Abstract
Rat submandibular glands can recover their function and secretory protein content following ductal ligation-induced atrophy. Morphological studies have established that following ligation, deligation of the gland regenerates new salivary gland tissue. However, little is know about changes happening during early regeneration following intra-oral duct ligation, which does not damage the parasympathetic nerves. Gland that had been 2 weeks ligated or 2 weeks ligated + 3 days deligation glands were compared. Tissue was prepared for histological, immunohistochemical (SMG-B and Ki 67) and immunocytochemical analyses (smooth muscle actin, aquaporin 5). H&E staining of deligated glands showed some acini have regained their cytoplasmic volume; moreover, the loss of AB/PAS staining from the lumen of ducts suggested successful deligation. The deligated gland was characterized by atypical acinar-ductal branched structures, which were less frequent in the ligated gland and rarely seen in normal unoperated tissue. Myoepithelial cells were also investigated since changes in their morphology reflected changes in the acini morphology not readily detected by conventional staining. Actin staining revealed the presence of some shrunken acini in the atrophic tissue whereas they had regained their normal morphology in the deligated gland suggesting the acini were recovering. Some acini during deligation regained aquaporin 5 expression which was decreased during atrophy. Furthermore SMG-B protein, located in the pro-acinar cell during gland development and usually located in the intercalated duct cells in the adult, has been detected in the newly formed acini of the deligated gland. This study suggests that morphological markers of regeneration are apparent after just 3 days following ligation removal.
Keywords: salivary gland, regeneration, myoepithelial cell, SMG-B, aquaporin 5 (AQP5)
INTRODUCTION
Obstructive sialadenitis, attributable for example to calculi formation, leads to salivary gland inflammation and eventually to atrophy (Harrison and Badir 1998;Matthews and Dardick 1988). The development of animal models involving ligation of the main excretory ducts of salivary glands, has contributed to the understanding of inflammation and atrophy (Cummins et al. 1994;Harrison and Garrett 1976;Tamarin 1979). Previous studies indicate that atrophy following ligation of the excretory duct with the inclusion of the chorda lingual nerve (extra-oral duct ligation) is characterized by inflammation, loss of acini, ductal cell proliferation (Harrison and Garrett 1976;Norberg et al. 1988;Walker and Gobe 1987), and secretory dysfunction (Martinez et al. 1982). Extra-oral duct ligation-induced atrophy has been shown to affect the behaviour of the myoepithelial cell, since these cells have been reported to proliferate during atrophy of the submandibular and parotid glands (Burgess et al. 1986;Takahashi et al. 2001), and to change their position from the small to the large ducts in sublingual glands (Takahashi et al. 2002).
More recently, in rat submandibular gland, ligation of the main excretory duct without the inclusion of the chorda lingual nerve (intra-oral duct ligation) has been shown to lead to less severe atrophy than extra-oral duct ligation (Osailan et al. 2006b). Intra-oral duct ligation, unlike extra-oral duct ligation, avoids damage to the chorda lingual nerve (Osailan et al. 2006b;Harrison et al. 2001), which is involved in normal salivary secretion (Garrett 1987). For this reason intra-oral duct ligation appears to be a more appropriate model for studying the effects of obstructive diseases on salivary glands.
After removal of the obstruction, both extra-oral and intra-oral duct ligation induced atrophy is reversible, since the glands are able to recover their functionality, secreting normal amounts of saliva with a broadly normal composition (Scott et al. 1999;Osailan et al. 2006a;Carpenter et al. 2007). Morphological studies of salivary gland regeneration after extra-oral duct ligation have reported an increase in the proportional volume of the acini, although a higher duct-to-acinar ratio than the normal gland still persisted (Scott et al. 1999). In regenerating submandibular glands acinar cell proliferation demonstrated a biphasic peak between day 2 and day 4 after deligation, suggesting that the residual acini proliferate first followed later by proliferation of newly formed acini (Takahashi et al. 2004b). During glandular regeneration newly formed acinar cells are thought to differentiate from intercalated duct cells (Tamarin 1971b;Takahashi et al. 1998). Whether these new acinar cells originate from de-differentiated intercalated duct cells (Tamarin 1971b;Man et al. 1995;Man et al. 2001), or from multipotent cells (Kishi et al. 2006), is still unclear.
In contrast to extra-oral ligation little information is available about the morphological and molecular changes occurring during glandular regeneration after intra-oral duct ligation. Therefore the aim of this paper has been to identify the earliest morphological and molecular markers of regeneration occurring in rat submandibular gland after intraoral ligation, with a view to examining some of the signalling events in a subsequent study. The characterization of the early regeneration process should provide useful information for further molecular analysis, and should also allow us to investigate possible correlation with the glandular embryonic/ perinatal development.
