Abstract
Interleukin-21 (IL-21) and its receptor represent the sixth cytokine system whose actions were recognized to require the common cytokine receptor γ chain. IL-21 is produced by activated CD4+ T cells, natural killer T cells, and follicular T helper cells and has actions on a range of lymphohematopoietic lineages. Among its many effects, IL-21 serves a critical role for immunoglobulin production and terminal B cell differentiation, acts as a T cell comitogen and can drive the expansion of CD8+ T cells, can negatively regulate dendritic cell function and plays an essential role in the differentiation of Th17 cells. Importantly, IL-21 is implicated in the pathogenesis of autoimmunity and exhibits potent actions as an antitumor agent. The ability to regulate and manipulate the actions of IL-21, therefore, has important implications for immunoregulation and the therapy of human disease.
BACKGROUND OF THE DISCOVERY OF THE IL-21/IL-21R SYSTEM
The discovery of the IL-21 receptor as an orphan type I cytokine receptor was first reported in 2000 [1, 2]. The IL-21 binding protein, denoted IL-21R, was discovered in two types of gene discovery programs that were based on genomic [1] or EST-based [2] sequencing projects. From both approaches, it was evident that a gene/cDNA had been detected with a predicted open reading frame that exhibited properties of a type I cytokine receptor, including four conserved extracellular cysteine residues, an extracellular membrane-proximal Trp-Ser-X-Trp-Ser motif, and a cytoplasmic domain that includes a “Box 1” motif. This putative orphan receptor, termed “novel interleukin receptor” (NILR) by one group [1], was most related to the IL-2 receptor β chain (27% similar), and its gene was located immediately 3′ of the IL4R locus on human chromosome 16. On the basis of multiple tissue Northern blots, IL-21R was primarily expressed by spleen and thymus, consistent with expression primarily in lymphohematopoietic lineages, and IL-21R expression was augmented in T cells following stimulation by mitogens, activation via the T cell receptor, or following transformation with human T cell lymphotropic virus, type I (HTLV-I), the cause of adult T cell leukemia (ATL), and tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM) [1, 2].
The cloning of the ligand for this novel receptor by one of the groups revealed the discovery of a cytokine that was, as expected, an up-up-down-down four α-helical bundle type I cytokine, and it was termed IL-21; accordingly, the receptor was termed IL-21R [2]. IL-21 was observed to be most similar to IL-2, IL-4, IL-15, and the IL21 gene was found to be adjacent to the IL2 gene on human chromosome 4 [2].
IL-21 was first noted to be produced by activated CD4+ T cells, whereas expression of its receptor was somewhat broader [1, 2]. IL-21R expression was constitutive on B and natural killer (NK) cells; its expression was absent to low on resting T cells but was potently induced following T cell activation [1,2,3]. IL-21 was observed to be capable of inducing the proliferation of both T and B cells and to be capable of activating NK cells [2]. The source of IL-21, its chromosomal location, and principal target cells were highly reminiscent of features of IL-2, and consistent with this, IL-21R shared greater homology than any other receptor to IL-2Rβ [1]. The biological distinction between IL-2 and IL-21 was, therefore, initially unclear.
IL-21 IS A γC FAMILY CYTOKINE, MEDIATING THE ACTIVATION OF Jak1 AND Jak3 AND OF Stat1, Stat3, Stat5a, AND Stat5b
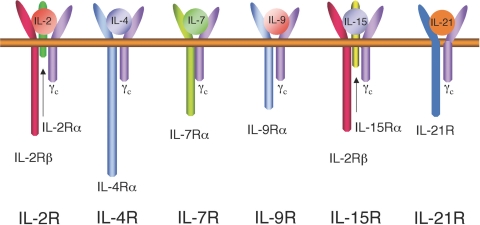
The similarity of IL-21 to IL-2, IL-4, and IL-15, as well as the other features noted above, suggested the possibility that IL-21, like IL-2, IL-4, IL-7, IL-9, and IL-15, might share as an essential component of its receptor the common cytokine receptor γ chain, γc (Fig. 1). γc is encoded by IL2RG, the gene that is mutated in humans with X-linked severe combined immunodeficiency (XSCID) [4]. XSCID is a disease characterized by an absence of T and NK cells; B cells are present in normal numbers but are nonfunctional due both to the lack of T cell help and an intrinsic B cell defect [5], an issue further discussed below. A series of studies confirmed that the IL-21 receptor complex shares γc [1, 6, 7].
Fig. 1.
Cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) whose receptors share the common cytokine receptor γ subunit, γc. Each of these cytokine receptors also contains one or two more distinctive receptor subunits.
