Abstract
Tuberculosis continues to be one of the most important global infectious causes of morbidity and mortality. Development of a more effective vaccine is a high worldwide priority and depends on a thorough understanding of the host response to infection. In this review, we highlight recent advances in our understanding of the innate immune response to MTb infection. We also describe recent discoveries in immunogenetics that are generating insight into the potential development of immunomodulatory therapies.
Introduction
Mycobacterium tuberculosis (MTb) infects approximately one-third of the world's population, kills more than 2 million people a year and is comparable only to human immunodeficiency virus (HIV) as an infectious cause of death. Despite the development of effective drug therapy for Mtb over 50 years ago, there remain a number of formidable challenges for controlling MTb infection. These obstacles include lengthy treatment regimens of 6-9 months duration, drug resistance, the lack of a highly efficacious vaccine, and an incomplete understanding of what controls infectivity and progression of disease. Although vaccination with Bacille-Calmette-Guerin (BCG) has been widely used since 1921 and does offer some protection against MTb, its efficacy is suboptimal and not adequate for disease control [1]. Development of a more effective vaccine is a high worldwide priority and depends on a thorough understanding of the host response to infection.
This review will focus on the innate immune response to MTb and recent discoveries in immunogenetics that are leading to insights into novel immunomodulatory therapies. We will review the research regarding innate host immune receptors on macrophages and dendritic cells and their role in regulating the initial detection of MTb as well as the subsequent development of adaptive immunity. Rather than providing a comprehensive review, we will highlight factors that may influence partial immunity to MTb and how modulation of the innate immune response may be incorporated into future MTb vaccines and therapeutic strategies. In-depth review of subtopics within this manuscript include work on the immune response to Tb [2,3], genetic influences on Tb susceptibility [4-7], and the innate immune response [8-10].
Disease Mechanism 1: Macrophages, genetics, and the innate immune response to MTb
Initial detection of MTb involves a number of receptors on phagocytes, including several Toll-like receptors (TLRs), Nod-like receptor 2 (NOD2), complement receptors, the mannose receptor (MR), dendritic cell-specific intracellular adhesion molecule-3 (ICAM-3)-grabbing non-integrin (DC-SIGN) (CD209), surfactant protein A (Sp-A) receptor, the class A scavenger receptor, mannose-binding lectin (MBL), and possibly dectin-1 [4,11-14]. The TLRs are a family of transmembrane proteins that are known as pattern recognition receptors (PRRs). Through recognition of specific microbial ligands, TLRs activate inflammatory signaling pathways [8-10]. There are a number of components of the mycobacterial cell wall that stimulate TLRs, including lipoproteins, the MTb cell wall core structure mucolylarabinogalactan-peptidoglycan complex (mAGP), lipids, and LAM [11,12]. In vitro studies indicate that TB is recognized by several TLRs, including TLR2 (as a heterodimer with TLR1 or TLR6), TLR9, and possibly also TLR4 [4,11,12]. Stimulation of TLRs on the surface of macrophages leads to the recruitment of several adaptor proteins including MyD88 (Myeloid differentiation primary response gene 88), TIRAP/MAL (TIR domain containing adapter/MYD88 adaptor-like), TICAM1/TRIF (TIR-Domain-containing adaptor molecule 1/TIR domain-containing adaptor inducing interferon beta), and TRAM (TRIF-related adaptor molecule). This subsequently leads to activation of a series of signaling pathway molecules that culminates in translocation of nuclear factor kappa B (NF-κB) into the nucleus and activation of immune response genes such as cytokines, chemokines, and nitric oxide synthase.
The ability of MTb to survive and proliferate inside human macrophages is influenced by pathogen factors as well as the host's innate and adaptive immune response. Several lines of evidence from a series of studies over the past 50 years suggest that host genetics influences susceptibility to tuberculosis and other Mycobacteria. First, twin studies indicate that TB rates among monozygotic twins are more than twice the rate of dizygotic twins [15]. Second, several primary immunodeficiency disorders are associated with susceptibility to mycobacteria in a Mendelian fashion attributable to rare single gene mutations with high penetrance [5]. Third, several genome-wide studies of susceptibility to TB have been performed with family-based linkage studies and have identified several promising chromosomal loci [16-19]. Fourth, candidate gene association studies, which evaluate whether common polymorphisms in candidate genes are associated with susceptibility to disease, suggest several loci that are associated with Tb susceptibility. Although previous studies have uncovered some of the genes involved in human predisposition to mycobacterial infections, a comprehensive understanding of genetic susceptibility factors remains an elusive and important goal [5].