MATERIALS AND METHODS
Experimental procedure
14 rats, Wistar strain (250-350g) were used. All experimental and killing procedures were conducted with approval of the local ethics committee and Home Office license. The rats were divided in three groups: one group (5 rats) experienced 2 weeks ligation only, another group (5 rats) underwent 2 weeks ligation followed by 3 days de-ligation, and a third group (4 rats) of unoperated rats as controls. Contralateral glands were not used as controls in this study because compensatory hyperplasia occurred when the other gland was extirpated (Shwartz-Arad et al. 1991), or ligated (Walker and Gobe 1987).
Atrophy was induced following the intraoral duct ligation procedure as previously described (Osailan et al. 2006b). Under recovery anaesthesia (combined Ketamine 75 mg/kg and Xylazine 15 mg/kg intraperitoneally [ i.p.]) a metal clip was applied to the main excretory ducts of the submandibular and sublingual glands through a small incision in the floor of the mouth, which was then sutured. A small plastic tube was applied together with the clip in order to avoid fibrosis of the ducts (Osailan et al. 2006a). Ligation performed in this manner did not include the chorda lingual nerve. Prior to the collection of the glands following the ligation-only procedure, the presence of the clip and the tube on the duct was confirmed in each animal. After 2 weeks the glands of the ligation only group were removed under terminal anaesthesia (pentobarbitone 60 mg/kg i.p) and weighed. In the other group (ligation followed by deligation), the duct was de-ligated (under recovery anaesthesia), and the glands were collected after 3 days. The collection of the glands was been performed under terminal anaesthesia (pentobarbitone 60 mg/kg i.p.).
After removal the submandibular gland was carefully dissected from the sublingual gland and weighed and a small portion was fixed in formal sucrose for histological sections. The rest of the gland was finely minced and incubated in cell culture media plus collagenase for cell preparation (see below).
Histochemical staining of tissue sections
Tissue fixed in formal sucrose was embedded in wax and 5μm sections were cut and mounted on slides. For general morphology tissue sections were then stained with Mayer's Haematoxylin and 1% Eosin (H&E). The secretory granules inside the acinar cells were identified by Alcian Blue period acid Schiff's (AB/PAS) staining.
Immunohistochemistry on tissue section
The tissue sections were first de-waxed and then incubated in a solution of 3% hydrogen peroxide to inhibit the endogenous peroxidase. After being washed in phosphate-buffered saline (PBS; 0.1 M), the sections were incubated with normal goat serum (DAKO, Ely UK, 1:5 dilution of PBS) to avoid non-specific binding of the primary antibody. To investigate the presence of the perinatal protein SMG-B, tissue sections were incubated with rabbit anti-ZZ3 polyclonal antibody (kind gift of Dr Hand, University of Connecticut, Farmington, USA) in a 1:50 dilution of PBS. The secondary antibody was biotinylated goat anti-rabbit polyclonal (DAKO, Ely UK, 1:400 dilution); then the section were reacted with streptavidin-biotin horse-radish peroxidase complex (DAKO , Ely UK). The peroxidase activity was visualized with diaminobenzidine tetrahydrochloride (DAB) (0.5 mg/ml) and counterstained with Mayer's Haematoxylin. Immunohistochemistry on tissue section was a better technique for investigating the presence of SMG-B than immunocytochemistry on cell preparations. A similar method for Ki-67 staining (1:50 dilution of rabbit polyclonal anti- Ki67, Thermo Scientific, Runcorn, Cheshire, UK) was used except sections were incubated at 95 °C for 15 mins in citric acid pH 6.0 before being incubated with the primary antibody.
Cell preparation
A portion of the gland was minced and digested with collagenase (0.5 mg/ml Worthington Biochemical Corporation, Freehold, N.J.) as previously described (Xu et al. 1996). The tissue was incubated at 37°C for 45 minutes in the presence of 100% oxygen and then centrifuged (1000 g) and cells re-suspended in 10 ml of new media without collagenase and incubated in presence of oxygen at 37°C for another 30 minutes.
Immunocytochemistry on Confocal Laser Microscope
After incubation in buffer without collagenase the cells were washed with PBS and then fixed in 100% methanol for 10 minutes at room temperature. After being washed in PBS they were sequentially incubated in blocking buffers with various concentrations of bovine serum albumin (BSA; 0.1% and 1% w/v) followed by mouse anti-human smooth muscle actin monoclonal antibody (DAKO clone 1A4, Ely UK, 1:200 dilution), or with goat anti AQP5 monoclonal antibody (Santa Cruz, CA USA, 1:50 dilution). The negative controls were incubated with 1% BSA blocking buffer and secondary antibody only. After washes with PBS the secondary fluorescent antibodies (anti-mouse Alexa 568 or anti-goat Alexa 568; Invitrogen, Paisley, UK; 1:2000 dilution) were applied to the cells. Finally, the cells were washed and mounted on slides with MOWIOL solution.