Analogous to the other γc family cytokines, IL-21 activates both Jak1 and Jak3, with IL-21R interacting with Jak1 (Jak3 interacts with γc) [1, 6, 7]. Interestingly, whereas IL-2, IL-7, IL-9, and IL-15 dominantly activate Stat5a and Stat5b and more weakly activate Stat3 and Stat1, and whereas IL-4 dominantly activates Stat6 and more weakly Stat5 proteins, IL-21 primarily activates Stat3 and Stat1, with weaker activation of Stat5 proteins [8].
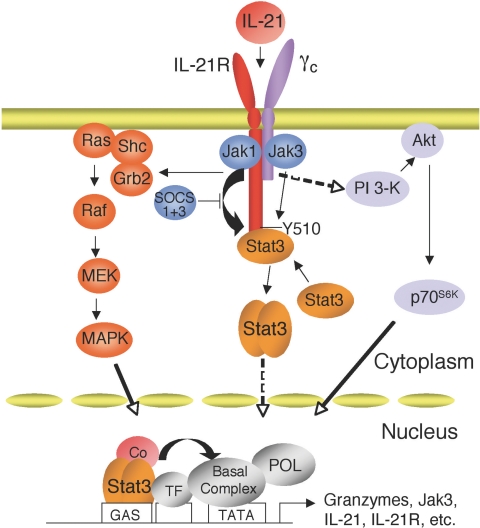
The basis for STAT protein activation by IL-21 has been elucidated [9]. IL-21R contains six tyrosine residues in its cytoplasmic domain, and analysis of a series of IL-21R mutants in which Tyr to Phe mutations were made alone or in combination revealed that Tyr 510 is responsible for the activation of Stat1 and Stat3, presumably serving as a phospho-tryosine docking site for these STAT proteins [9]. In fact, Tyr 510 is part of a YXXQ motif (Fig. 2), which is known to mediate the activation of Stat3 [10]. Interestingly, none of the tyrosines appear to be important for the activation of Stat5 [9]; instead, Stat5 activation perhaps is mediated by its interaction with the Jaks, analogous to what has been previously reported in other systems [11, 12]. The activation of Stat1 and Stat5 by IL-21 in peripheral T cells is relatively transient, whereas its activation of Stat3 is more sustained [9, 13]. It is interesting that Stat1 has been suggested to promote apoptosis, whereas Stat3, and to a lesser degree Stat5, have been associated with oncogenesis [14,15,16], so the balance of activation of these different STAT proteins in different biological contexts presumably influences the actions induced by Il-21. The importance of this selective activation of STAT proteins remains to be determined, especially with regard to cell type-specific and signal-specific regulation by IL-21.
Fig. 2.
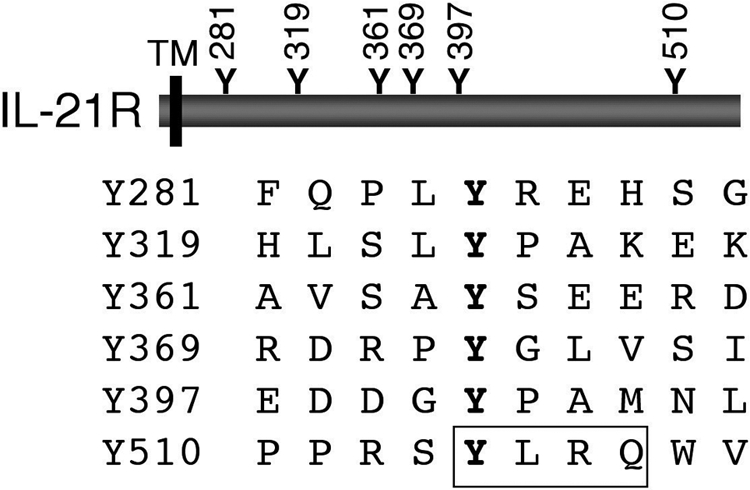
The mouse IL-21 receptor (IL-21R) contains six tyrosine residues in its cytoplasmic domain. Y510 is within a consensus Stat3-bibding motif, YXXQ (boxed). TM, transmembrane domain.
The use of T cells lacking Stat1 and/or Stat3 underscored the importance of Stat3 for at least some of the actions of IL-21 [9]. As would be anticipated, however, IL-21 can also activate other pathways, including phosphoinositol 3-kinase/Akt and Ras/MAP kinase pathways, which also contribute to the actions of this cytokine [9]. Presumably, these signaling pathways as well as potentially others, cooperate to orchestrate the range of biological actions induced by IL-21 [8] (Fig. 3).
Fig. 3.
A model for IL-21R signaling. IL-21 can activate at least three signaling pathways: Jak-STAT, MAPK, and PI 3-kinase pathways.