Human genetic studies indicate that variants in TLR pathway genes regulate the cellular immune response and may influence susceptibility to MTb [4,5,7,20]. A polymorphism in the signaling domain of TLR2 (G2258A (R753Q)) alters signaling in response to stimulation with lipopeptides as measured by NF-κB luciferase assays [21]. Homozygous (753QQ) and heterozygous (753RQ) genotypes were associated with an increased risk of MTb [22]. A GT dinucleotide repeat poylmorphism in intron II was associated with increased susceptibility to TB as well as altered TLR2 promoter activity [23]. We found that a synonymous polymorphism in the TLR2 domain (T597C, N199N) was associated with increased susceptibility to TB meningitis in a cohort of patients from Vietnam [24].
TLR2 forms a heterodimer with TLR1 or TLR6 to mediate host responses to lipopeptides from several classes of pathogens. We and others recently described a transmembrane SNP, T1805G (I602S) in TLR1 that regulates lipopeptide-induced signaling [25,26]. Individuals with the 602SS genotype had a greater than ten-fold reduction in levels of IL-6 after whole blood stimulation with tri-acylated lipopeptide compared to 602SI and II individuals [25]. In addition, there was diminished cell surface TLR1 staining of monocytes in 602SS individuals, but normal total cellular levels of TLR1 [26]. This finding suggested a defect in TLR1 trafficking to the cell surface in 602SS individuals. In a cohort of leprosy patients from Turkey, the G allele at 1805 (602S) was associated with protection against leprosy (odds ratio 0.48, 95% CI 0.29–0.80, p = 0.004) [26]. We have independently investigated the association of this SNP with leprosy susceptibility in a separate cohort and found that allele 602S is associated with protection from reversal reaction (EA Misch & TR Hawn, unpublished observations). There is high potential clinical impact for this polymorphism given its clear role in regulating the cellular immune response, its high frequency and the diverse array of pathogens recognized by TLR1/2 heterodimers.
Recent studies of TIRAP/MAL suggest an association of SNPs in this gene with MTb. A polymorphism in the TIRAP gene which changes a serine to a leucine at amino acid 180 (S180L, C539T) has been shown to impair TLR2-mediated NF-κB signaling in in vitro experiments [27]. The 180L variant was less able to bind TLR2 in comparison to the 180S variant. The heterozygous state was associated with protection from MTb in Guinea-Bassau. We recently examined variants of TIRAP in a cohort of Vietnamese patients [28]. Although we did not find an association of the S180L polymorphism with susceptibility to Mtb, the frequency of this SNP was too low for proper statistical evaluation. We did find a synonymous SNP (C558T, A186A) that was associated with increased susceptibility to meningeal TB and decreased lipopeptide-induced whole blood IL-6 production. These findings suggest that TIRAP variants influence susceptibility by modulating the inflammatory response. In addition, the TLR pathway may influence susceptibility to meningeal and pulmonary TB by different immune mechanisms. Although many of these genetic findings require replication and further cellular experiments to better understand the impact of the polymorphism on gene function, together these studies suggest that common variants in the TLR pathway regulate innate immune signaling responses and are associated with susceptibility to tuberculosis.