Following collagenase-digestion and immunocytochemistry discrete acinar-ductal units were analyzed with the laser confocal microscopy. Optical sections (1 μm) were captured at various depths, projected as a three-dimensional (3D) image and colour-coded according to the depth of field. Preliminary studies had indicated that the 3D images of Actin staining provided information not only about myoepithelial cell morphology but, because of their localization, also about acinar cell area. Likewise, for AQP5, capturing optical sections at different depths allowed us to track the expression of AQP5 all along the acinar-ductal unit under various experimental conditions. However, similar results were not obtained for SMG-B and so standard immuno-histochemistry was performed (see above).
Measurement of acini area
Samples prepared for the actin immunostaining were also used for measuring the area (μm2) of the acini by means of Leica TCS SP2 confocal microscope software, version 2.1 (Leica Microsystems Heidelberg GmbH). The shape of the acini was deduced from the morphology of the myoepithelial cell surrounding them.
Statistical analysis
Results were expressed as means ± SEM (Standard Error of the Mean), and were statistically compared by ANOVA and paired Student's t-test; p< 0.05 was considered statistically significant.
RESULTS
Gland weights
Following 2 weeks of ligation submandibular glands weighed less than 50% of the unoperated controls (figure 1). However, once deligated for 3 days the glands showed a significant increase in weight of about 24% above ligated glands, although still 2 fold less than the mean weight of normal gland (figure 1).
Fig. 1.
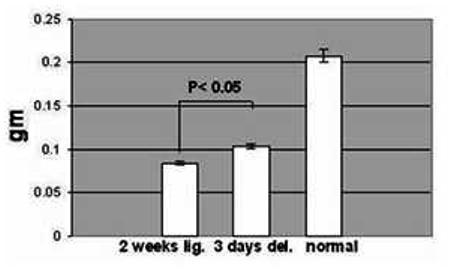
Mean weight (± SEM) of 2 weeks ligated (2 weeks lig.), of 2 weeks ligated plus 3 days de-ligated (3 days del.) and unoperated (normal) adult submandibular glands. The 2 weeks ligated plus 3 days de-ligated gland (n=5) showed an increase in weight (p < 0.05) compared to the 2 weeks ligated (n=5), but were nevertheless half the typical weight of a normal gland (n=4).
Light microscopy
Compared with unoperated adult submandibular glands both the 2 weeks ligated and the 3 days de-ligated glands showed increased inflammatory cell infiltration in the connective tissue between the lobules and among the parenchymal elements, although this seemed less severe in the de-ligated glands (figure 2c, e). H&E staining of the ligated glands suggests that the acini had almost completely disappeared, whereas many residual ducts-like structures were still visible with a considerably dilated lumen compared with the control (figure 2a, c). Accordingly, AB/PAS revealed an almost complete absence of secretory granules from both acini and ductal cells in ligated glands. In addition this stain also showed the presence of some secretory products in the lumen of the ducts (figure 2 d) in contrast with the de-ligated glands where the AB/PAS staining in the lumen was lost (figure 2 f).
Fig. 2.
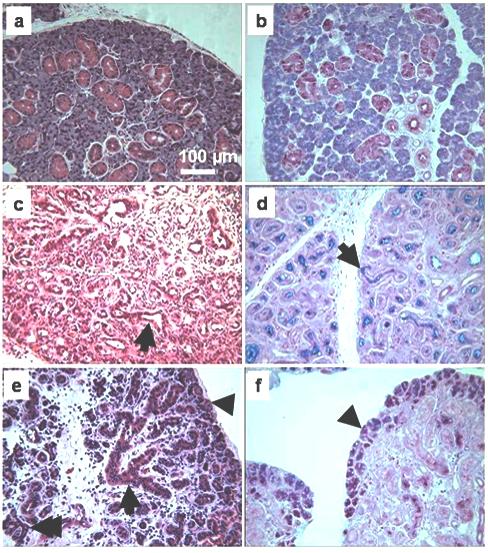
Histological comparison among unoperated gland, ligated, and de-ligated submandibular gland. a, b) Unoperated gland H&E and AB/PAS, respectively, showing typical appearance of acinar and duct cells. c) H&E stained section of ligated gland, showing luminal dilatation of the duct (arrow), absence of acini and extensive inflammation. d) AB/PAS in ligated gland, showing loss of cellular secretory granules and material in the lumen of the ducts (arrow). e) 3 days de-ligated gland H&E section; some acini (arrow head) and ductal cells (arrow) have recovered some of their size. Acinar-ductal branched structures are often visible (double arrow). f) AB/PAS in de-ligated gland, showing some acini that have recovered their glycoproteins content (arrowhead).
In the 3 days de-ligated glands the H&E staining showed that some acini, mostly on the edge of the lobules, have recovered some of their size; also the lumen of the ducts appeared less obvious (figure 2 e). This was supported by the AB/PAS staining showing that several acini, often located at the edge of the lobules, had recovered some of their glycoproteins content (figure 2 f).
In both ligated and de-ligated glands H&E staining revealed an apparent increase in the proportion of ducts together with the presence of abnormal branched entities characterized by short duct-like structures ending with small acini (figure 3 a, b & c). These branched structures were more frequent in the de-ligated glands than in the ligated glands (figure 4), and strongly resembled structures occurring during branching morphogenesis in embryonic development (embryonic day 18) of the submandibular gland (figure 3f for purpose of comparison). In the de-ligated gland some of the acini at the end of the branched structures were stained with AB/PAS showing an increased presence of glycoproteins (figure 3d, e).