THE BIOLOGY OF IL-21: THE PHENOTYPE OF Il21r-DEFICIENT MICE AND THE CLARIFICATION OF A CRITICAL ROLE OF IL-21 FOR IMMUNOGLOBULIN PRODUCTION
To elucidate the biology of IL-21, Il21r-deficient mice were generated [17, 18]. The initial analyses of these animals revealed no obvious developmental defects. Progeny were born according to normal Mendelian genetics, with fertility and lifespan being normal. No developmental abnormalities were evident and the lymphoid populations of both thymus and spleen were in normal limits. There were, however, abnormalities, within B cell function, in that IgE was elevated, and particularly after immunization, IgE was profoundly elevated. Conversely IgG1 was markedly diminished, with partial decreases in IgG2b and IgG3, whereas the other immunoglobulin classes were not substantially affected. This pattern of immunoglobulin expression was surprising in that typically a decrease in the levels of IgG1 is associated with diminished IgE rather than in an increase in IgE, as immunoglobulin class switching to γ1 and ε typically correlate, and each is dependent at least in part on the actions of IL-4.
To determine whether the elevated IgE in the Il21r KO mice was indeed dependent on the actions of IL-4, these mice were crossed to Il4 KO mice to yield Il21r/Il4 double KO mice [17]. These animals no longer produced any IgE following immunization, revealing that the mechanism of elevated IgE was dependent on IL-4. Unexpectedly, however, the mice also exhibited a pan-hypogammaglobulinemia, with defective production of IgG1, IgG2a, IgG2b, IgG3, and IgG4, although some IgM was still produced. These results established that IL-4 and IL-21 cooperated with each other for IgG production in vivo [17]. Moreover, it was striking that the immunoglobulin pattern was typical of that observed in humans with XSCID. These results strongly suggested that the intrinsic B cell defect in XSCID resulted from the simultaneous inactivation of IL-4 and IL-21. Interestingly, the germinal centers in the Il4/Il21r double KO mice were abnormal, exhibiting diminished numbers of tingible body macrophages, whereas both of the Il21r and Il4 single KO mice had normal germinal centers, suggesting a cooperative function for IL-4 and IL-21 for germinal-center formation. Overall, these data indicate that IL-4 and IL-21 cooperate to globally regulate immunoglobulin production, although each may have a specific and opposing effect on IgE production [17]. The mechanism of this selective action remains unclear, but the elevated IgE in the Il21r KO mice can be explained, at least in part, by the observation that IL-21 can inhibit germline Cε transcription induced by the combination of lipopolysaccharide (LPS) and IL-4 [19].
REGULATION OF EXPRESSION OF IL-21 AND IL-21R
As noted above, IL-21 was initially observed to be produced by activated CD4+ T cells. An analysis of the expression of IL-2 and IL-21 revealed that they were both induced in these cells. Interestingly, the use of preactivated T cells allowed the elucidation of conditions wherein the induction of IL-2 mRNA required two signals—both PMA and calcium ionophore, whereas IL-21 mRNA was potently induced following stimulation with calcium ionophore in the absence of PMA [20]. Expression of both IL-2 and IL-21 mRNA was inhibited by treatment with cyclosporine A or FK506 but not by rapamycin. Analysis of the 5′ regulatory region of the IL-21 gene revealed three motifs typical of those that bind NFAT (nuclear factor of activated T cells) proteins, and it was shown that NFAT2 can bind to these sites in vivo. Mutation of the sites confirmed the critical role of the NFAT sites for IL-21 promoter activity [20, 21].
Regulation of IL-21R expression is interesting in that it exhibits a biphasic response [22]—an early induction in T cells in response to TCR signaling, with subsequent decline, but then with a secondary “spontaneous” induction that putatively is dependent on the production of cytokine(s), including IL-21, which can induce expression of its own receptor [22, 23]. The induction of IL-21R in response to TCR stimulation depends, at least in part, on TCR-mediated induction and dephosphorylation of Sp1, which binds in vivo to a site in the IL-21R promoter region. The regulation of IL-21R expression not only by TCR but also by IL-21 underscores the fine tuning of this system. In this sense, IL-21 is similar to IL-2 and IL-4, which each induces expression of its distinctive receptor component, IL-2Rα, IL-2Rβ, and IL-4Rα, respectively, whereas it contrasts to IL-7, which negatively regulates expression of IL-7Rα.
THE RANGE OF CELLULAR POPULATIONS THAT EXPRESS IL-21R
In addition to its expression on B, T, and NK cells [1,2,3], IL-21R is now known to be expressed on dendritic cells, macrophages, epithelial cells, and keratinocytes as well [24,25,26,27], consistent with the ability of these cells to respond to IL-21. In thymic development, immature CD4−CD8− double-negative cells do not express IL-21R, but IL-21R expression is found in CD4+CD8+ double-positive cells, with a further increase in expression in both CD4+ and CD8+ single-positive thymocytes [3]. As noted above, however, IL-21R KO mice exhibit normal lymphoid development, so this IL-21R expression is not critical for the basic maturation and development of key populations, although it is conceivable that it indicates a partially redundant action. In the periphery, IL-21R is weakly expressed on naïve CD4+ and CD8+ T cells, and its expression is increased following TCR stimulation [3, 22].