Disease Mechanism 2- Dendritic cells, genetics, & MTb infection—bridging the innate-adaptive interface
Recognition of MTb by dendritic cells induces an inflammatory response that leads to the development of adaptive immunity. One arm of this immune response is Th1 type immunity with T-cell production of IFN-γ that can activate macrophages to produce nitric oxide and restrict mycobacterial growth [2,3]. IL-12, which is secreted by monocytes, macrophages, and dendritic cells, promotes T-cell differentiation into Th1 cells and production of IFN-γ. IL-23 has recently been shown to be secreted by dendritic cells and to stimulate IL-17 production by T cells and a Th17 type response. MTb stimulates production of both IL-12 and IL-23, which contribute overlapping, but distinct roles in the host immune response to MTb through Th1 and Th17 type T cell responses [29,30]. Development of an effective adaptive immune response includes maturation of dendritic cells for optimal antigen presentation and activation of T cells. Although stimulation of TLRs enhances maturation of DCs and influences T cell responses, the degree and type of DC response may be influenced by which TLRs and DCs are stimulated. Dendritic cells display diversity in both the number and type of TLR molecules they express. For example, TLR4, which recognizes bacterial LPS, is located on the cell membrane of human myeloid dendritic cells and monocytes. In contrast, TLR9 is expressed on endosomal membrane of plasmacytoid dendritic cells [31]. Stimulation through TLR3, 4, & 5 on myeloid DCs leads to high levels of IL-12p70 and favors a Th1 response in contrast to stimulation through TLR2 (in conjunction with 1 or 6) which leads to lower levels of IL-12p70, higher levels of IL-10 and favors a Th2 response [31,32]. Stimulation through TLR9 occurs on plasmacytoid DCs and leads to production of IFN-α and Il-12p70 and primarily a Th1 type response [33]. ManLAM induces IL-10 secretion and suppression of DC-activation in a DC-SIGN-dependent manner [34]. Recent data suggests that Dectin-1 mediates MTb-stimulated DC production of IL-12 raising the possibility that it influences a Th1 type T cell response [13]. NOD2 stimulation of DCs has been shown to stimulate IL-23 production and foster a Th17 type T-cell response [35]. Further studies will illuminate the degree to which Th1 and Th17 T-cell responses to MTb are influenced by selective stimulation of innate immune receptors. Given that MTb activates DCs through TLR1,2,6,9, NOD2, DC-SIGN and possibly TLR4 and Dectin-1, there are multiple potential adaptive immunity phenotypes that may result from MTb interaction with the innate immune system. Together, these results suggest that modulation of the interaction of Tb with these innate immune pathways may lead to altered adaptive immune responses.
Several lines of evidence suggest that host genetic variation influences dendritic cells and the adaptive immune response to MTb. Early studies suggested associations of MHC variants and MTb susceptibility [7,20,36]. In addition, the discussion of variants in TLR pathway genes described above may impact DC function. More recently, genetic studies of human DC-SIGN in South Africa have identified two promoter variants, -871G and -336A, that are associated with TB susceptibility [37]. The -336 SNP has also been found to be associated with Dengue fever and alters transcriptional activity at a Sp1-binding site, where the SNP resides [38]. In a study from Colombia, there was no association of the -336 SNP with TB susceptibility [39]. Together, these results suggest a possible role for DC-SIGN variation and susceptibility to human TB, although the magnitude of the effect is small and has not been validated in a second TB population. A mutation in the IL-12p40 subunit, which abrogates IL-12 production in the homozygous state is associated with a primary immunodeficiency in humans that manifests with increased susceptibility to mycobacterial disease [5]. In addition, candidate gene studies suggest that several common IL-12 polymorphisms may be associated with MTb susceptibility [40,41]. However, further studies on these polymorphisms in a Korean population and a Moroccan population failed to show an association with susceptibility to [42,43]. Together, these results suggest that genetic variation in genes regulating dendritic cell function may be associated with susceptibility to MTb. Furthermore, specific modulation of DC function provides a promising strategy to modulate anti-mycobacterial T-cell responses.
Conclusions: can partial immunity to MTb and BCG vaccination be overcome by innate immunomodulatory therapies?