Fig. 3.
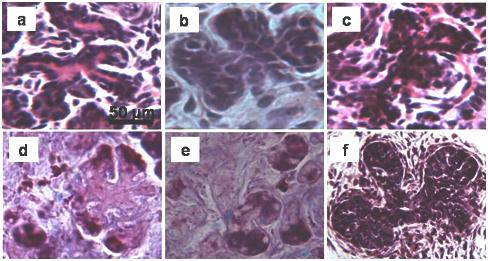
Branched structures occurring in the 3 days deligated tissue (a, b, d, e) and in the 2 weeks ligated tissue (c). The AB/PAS staining shows presence of glycoproteins inside the immature acini at the end of the branched structures (d, e). (f) Branched structures occurring during the embryonic development of rat submandibular gland, (embryonic day 18).
Fig. 4.
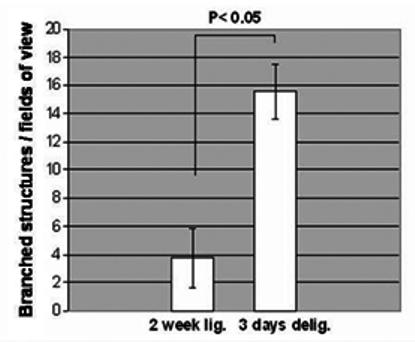
Mean (± SEM) number of branched structures per field of view (200 × magnification) in the 2 weeks ligated (2 weeks lig.) and in the 2 weeks ligated plus 3 days de-ligated (3 days del.) submandibular gland stained by H&E. The 2 weeks plus 3 days de-ligated gland showed an increase in the frequency of the branched structures (p< 0.05, 5 observations from 5 rats, n=25) compared to the 2 weeks ligated. In the unoperated tissue, no branched structures were identified.
Immunocytochemistry
Actin immunofluorescence
In the unoperated submandibular glands anti-actin antibody showed star-shaped myoepithelial cells surrounding the acini, but not the big ducts (figure 5a). In the ligated only tissue the immunostaining revealed, in contrast with the H&E staining (figure 2c), the presence of acini groups albeit shrunken (figure 5b). They appeared to be fully covered by myoepithelial cells and without their characteristic star-shape. In the de-ligated glands the myoepithelial cells regained some of their normal morphology (figure 5 c); the gaps between the cellular processes became visible, and the acini seem to have recovered some of their size. Morphometric analysis confirmed a significant increase (more than 80%) in the size of the acini of the deligated gland, compare to the ligated only gland (figure 6).
Fig. 5.
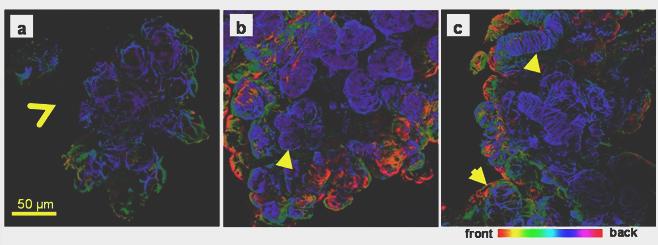
Actin immunofluorescence in adult rat submandibular gland. Collagenase-digested cells were incubated with an anti-smooth muscle actin antibody and viewed by confocal microscopy. Optical sections (approximately 1μm) were taken and projected to create a 3D image and colour-coded according to the depth of field. a) Unoperated submandibular gland, star-shaped myoepithelial cells surround the acini, but not the big ducts (open arrowhead). b) In the ligated gland myoepithelial cells have lost their typical morphology but reveal the presence of numerous shrunken acini (arrowhead). c) In the de-ligated gland the myoepithelial cells on the acini recovered their normal star-shaped morphology (arrow). In ligated and deligated tissue myoepithelial cells were found on the big ducts (arrow head).
Fig. 6.
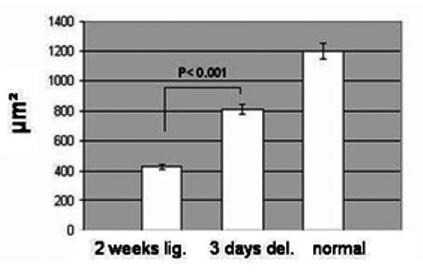
Mean (± SEM) area of the acini from the 2 weeks ligated (2 weeks lig.), from the 2 weeks ligated plus 3 days de-ligated (3 days del.) and from unoperated (normal) submandibular gland. The 3 days de-ligated gland showed a significant increase in the acini area (p < 0.001, 20 observations from 3 samples n=60) compared to the 2 weeks ligated only glands.
The actin staining also reveals that in the ligated and de-ligated glands some big ducts appear to be fully covered by spindle-shape myoepithelial cells (figure 5 c).