Within the B cell lineage, IL-21R is not expressed on immature pro-B cells, but high-level IL-21R expression is found at the B220highIgMlow stage, as well as by mature B220highIgMhigh B cells [3]. Within B cells, IL-21R can be potently induced by LPS [3], which acts via TLR9. In contrast to the above noted signals that can augment expression of IL-21R on T or B cells, interferon-α (IFN-α) decreases levels of IL-21R mRNA in both T cells and NK cells, underscoring careful control of IL-21R expression in different biological situations [28].
ROLE OF IL-21 IN B CELL BIOLOGY: DEATH VS. PLASMA CELL DIFFERENTIATION
Above, we have already discussed the role of IL-21 in immunoglobulin production. This was further investigated using IL-21 transgenic mice and in mice receiving hydrodynamic electroporation of IL-21 plasmid DNA [29]. In these animals, there was a striking increase in post-switch cells (cells that have lost expression of IgD and IgM), memory B cells (surface IgG+), and Syndecan-1+ plasma cells. The increase in plasma cells corresponded to a substantial elevation of serum IgG1 and IgM; interestingly, IgE was not evidently altered. The induction of plasma cell differentiation reinforced the idea of a critical role of IL-21 for immunoglobulin production [29]. In an in vitro analysis of human B cells, IL-21 was also shown to induce class switching to IgG1 and IgG3, consistent with the basic effect of IL-21 in murine cells [30].
Unexpectedly, however, it was also evident that IL-21 also increased B cell apoptosis [3, 29, 31]. The addition of IL-21 to freshly isolated B cells or to cells that have been stimulated with LPS, anti-CD40, or anti-IgM antibodies resulted in apoptosis of these cells. Although Bcl-2 mRNA levels are diminished, Bcl-2 protein levels are not affected [29]; instead, the pro-apoptotic effect appears to result, at least in part, from an increase in expression of the proapoptotic factor, Bim [3]. Although γc-dependent cytokines generally mediate survival effects, the proapoptotic effect of IL-21 is perhaps analogous to the ability of IL-2 to induce death in the context of a process known as activation-induced cell death (AICD).
Thus, the apparent conundrum is that IL-21 is proapoptotic for B cells but nevertheless, it is required for immunoglobulin production. A potential explanation results from the observation that the addition of IL-21 results in more proliferation and less death in settings where a T-cell signal is also provided (e.g., anti-IgM plus anti-CD40) as opposed to only a B-cell signal (e.g., anti-IgM alone) [29]. This suggests that in settings of incomplete B cell activation that can lead to the development of potentially autoreactive cells, IL-21 may be a “gatekeeper” to eliminate these potentially harmful cells. In this sense, IL-21 may be for B cells what IL-2 is for T cells in the process of activation-induced cell death (AICD). In contrast, in a more effective immune response, IL-21 can drive plasma cell differentiation [29]. This model is not rigorously proven, but it provides an attractive framework to rationalize the ability of IL-21 to both eliminate cells and to be required for immunoglobulin production. The ability of IL-21, particularly in combination with anti-IgM and anti-CD40, to drive plasma cell differentiation has been shown for normal human peripheral CD27+ memory B cells, as well as cord blood human B cells, a dramatic finding not evident for any other cytokine [32].
The ability of IL-21 to induce terminal differentiation to plasma cells can be explained by its potent induction of Blimp-1 [29, 32], a transcription factor whose action is required for plasma cell differentiation [33]. It is interesting that Blimp-1 and Bcl-6 can negatively regulate each other’s expression, but IL-21 is the first and perhaps only cytokine that is known to be capable of inducing expression of both Bcl-6 and Blimp-1 [29]. It remains unclear, however, whether these proteins are coinduced within the same cells or whether they are instead being induced by IL-21 in distinct cell populations. Whether the induction of Bcl-6 or Blimp-1 dominates may determine the differentiation fate of the cell.
IL-21 AND AUTOIMMUNITY
Given a role for IL-21 for immunoglobulin production, what then is the potential contribution of IL-21 to autoimmunity? A hint of a disease-promoting effect of IL-21 was suggested by the observation that transgenic mice expressing very high levels of IL-21 did not survive, so that the only founders that persisted were those with more modest levels of IL-21 production [29]. It was then further suggested by the elevated levels of IL-21 that were observed in NOD mice [34], which develop an autoimmune form of diabetes, and by elevated IL-21 in the BXSB-Yaa mouse model [29], which is associated with duplication of the Tlr7 gene and increased expression of TLR7. In the BXSB-Yaa disease, affected animals develop very severe lupus and die at approximately 6 months of age. Before week 12, IL-21 levels are low, but then the levels of IL-21 mRNA and serum protein progressively increase with age and correlate with elevated levels, particularly of IgG1 and IgG3 but also of IgG2b, as well as with the progression of disease [29]. Corresponding to the augmented IL-21 expression in these mice, approximately half of humans with SLE have elevated IL-21 (Dr. Peter Lipsky, personal communication), and there is a genetic association of IL-21 polymorphisms with SLE in humans [35], consistent with a role of IL-21 in the pathophysiology of at least some patients with this disease.