There are a number of critical questions about the host immune response to tuberculosis that are not well understood. First, why do some exposed individuals never become infected and others rapidly develop symptoms (primary progressive disease)? (Fig. 1). Second, why do only 10% of individuals who are latently infected with MTb develop active disease? Third, why do some individuals have disseminated tuberculosis that spreads to the meninges and central nervous system, while most people have localized disease in the lungs? Finally, why is there wide variation in efficacy of the BCG vaccine [1]. Each of these problems is due to partial immunity—either from natural infection or from vaccination with BCG. Recent advances in our understanding of the innate immune response to MTb suggest that such “partial” immunity may result from genetic variation in innate immune response genes. Although these studies are in their early stages and require replication in additional populations, they suggest that common variation in innate immune genes may provide a explanation for the partial immunity that is observed in the host response to MTb. In addition, these studies suggest possible therapeutic interventions that may alter or augment the existing innate immune response in a host. For example, selective stimulation of TLR4 and Dectin-1 may enhance Th1 adaptive immune responses. In contrast, stimulation of TLR2 may culminate in a Th2 type response while stimulation of DC-SIGN may suppress DC-function. Finally, NOD2 agonists may promote Th17 type immunity. We speculate that such immunomodulatory strategies could enable the host to boost its immune response with various potential outcomes. For example, current strategies for treatment of latent Tb require 9 months of INH therapy. Selective stimulation of an innate immune receptor may lead to activation of the adaptive immune response and an ability to eliminate the bacillus with a shorter course of therapy. Alternatively, innate immunomodulatory strategies may enable the host to prevent disseminated disease. Finally, innate immune receptor ligands serve as adjuvants and could potentially be used to alter the nature and efficacy of the BCG vaccine. The vaccine for yellow fever, which is highly effective, has been shown to stimulate TLR2, 7, 8 and 9 [44]. The BCG vaccine, conversely, is unlikely to stimulate through TLR7/8 and will likely stimulate through TLR1,2,4,6,9, NOD2, DC-SIGN, and Dectin-1. It is possible that stronger or more selective stimulation of the innate immune system with TLR and other innate immune pathway adjuvants will confer a more robust adaptive immune response. However, stimulation could also lead to adverse outcomes.
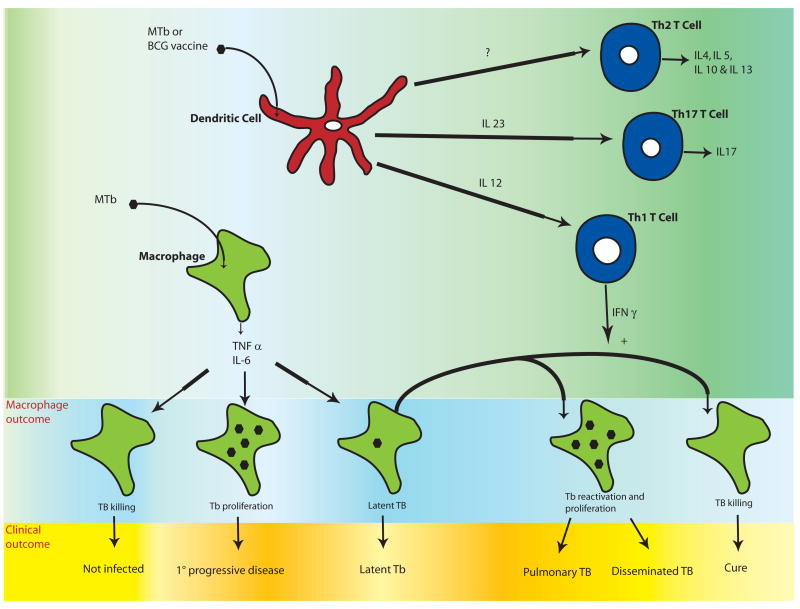
Figure 1. Clinical and cellular outcomes of Mycobacterium tuberculosis (MTb) and the role of the adaptive immune system.
Initial MTb infection of the macrophage results in production of pro-inflammatory cytokines such as IL-6, IL-1β, IL-12, and TNF-α. Unknown factors in host defense lead to a spectrum of initial clinical outcomes including resistance to infection, primary progressive disease, and latency. In latent infection, there is a balance of mycobacterial proliferation and host defense and MTb is contained in granulomas with no clinical symptoms. Ten percent of those with latent MTb infection will eventually develop clinically active disease that manifests as localized pulmonary infection (in 80% of individuals) or disseminated disease. These diverse clinical outcomes may be regulated by variation in the innate immune response of macrophages and/or dendritic cells. T cell responses are shaped by interactions with dendritic cells, which depend on the innate immune response to MTb or BCG. Th1 T cells produce IFN-γ and promote mycobacterial killing of TB-infected macrophages. Th17 T cells secrete IL-17 and may be important for protective vaccine-induced responses. The role of Th2 T cells in host defense to MTB infection is less clear. The combination of innate and adaptive responses influences the macrophage response to TB infection as well as the clinical outcome. Immunomodulatory therapy directed at innate immune receptors could potentially alter clinical outcomes by affecting several steps in disease pathogenesis outlined in this figure.