Aquaporin 5 (AQP5) immunofluorescence
In the unoperated tissue AQP5 was localized along the apical membrane, including the intercellular secretory canaliculi, and the intercalated ducts (figure 7 a). After 2 weeks ligation the expression of AQP5 decreased and the labels for this water channel showed a dot-like patterns mainly restricted on the apical membrane of presumed acinar cells (figure 7 b).
Fig. 7.
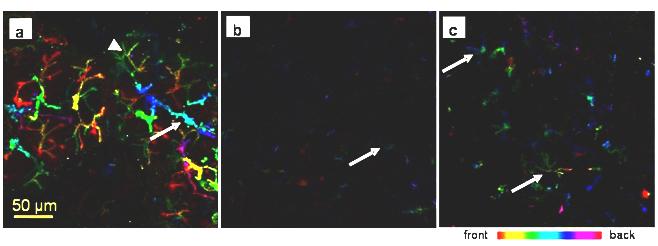
AQP5 immunofluorescence in rat submandibular gland. Collagenase-digested cells were incubated with an anti-AQP5. All images were taken using the same settings; different colours represent differences in depth of field. a) In the control gland AQP5 is strongly expressed along the apical membrane, the intercellular secretory canaliculi (arrowhead) and along the intercalated ducts (arrow). b) In the ligated tissue weak AQP5 expression is restricted to the apical membrane of presumed acinar cells (arrow). c) Some acini in the deligated tissue have regained some AQP5 expression along the intercellular secretory canaliculi (arrow)
Interestingly after 3 days following the removal of the obstruction AQP5 expression increased presumably at newly formed/regenerating acini (figure 7 c). AQP5 was never found in the duct system downstream from the intercalated ducts.
SMG-B and Ki-67 immunohistochemistry
Unoperated adult tissue showed immunoreactivity in several intercalated ducts, but not in the acini; (figure 8a), whereas in the ligated gland no specific staining of the parenchyma was observed (figure 8 b). In the de-ligated glands some acini on the edge of the lobules and few acini in the middle of the parenchyma were stained (figure 8 c). Ki-67 positive nuclei were present on the edges of lobes (figure 8d) suggesting an association with SMG-B positive acini although this needs to be confirmed by double immunostaining.
Fig. 8.
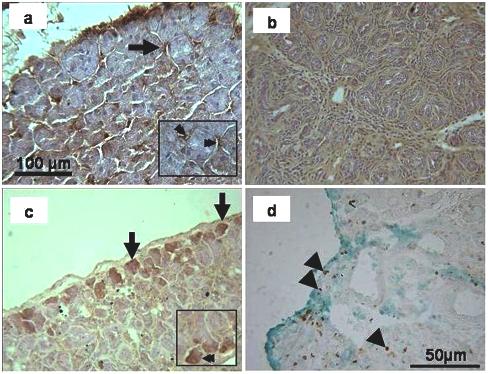
SMG-B immunohistochemistry of the submandibular gland. a) In the unoperated control the staining appeared in some intercalated ducts (arrow; see also double arrow in inset) but not in the acini. b) The 2 weeks ligated gland did not show any localised staining in the parenchyma. c) In the de-ligated tissue, some acini on the edge of lobule showed positive immunoreactivity (arrow; see also double arrow in inset). d) Ki-67 staining (counterstained with Light Green) on 3 day de-ligated plus 2 week ligated submandibular gland. Some cells on the edge of lobules (arrowheads) and in the centre of glands (arrowheads) were labelled.
DISCUSSION
The functional unit of rat submandibular gland consists of acini and various sets of branched ducts, viz. intercalated, striated and excretory ducts (Tandler 1993). During the embryonic stage, the major salivary glands originate from an interaction between the epithelium and the surrounding mesenchyme (Cutler and Chaudhry 1973;Cutler and Chaudhry 1974). Branching morphogenesis gives rise to the ductal system and marks the start of the cytodifferentiation which continues during the perinatal stage of development (Ball et al. 1991;Patel et al. 2006). Acinar cells are mainly responsible for fluid and protein secretion, whereas the ductal system is primarily involved in the re-absorption of sodium chloride (Martinez 1987;Thaysen et al. 1954). In addition myoepithelial cells, which are typically star-shaped, are usually found surrounding the acini and intercalated ducts (Nagato et al. 1980;Redman 1994).