Elevated levels of IL-21 were also noted in another autoimmune mouse model, namely the Sanroque mutant, which has defective expression/function of the protein Roquin, which negatively regulates the production of a population of follicular T helper cells that produce high levels of IL-21 [36]. These cells produce high levels of IL-21, which is correlated with elevated antinuclear antibodies, as well as with the development of lymphadenopathy and glomerulonephritis. The MRL/lpr mouse model of SLE is also associated with elevated production of IL-21 by CD4+ T cells [37]. Interestingly, treatment of these animals with an IL-21R-Fc fusion protein resulted in a partial decrease in IgG levels, diminished lymphadenopathy, and less severe renal pathology, indicative of a pathophysiological role of IL-21 in this disease. The basis for the partial effect remains unclear but could reflect that IL-21 is only one key factor for the development of disease and/or alternatively that insufficient levels of the IL-21R-Fc fusion protein were achieved in vivo at the relevant anatomic sites under the experimental conditions selected. The same agent also exhibited a partial effect in a mouse model of collagen-induced arthritis [38].
Another disease in which elevated levels of IL-21 were observed was in the nonobese diabetic (NOD) mouse model of type I diabetes [34]. In these mice, IL-21 was suggested to promote a homeostatic expansion of CD8+ T cells, which are believed to be involved in destruction of the β islet cells of the pancreas. Interestingly, the NOD disease is associated with the insulin-dependent diabetes susceptibility locus 3 (Idd3); however, rather than being at the Il21 gene, the Idd3 locus instead was recently reported to be at the adjacent Il2 locus [39]. Nevertheless, it remains possible that IL-21 is pathophysiologically involved in the development of type I diabetes in the NOD mouse.
ROLE OF IL-21 IN T CELL EXPANSION, PROLIFERATION, AND CYTOLYTIC ACTIVITY
IL-21 was first observed to be produced by activated CD4+ T cells and to be capable of serving as a T cell comitogen, augmenting proliferation in response to mitogens [2]. In addition to serving as a comitogen, the ability of IL-21 to directly influence the proliferation of T cells was examined [23]. Interestingly, when added to freshly isolated splenic T cells, IL-21 alone had little or no effect, but it potently synergized with either IL-7 and IL-15 to drive the expansion of CD8+ T cells, while having no significant effect on CD4+ T cells. This effect occurred for both naïve (CD44low) and memory phenotype (CD44high) CD8+ T cells, and the cells produced high levels of IFN-γ and exhibited augmented cytolytic activity [23]. These results are consistent with a role for IL-21 in the initiation as well as the maintenance of a CD8+ T cell response. Although Il21r KO mice have normal levels of peripheral CD8+ T cells, the mice exhibit diminished responsiveness to a vaccinia-encoded antigen [23], underscoring the role of IL-21 in antigen-specific expansion of CD8+ T cells.
IL-21 AS AN ANTITUMOR AGENT
In view of the augmented cytolytic effect mediated by IL-21, IL-21 was evaluated in an antitumor model dependent on CD8+ T cells [23]. In this system, mice were implanted with B16 melanoma that was allowed to establish, and then animals were treated with TCR-transgenic pmel-1 CD8+ T cells that have a specificity for the gp100 melanoma antigen and vaccinated with gp100. Although this treatment alone had no effect, the addition of IL-15 or IL-21 had an antitumor effect, and the combination of these cytokines had a dramatic cooperative antitumor effect with complete cures in some animals. The basis for the synergistic effect of IL-21 with either IL-7 or IL-15 is unknown but may, in part, relate to the cooperative effect of Stat3 (induced by IL-21) and Stat5 proteins (induced by IL-7 or IL-15). However, it is clear that this is not the complete answer given the inability of IL-21 to exhibit a synergistic effect with IL-2, which like IL-7 or IL-15, can activate Stat5 proteins.
Interestingly, when cells were primed by exposure to IL-21 prior to adoptive transfer, they exhibited very potent antitumor activity after transfer, even without the continued presence of the cytokine [40]. Although the mechanism of this effect is unclear, the effect of IL-21 was most distinctive in this regard, in that no other cytokine had a similar effect. The basis for the priming effect is unclear, but the result is dramatic and correlates with preferential expansion of IL-21-treated cells in both spleen and draining lymph nodes.
IL-21 has been observed to have potent antitumor activity in a range of model systems, as summarized previously [41, 42]. The antitumor activity has been observed to be mediated by NK cells and by CD8+ T cells. For example, NK-mediated killing of tumors was documented in studies demonstrating the inhibition of established fibrosarcomas [43, 44], melanomas [43,44,45], pancreatic carcinoma [46], and colon carcinoma [47], whereas CD8+ T cells are clearly the mediators in the pmel-1 TCR transgenic system discussed above [23, 40].