Efforts to develop agonists and antagonists of TLRs are underway for a number of diseases. For example, CpG DNA TLR9 agonist compounds are currently under development for a number of applications including infections, cancer, and allergic and atopic disease [33]. TLR4 agonists are also being developed as adjuvants for a number of applications [45]. Before implementing innate immunomodulatory strategies for MTb treatment, further studies of the correlates of protective immunity to MTb and BCG vaccination will be needed. Given the dramatic progress in our understanding of the innate immune response to MTb over the past 10 years, such novel treatment strategies are becoming realistic hopes.
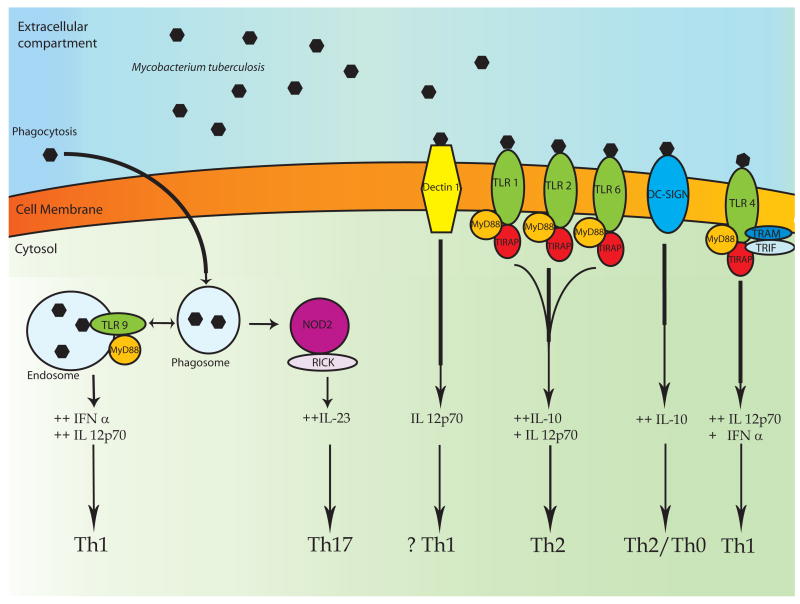
Figure 2. MTb, innate immunity pattern recognition receptors and the adaptive immune response.
MTb is recognized by PRRs that activate the immune response through detection of mycobacterial molecules. Receptors involved in MTb recognition include several TLRs, NOD2, DC-SIGN, and Dectin-1. As explained in the text, several lines of evidence suggest that the type of adaptive immune response is influenced by which PRR is stimulated on macrophages and dendritic cells. The stimulation of multiple PRRs by MTb raises the possibility that agonists or antagonists of each receptor may selectively alter different aspects of the innate and/or adaptive immune response to MTb and/or BCG vaccination.
Table 1. Therapeutic targets in the innate immune response to MTb, and their effect on adaptive immune outcomes.