Characterization of the early regeneration of submandibular gland following ligation-induced atrophy is a crucial step for fully understanding the mechanisms leading to the recovery of their parenchymal elements and eventually their secretory ability. In this study we have used the intra-oral ductal ligation model for inducing atrophy and have identified some novel morphological and cellular markers of regeneration occurring as early as 3 days after deligation of the duct. In the 2 week ligated glands dramatic morphological changes occurred, all of them suggesting that ligation of the excretory ducts leads to atrophy of the glands as previously described in the literature (Norberg et al. 1988;Osailan et al. 2006b;Scott et al. 1999;Shiba et al. 1972). The luminal dilatation of the ducts suggests secretory pressure is maintained, whereas the proportion of duct-like structures (characterized by the presence of a lumen) seem to be increased because of the proliferation activity of the ductal cells (Walker and Gobe 1987;Takahashi et al. 2004b). In addition to the loss of cellular secretory products, the presence of some secretory material in the lumen of the ducts, as shown by AB/PAS, suggests that the acini and ductal cells have degranulated into the ductal lumen adding further to secretory pressure (Osailan et al. 2006a). After the removal of the duct ligation some acini, mostly on the edge of the lobules, recover their size. The lumen of the ducts is also less obvious suggesting a recovery of the cytoplasm of the ductal cells. Moreover, the absence of secretory material in the lumen of the ducts suggests the presence of active salivary flow, although further studies are needed to confirm this hypothesis.
Myoepithelial cells still persist in atrophic glands (Emmelin et al. 1974) and undergo morphological and proliferative changes during both atrophy and regeneration (Emmelin et al. 1974;Takahashi et al. 2001;Takahashi et al. 2002;Takahashi et al. 2004a). In addition they surround the acini and therefore changes in their morphology reflect changes in acini morphology. For these reasons, we decided to investigate the behaviour of myoepithelial cells in our study. The use of smooth muscle actin immunostaining of glands digested with collagenase permitted us to observe discrete acinar units, and to measure cellular areas by means of confocal laser microscopy.
In the ligated glands actin immunostaining indirectly showed the presence of several shrunken acini fully covered by myoepithelial cells; these acini were hard to identify after H&E staining. Previous papers have reported the disappearance of almost all the acini from the atrophic tissue in H&E sections (Walker and Gobe 1987;Tamarin 1971b;Tamarin 1971a), since they lose their secretory products and much of their cytoplasm. By observing acini indirectly by staining myoepithelial cells we believe that we have increased the sensitivity of the method and have obtained a better estimate of the acinar cells. Although this technique does not give us a quantitative measure of acinar numbers, it is clear from actin staining that considerable numbers of acini (but fewer than normal) are present, principally occurring at the end of extended ducts rather than throughout the highly branched structure of a normal adult gland.
The presence of significant numbers of acini in the atrophic glands suggests the glands maintain their secretory potential; these residual acini have also have been suggested to participate in the early stage of glandular regeneration, followed later by the proliferation of newly formed acini (Takahashi et al. 2004b). Spindle-shaped myoepithelial cells have also often been found in the ducts of the regenerated glands and occasionally in the ducts in the atrophic glands. This finding probably reflects the increase in the number of these cells, which have been shown to have proliferative activity under both these conditions (Takahashi et al. 2001;Takahashi et al. 2004a). However the way that myoepithelial cells appear on the ducts remains unclear, i.e. whether they move onto the ducts (Takahashi et al. 2002;Takahashi et al. 1999), or whether they differentiate from the basal cells located in the duct-like structures (Takahashi et al. 1997).
During this study we noticed the presence of branched structures, in both ligated and 3 day deligated tissue, although they appear to be significantly more frequent in the latter. These structures consisted of short branched ducts terminating with small, probably immature, acini and were comparable with those seen during the development of the ductal tree at the embryonic stage (Borghese 1950), thus providing a preliminary link between the regeneration process and embryonic development. In order to investigate further similarities with glandular embryonic/perinatal development, we decided to assess the presence of the perinatal protein SMG-B in the tissue via immunohistochemistry. In rat submandibular gland this protein has been shown to change its localization according to developmental stage. Indeed significant evidence has been provided showing that cells expressing SMG-B during the perinatal stage are pro-acinar cells (Ball et al. 1988;Moreira et al. 1991), whereas in adult tissue SMG-B expression is mostly restricted to the intercalated ducts (Ball et al. 1988;Man et al. 1995). In our experiments on adult unoperated tissue we have confirmed the presence of SMG-B as being restricted to the intercalated duct cell. However in the 3 day deligated tissue this protein was expressed in some of the immature acini. The presence of SMG-B in the acinar cells and its absence from the duct system in the deligated glands, suggest that during regeneration some of the immature acini exhibit a perinatal-like immunoreactive phenotype. We consider that these immature SMG-B positive acini are derived from intercalated duct cells which in the adult gland express the perinatal protein SMG-B, although further studies are needed to establish this hypothesis. No SMG-B immunoreactivity has been detected in ligated tissue; this is probably related to the considerable reduction of secretory protein synthesis that affects most secretory cells during atrophy.
APQ5 has been previously found to be expressed in the acinar cells and along the intercalated ducts in submandibular glands (Matsuzaki et al. 1999) and its importance in saliva secretion for water movement across the plasma membrane has been establish (Ma et al. 1999). The reduced expression of AQP5 that we have found in the ligated gland agrees with the defective fluid secretion previously reported for atrophic glands (Martinez et al. 1982;Osailan et al. 2006b). Our finding that the regenerating glands regained some AQP5 expression on the apical membrane and along the intercellular canaliculi may be linked to the ability of this tissue to recover its secretory activity as has been previously determined by functional studies at earlier (Carpenter et al. 2007) and later (Osailan et al. 2006a) time points.