IL-21 has already entered human clinical studies. Two phase I trials in patients with metastatic melanoma (MM) or renal cell carcinoma (RCC) showed a favorable safety profile and signs of antitumor activitiy [48, 49]. IL-21-induced in vivo immune activation of CD8+ T cells, and NK cells were also evident in patients with MM or RCC [48, 50]. Currently, IL-21 is being evaluated in phase II trials either as monotherapy or combination therapy for MM, RCC, non-Hodgkin’s lymphoma, and metastatic colon cancer [42, 50].
THE ROLE OF IL-21 RELATED TO Th1, Th2, AND Th17 CELLS
In addition to the production of IL-21 by mitogen/antigen-activated peripheral T cells, subsequent analysis has revealed that IL-21 can be produced by various functional subsets of CD4+ T cells, including Th1 cells, which are involved in the control of viruses and intracellular pathogens, Th2 cells, which mediate antibody responses to extracellular pathogens, including helminthes, and are important in allergy/asthma, and Th17 cells, which mediate certain inflammatory processes and are involved in certain types of diseases, including Crohn’s disease and psoriasis, as well as bacterial and fungal infections at mucosal surfaces [8]. The relative importance of IL-21 in some of these responses has been unclear. For example, one study reported that IL-21 is produced by Th2 cells but not by Th1 cells [51], whereas another study revealed the dominant expression in Th1 instead of Th2 cells [52]. More recent studies have indicated that Th17 cells may, in fact, produce higher levels of IL-21 [53,54,55], which will be discussed in more detail below. The regulation of Th1 vs. Th2 responses in vivo by IL-21 has been studied primarily using Il21r KO mice [17]. No obvious difference was observed in the levels of IL-4 or IFN-γ between wild-type and Il21r KO mice. In a delayed type hypersensitivity model, the Il21r KO mice had higher inflammatory responses than did the WT mice, including higher production of IFN-γ [51]. Interestingly, in an infection model using Schistosoma mansoni, which induces a Th2-dependent granuloma, IL-21 levels were measured in strains of mice that develop massive Th1 vs. Th2 disease and were present in both situations, demonstrating that IL-21 is not a classical Th1 or Th2 cytokine [27]. There was a diminished Th2 response (decreased IL-4, IL-10, and IL-13), without a concomitant increase in Th1 cytokines. Infection of mice with Heligmosomoides polygyrus resulted in fewer/smaller granulomas and diminished eosinophilia, which was consistent with a partially defective Th2 response, even though measuring cytokines ex vivo did not reveal differences between IFN-γ and IL-4 [56]. Thus, whereas IL-21 can apparently influence Th1/Th2 responses to some degree, the effects are partial.
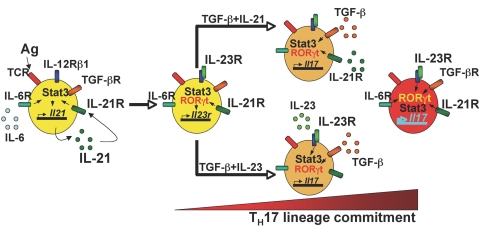
In contrast to the partial effects related to Th1/Th2, IL-21 is absolutely vital for Th17 differentiation. The hallmark of Th17 differentiation is the production of IL-17 [57]. Differentiation of Th17 cells, like Treg cells, can occur in the context of the immunosuppressive cytokine, TGF-β. The addition of IL-6 diminishes the number of Treg cells and augments the number of Th17 cells. Th17 differentiation is dependent on expression of the transcription factor RORγt. It was observed that both IL-6 and RORγt potently induce the production of IL-21 [55]. This led to the hypothesis that IL-21 might be involved in the differentiation of these cells. Indeed, Th17 differentiation was markedly defective in Il21r KO mice. Moreover, so was the induction of IL-23R by either IL-6 or IL-21. IL-23R is a critical component of the IL-23 receptor, which is known to play a critical role in Th17 differentiation. Consistent with the activation of Stat3 by IL-21, Stat3-deficient T cells were also dramatically defective in their ability to mediate Th17 differentiation, with defective IL-17 induction and IFN-γ down-regulation in response to IL-6 or IL-21 in Stat3-deficient T cells [55]. Overall, these observations allowed a model of sequential roles for IL-6, IL-21, and IL-23 in Th17 differentiation (Fig. 4). In particular, IL-6 via Stat3 induces IL-21 expression, then IL-21 via Stat3 induces IL-21, IL-23R, and IL-21R induction, with IL-23 then being required for stable Th17 differentiation.
Fig. 4.