| # | Therapeutic Targets | Approach | Expected innate response | Possible adaptive response | References |
|---|---|---|---|---|---|
| 1 |
TLR 2
(+1 or 6) |
Agonist | Strong IL-10
Weak IL 12p70 |
Th2 | 31,32 |
| 2 | TLR 4 | Agonist | Strong IL-12p70
IFN α |
Th1 | 31, 32, 45 |
| 3 | TLR 9 | Agonist | Strong IFN α
Strong IL-12p70 |
Th1 | 31, 32, 33 |
| 4 | NOD2 | Agonist | IL-23 | Th17 | 35 |
| 5 | DC-SIGN | Agonist | IL-10 | Th2/Th0 | 34 |
| 6 | Dectin-1 | Agonist | IL-12p40 | ? Th1 | 13, 14 |
Abbreviations
- BCG
Bacille-Calmette-Guerin
- DC-SIGN
dendritic cell-specific intracellular adhesion molecule-3 (ICAM-3)-grabbing non-integrin
- MTb
Mycobacterium tuberculosis
- PRR
pattern-recognition receptor
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colditz GA, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 2.Kaufmann SH. Tuberculosis: back on the immunologists' agenda. Immunity. 2006;24(4):351–357. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev. 2007;219:167–186. doi: 10.1111/j.1600-065X.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–486. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 7.Remus N, et al. Human genetics of common mycobacterial infections. Immunol Res. 2003;28(2):109–129. doi: 10.1385/IR:28:2:109. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, et al. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 11.Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 2002;4(9):937–944. doi: 10.1016/s1286-4579(02)01611-8. [DOI] [PubMed] [Google Scholar]
- 12.Quesniaux V, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6(10):946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Rothfuchs AG, et al. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol. 2007;179(6):3463–3471. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 14.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108(9):3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comstock GW. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117(4):621–624. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood CM, et al. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000;67(2):405–416. doi: 10.1086/303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baghdadi JE, et al. An autosomal dominant major gene confers predisposition to pulmonary tuberculosis in adults. J Exp Med. 2006;203(7):1679–1684. doi: 10.1084/jem.20060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson SE, et al. Evidence for a cluster of genes on chromosome 17q11-q21 controlling susceptibility to tuberculosis and leprosy in Brazilians. Genes Immun. 2004;5(1):46–57. doi: 10.1038/sj.gene.6364029. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy R, et al. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc Natl Acad Sci U S A. 2000;97(14):8005–8009. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernando SL, Britton WJ. Genetic susceptibility to mycobacterial disease in humans. Immunol Cell Biol. 2006;84(2):125–137. doi: 10.1111/j.1440-1711.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 21.Schroder NW, et al. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol. 2005;175(4):2534–2540. doi: 10.4049/jimmunol.175.4.2534. [DOI] [PubMed] [Google Scholar]
- 22.Ogus AC, et al. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23(2):219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 23.Yim JJ, et al. The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun. 2006;7(2):150–155. doi: 10.1038/sj.gene.6364274. [DOI] [PubMed] [Google Scholar]
- 24.Thuong NT, et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8(5):422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 25.Hawn TR, et al. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol. 2007;37(8):2280–2289. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CM, et al. Cutting edge: A common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J Immunol. 2007;178(12):7520–7524. doi: 10.4049/jimmunol.178.12.7520. [DOI] [PubMed] [Google Scholar]
- 27.Khor CC, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawn TR, et al. A polymorphism in toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J Infect Dis. 2006;194(8):1127–1134. doi: 10.1086/507907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper AM, et al. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol. 2007;19(4):441–447. doi: 10.1016/j.coi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 31.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 32.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174(5):2457–2465. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 33.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 34.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27(4):660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2(12):967–977. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 37.Barreiro LB, et al. Promoter Variation in the DC-SIGN-Encoding Gene CD209 Is Associated with Tuberculosis. PLoS Med. 2006;3(2):e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakuntabhai A, et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet. 2005;37(5):507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez LM, et al. Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum Immunol. 2006;67(10):808–811. doi: 10.1016/j.humimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Akahoshi M, et al. Influence of interleukin-12 receptor beta1 polymorphisms on tuberculosis. Hum Genet. 2003;112(3):237–243. doi: 10.1007/s00439-002-0873-5. [DOI] [PubMed] [Google Scholar]
- 41.Tso HW, et al. Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population. J Infect Dis. 2004;190(5):913–919. doi: 10.1086/422693. [DOI] [PubMed] [Google Scholar]
- 42.Lee HW, et al. Lack of an association between interleukin-12 receptor beta1 polymorphisms and tuberculosis in Koreans. Respiration. 2005;72(4):365–368. doi: 10.1159/000086249. [DOI] [PubMed] [Google Scholar]
- 43.Remus N, et al. Association of IL12RB1 polymorphisms with pulmonary tuberculosis in adults in Morocco. J Infect Dis. 2004;190(3):580–587. doi: 10.1086/422534. [DOI] [PubMed] [Google Scholar]
- 44.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124(4):849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Alderson MR, et al. TLR4 agonists as immunomodulatory agents. J Endotoxin Res. 2006;12(5):313–319. doi: 10.1179/096805106X118753. [DOI] [PubMed] [Google Scholar]