In conclusion, our study has identified the morphological and molecular markers of glandular regeneration occurring 3 days after the removal of ligation of submandibular salivary glands. The most significant results is the increased weight of the glands, reflecting the increased acinar cell size and secretory glycoprotein content, and the increase in the number of embryonic-like branched structures in the regenerated gland. The expression of the perinatal protein SMG-B in some of the newly formed acini and novel acinar-duct structures have provided preliminary evidence of a correlation between submandibular gland regeneration and embryonic/perinatal development, in terms of morphological changes and cytodifferentiation. The recovering AQP5 expression in some acinar cells, and the return of the typical star-shaped morphology of the myoepithelial cells surrounding the acini are early signs of recovery of function.
ACKNOWLEDGEMENTS
We wish to thank Dr. A.R. Hand (University of Connecticut, Farmington, USA) for providing us with the rabbit anti-ZZ3 polyclonal antibody and the Wellcome Trust for funding.
The original publication is available at www.springerlink.com, DOI: 10.1007/s00441-008-0588-6
Reference List
- Ball WD, Hand AR, Johnson AO. Secretory proteins as markers for cellular phenotypes in rat salivary glands. Dev Biol. 1988;125:265–279. doi: 10.1016/0012-1606(88)90210-2. [DOI] [PubMed] [Google Scholar]
- Ball WD, Hand AR, Moreira JE. A neonatal secretory protein associated with secretion granule membranes in developing rat salivary glands. J Histochem Cytochem. 1991;39:1693–1706. doi: 10.1177/39.12.1940321. [DOI] [PubMed] [Google Scholar]
- Borghese E. The development in vitro of the submandibular and sublingual glands of Mus musculus. J Anat. 1950;84:287–302. [PMC free article] [PubMed] [Google Scholar]
- Burgess K, Dardick I, Cummins MM, Burford-Mason AP, Basset R, Brown DH. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Radiol Endod. 1986;82:674–680. doi: 10.1016/s1079-2104(96)80443-4. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Osailan SM, Correia PN, Paterson KL, Proctor GB. Rat salivary gland ligation causes reversible secretory hypofunction. Acta Physiol. 2007;189:241–249. doi: 10.1111/j.1365-201X.2006.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins M, Dardick I, Brown D, Burford-Mason A. Obstructive sialadenitis: a rat model. J Otolaryngol. 1994;23:50–56. [PubMed] [Google Scholar]
- Cutler LS, Chaudhry AP. Intercellular contacts at the epithelial-mesenchymal interface during the prenatal development of the rat submandibular gland. Dev Biol. 1973;33:229–240. doi: 10.1016/0012-1606(73)90133-4. [DOI] [PubMed] [Google Scholar]
- Cutler LS, Chaudhry AP. Cytodifferentiation of the acinar cells of the rat submandibular gland. Dev Biol. 1974;41:31–41. doi: 10.1016/0012-1606(74)90280-2. [DOI] [PubMed] [Google Scholar]
- Emmelin N, Garrett JR, Ohlin P. Secretory activity and the myoepithelial cells salivary gland after duct ligation in cats. Arch Oral Biol. 1974;19:275–283. doi: 10.1016/0003-9969(74)90188-5. [DOI] [PubMed] [Google Scholar]
- Garrett JR. The proper role of nerves in salivary secretion: a review. J Dent Res. 1987;66:387–397. doi: 10.1177/00220345870660020201. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Badir MS. Chronic submandibular sialadenitis: ultrastructure and phosphatase histochemistry. Ultrastruct Pathol. 1998;22:431–437. doi: 10.3109/01913129809032278. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Fouad HM, Garrett JR. Variation in the response to ductal obstruction of feline submandibular and sublingual salivary glands and the importance of the innervation. J Oral Pathol Med. 2001;30:29–34. doi: 10.1034/j.1600-0714.2001.300105.x. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Garrett JR. Histological effects of ductal ligation of salivary glands of the cat. J Pathol. 1976;118:245–254. doi: 10.1002/path.1711180407. [DOI] [PubMed] [Google Scholar]
- Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Man YG, Ball WD, Culp DJ, Hand AR, Moreira JE. Persistence of a perinatal cellular phenotype in submandibular glands of adult rat. J Histochem Cytochem. 1995;43:1203–1215. doi: 10.1177/43.12.8537636. [DOI] [PubMed] [Google Scholar]
- Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263:202–214. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- Martinez JR. Ion Transport and water movement. J Dent Res. 1987;66:638–647. doi: 10.1177/00220345870660S206. [DOI] [PubMed] [Google Scholar]
- Martinez JR, Bylund DB, Cassity N. Progressive secretory dysfunction in the rat submandibular gland after excretory duct ligation. Arch Oral Biol. 1982;27:443–450. doi: 10.1016/0003-9969(82)90082-6. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- Matthews TW, Dardick I. Morphological alterations of salivary gland parenchyma in chronic sialadenitis. J Otolaryngol. 1988;17:385–394. [PubMed] [Google Scholar]