Schematic of the sequential roles of IL-6, IL-21, and IL-23 in Th17 lineage differentiation. IL-6 helps to initiate Th17 differentiation, and via Stat3 induces expression of IL-21 and IL-21R. IL-21 can then induce IL-23R expression, and IL-23 can then act. This overall cytokine cascade then can result in stable Th17 differentiation.
THE EFFECTS OF IL-21 ON NK CELL BIOLOGY
Although normal numbers of NK cells develop in Il21r KO mice, IL-21, nevertheless, can influence the activity of WT NK cells [2, 18]. Moreover, when IL-21 is combined with IL-7, IL-15, stem cell factor, and Flt3 ligand, it augments the generation of NK cells in vitro [58]. IL-21 can augment NK-cell cytolytic activity, and this is associated with IL-21-induced expression of killer inhibitory receptors (KIRs) on these cells. When combined with IL-15, IL-21 synergistically augments the production of IFN-γ by NK cells [13]. Interestingly, Il15 KO lymphocytes have diminished IL-21R expression, indicating a crosstalk between IL-15 and IL-21R and a role for IL-15 in regulating the responsiveness of the cells to IL-21 [59]. IL-21 can augment the proliferation of NK cells, although higher doses are often inhibitory, whereas low concentrations are stimulatory [60]. IL-21 can induce apoptosis of the cells, but the addition of IL-15 diminishes IL-21-mediated apoptosis [18, 45]. Thus, the effects of IL-21 are pleiotropic in part depending on its concentrations and the presence of other cytokines.
A NEGATIVE EFFECT OF IL-21 ON DENDRITIC CELLS
In addition to its ability to act on lymphocytes, IL-21 can also act on dendritic cells (DCs) and granulocytes. Injection of IL-21 can increase the number of Gr1+ and CD11b+ cells, indicative of effects on granulocytes and macrophage/monocytes [43]. In addition, it can affect the activity of DCs in a differential manner from IL-15 [24]. DCs expanded in the presence of IL-15 express high-affinity IL-2 receptors, whereas those expanded in the presence of IL-21 do not express either IL-2Rβ or IL-2Rα and have a phenotype characterized by low levels of MHC class II and low levels of the chemokine receptor CCR7. LPS cannot activate these DCs to augment their expression of CD80, CD86, or MHC Class II. Most importantly, DCs expanded with IL-15 stimulate T-cell responses, whereas those expanded in the presence of IL-21 exert negative inhibitory effects that presumably negatively affect T-cell responses.
IL-21 IN CANCER, AUTOIMMUNITY, AND ALLERGY
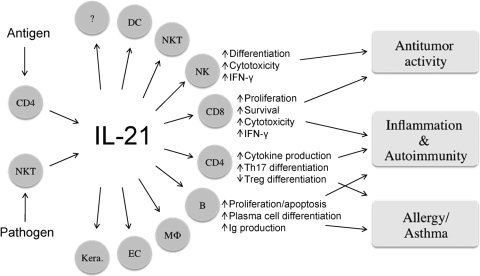
Above, we have discussed a broad range of immunological effects of IL-21, including its putative role in a number of different autoimmune syndromes, as well as some of the data supporting the role of IL-21 as a possible antitumor agent (Fig. 5). The hypothesis is that disrupting IL-21 signaling, if done in the proper fashion, could be therapeutic for autoimmune syndromes but conceivably might predispose to malignancy. In contrast, providing IL-21 might be therapeutic for cancer but could predispose to aggravation of an autoimmune problem. Thus, careful attention to these considerations is required in planning any therapeutic regiment for augmenting or inhibiting the action of IL-21. It is also evident that IL-21 has a potentially interesting is relationship to allergic diseases [56, 61, 62], and Il21r KO mice have elevated IgE [17]. More work is needed to determine whether manipulating the IL-21/IL-21R system may be therapeutic in allergic diseases.
Fig. 5.
Schematic showing the production of IL-21 by activated CD4+ T cells including follicular helper T (TFM) cell and natural killer T (NKT) cells and its action on a large array of target cells. Collectively, IL-21 can exert antitumor activity and additionally has effects in inflammation, autoimmunity, and allergic diseases. DC, Dentritic cells; MΦ, macrophages; EC, epithelial cells; Kera., Keratinocytes.
CONCLUDING REMARKS
The discovery of IL-21 and its receptor were first reported just eight years ago in 2000. Although this cytokine system was previously unsuspected (i.e., there was no obvious “biological activity” for which investigators were seeking to identify the activities subserved by IL-21), its discovery has already explained a great deal. The discovery of this sixth cytokine to share γc has 1) elucidated the basis for the intrinsic B cell defect in XSCID, 2) provided at least a partial answer to the basis for terminal B cell differentiation to plasma cells, 3) provided another key cytokine for the expansion of CD8+ T cells, at least in some circumstances, 4) clarified the basis for Th17 differentiation, 5) provided another activator of NK cells, and 6) strikingly revealed a new proapoptotic mediator for at least B cells and NK cells. Moreover, manipulating the IL-21/IL-21R system may provide a means for treating certain autoimmune and malignant diseases. This is an area of active investigation in which much work is still needed before efficacy is achieved in humans. Thus, the discovery of IL-21 and its receptor has provided many important scientific insights, as well as having provided an area of investigation for new therapeutic approaches for an array of diseases.
