 367.Endo K, Igarashi Y, Nisar M, Zhou QH, Hakomori S. Cell membrane signaling as target in cancer therapy: inhibitory effect of N, N-dimethyl and N, N, N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res. 1991;51:1613–1618. [PubMed] [Google Scholar]
- 368.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]