- Moreira JE, Ball WD, Mirels L, Hand AR. Accumulation and localization of two adult acinar cell secretory proteins during development of the rat submandibular gland. Am J Anat. 1991;191:167–184. doi: 10.1002/aja.1001910204. [DOI] [PubMed] [Google Scholar]
- Nagato T, Yoshida H, Yoshida A, Uehara Y. A Scanning Electron Microscope Study of Myoepithelial Cells in Exocrine Glands. Cell Tissue Res. 1980;209:1–10. doi: 10.1007/BF00219918. [DOI] [PubMed] [Google Scholar]
- Norberg LE, Abok K, Lundquist PG. Effects of ligation and irradiation on the submaxillary glands in rats. Acta Otolaryngol. 1988;105:181–192. doi: 10.3109/00016488809119463. [DOI] [PubMed] [Google Scholar]
- Osailan SM, Proctor GB, Carpenter GH, Paterson KL, McGurk M. Recovery of rat submandibular salivary gland function following removal of obstruction: a sialometrical and sialochemical study. Int J Exp Pathol. 2006a;87:411–423. doi: 10.1111/j.1365-2613.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osailan SM, Proctor GB, McGurk M, Paterson KL. Intraoral duct ligation without inclusion of the parasympathetic nerve supply induces rat submandibular gland atrophy. Int J Exp Pathol. 2006b;87:41–48. doi: 10.1111/j.0959-9673.2006.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Redman RS. Myoepithelium of salivary glands. Microsc Res Tech. 1994;27:25–45. doi: 10.1002/jemt.1070270103. [DOI] [PubMed] [Google Scholar]
- Scott J, Liu P, Smith PM. Morphological and functional characteristics of acinar atrophy and recovery in the duct-ligated parotid gland of the rat. J Dent Res. 1999;78:1711–1719. doi: 10.1177/00220345990780110801. [DOI] [PubMed] [Google Scholar]
- Shiba R, Hamada T, Kawakatsu K. Histochemical and electron microscopical studies on the effect of duct ligation of rat salivary glands. Arch Oral Biol. 1972;17:299–309. doi: 10.1016/0003-9969(72)90213-0. [DOI] [PubMed] [Google Scholar]
- Shwartz-Arad D, Michaeli Y, Zajicek G. Compensatory Hyperplasia of the rat submandibular gland following unilateral extirpation. J Dent Res. 1991;70:1328–1331. doi: 10.1177/00220345910700100301. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Domon T, Yamamoto T, Wakita M. Regeneration of myoepithelial cells in rat submandibular glands after yttrium aluminium garnett laser irradiation. Int J Exp Pathol. 1997;78:91–99. doi: 10.1046/j.1365-2613.1997.d01-244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Shinzato K, Domon T, Yamamoto T, Wakita M. Apoptosis and proliferation of Myoeithelial Cells in Atrophic Rat Submandibular Glands. J Histochem Cytochem. 2001;49:1557–1563. doi: 10.1177/002215540104901209. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Suzuki R, Domon T, Yamamoto T, Wakita M. Changing myoepithelial cell distribution during regeneration of rat parotid glands. Int J Exp Pathol. 1999;80:283–290. doi: 10.1046/j.1365-2613.1999.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Proliferation and distrubution of myoepithelial cells during atrophy of the rat sublingual gland. J Oral Pathol Med. 2002;32:90–94. doi: 10.1034/j.1600-0714.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004a;33:430–434. doi: 10.1111/j.1600-0714.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004b;33:23–29. doi: 10.1111/j.1600-0714.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Tamarin A. The leukocytic response in ligated rat submandibular glands. J Oral Pathol. 1979;8:293–304. doi: 10.1111/j.1600-0714.1979.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Submaxillary gland recovery from obstruction. II. Electron microscopic alterations of acinar cells. J Ultrastruct Res. 1971b;34:288–302. doi: 10.1016/s0022-5320(71)80073-4. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Submaxillary gland recovery from obstruction. I. Overall changes and electron microscopic alterations of granular duct cells. J Ultrastruct Res. 1971a;34:276–287. doi: 10.1016/s0022-5320(71)80072-2. [DOI] [PubMed] [Google Scholar]
- Tandler B. Structure of the duct system in mammalian major salivary glands. Microsc Res Tech. 1993;26:57–74. doi: 10.1002/jemt.1070260107. [DOI] [PubMed] [Google Scholar]
- Thaysen JH, Thorn NA, Schwartz IL. Excretion of Sodium, Potassium, Chloride and Carbon Dioxide in Human Parotid Saliva. American Journal of Physiology. 1954;178:155–159. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- Walker NI, Gobe GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol. 1987;153:333–344. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]
- Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signalling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996;491(Pt 3):647–662. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]