Acknowledgments
We thank Dr. Jian-Xin Lin for critical comments and Dr. Dan R. Littman for the design idea for Fig. 4. This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- Ozaki K, Kikly K, Michalovich D, Young P R, Leonard W J. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon S R, Nelson A, Hammond A, Sprecher C, Gross J A, Johnston J, Madden K, Xu W, West J. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Jin H, Carrio R, Yu A, Malek T R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Yi H, Rosenblatt H M, Filipovich A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- Leonard W J. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–8731. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard W J. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2007;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Casas E, Zhu W, Levy D E, Leonard W J. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Migone T S, Lin J X, Cereseto A, Mulloy J C, O'Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Hibi M, Fukada T, Takahashi-Tezuka M, Yoshida H, Yamaguchi T, Sugiyama K, Yamanaka Y, Nakajima K, Hirano T. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 1997;14:751–761. doi: 10.1038/sj.onc.1200907. [DOI] [PubMed] [Google Scholar]
- Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- O'Shea J J, Gadina M, Schreiber R D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Levy D E, Darnell J E., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng C G, Qi C F, Cheng J, Sher A, Morse H C, III, Liu C, Schwartzberg P L, Leonard W J. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Kasaian M T, Whitters M J, Carter L L, Lowe L D, Jussif J M, Deng B, Johnson K A, Witek J S, Senices M, Konz R F. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster D C, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- Kim H P, Korn L L, Gamero A M, Leonard W J. Calcium-dependent activation of interleukin-21 gene expression in T cells. J Biol Chem. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- Mehta D S, Wurster A L, Weinmann A S, Grusby M J. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc Natl Acad Sci USA. 2005;102:2016–2021. doi: 10.1073/pnas.0409512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kim H P, Xue H H, Liu H, Zhao K, Leonard W J. Interleukin-21 receptor gene induction in human T cells is mediated by T-cell receptor-induced Sp1 activity. Mol Cell Biol. 2005;25:9741–9752. doi: 10.1128/MCB.25.22.9741-9752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein S E, Oh S, Kovanen P E, Hinrichs C S, Pise-Masison C A, Radonovich M F, Brady J N, Restifo N P. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K, Bulfone-Paus S, Foster D C, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- Distler J H, Jungel A, Kowal-Bielecka O, Michel B A, Gay R E, Sprott H, Matucci-Cerinic M, Chilla M, Reich K, Kalden J R. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum. 2005;52:856–864. doi: 10.1002/art.20883. [DOI] [PubMed] [Google Scholar]
- Caruso R, Fina D, Peluso I, Stolfi C, Fantini M C, Gioia V, Caprioli F, Del Vecchio Blanco G, Paoluzi O A, Macdonald T T. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3α, by gut epithelial cells. Gastroenterology. 2007;132:166–175. doi: 10.1053/j.gastro.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Pesce J, Kaviratne M, Ramalingam T R, Thompson R W, Urban J F, Jr, Cheever A W, Young D A, Collins M, Grusby M J, Wynn T A. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strengell M, Julkunen I, Matikainen S. IFN-α regulates IL-21 and IL-21R expression in human NK and T cells. J Leukoc Biol. 2004;76:416–422. doi: 10.1189/jlb.1003488. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Ettinger R, Kim H P, Wang G, Qi C F, Hwu P, Shaffer D J, Akilesh S, Roopenian D C. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- Pene J, Gauchat J F, Lecart S, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron J C, Yssel H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- Mehta D S, Wurster A L, Whitters M J, Young D A, Collins M, Grusby M J. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- Ettinger R, Sims G P, Fairhurst A M, Robbins R, da Silva Y S, Spolski R, Leonard W J, Lipsky P E. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- Calame K L, Lin K I, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Sawalha A H, Kaufman K M, Kelly J A, Adler A J, Aberle T, Kilpatrick J, Wakeland E K, Li Q Z, Wandstrat A E, Karp D S. Genetic association of IL-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–461. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- Vinuesa C G, Cook M C, Angelucci C, Athanasopoulos V, Rui L, Hill K M, Yu D, Domaschenz H, Whittle B, Lambe T. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Herber D, Brown T P, Liang S, Young D A, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- Young D A, Hegen M, Ma H L, Whitters M J, Albert L M, Lowe L, Senices M, Wu P W, Sibley B, Leathurby Y. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner V E, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinrichs, C. S., Spolski, R., Paulos, C. M., Gattinoni, L., Kerstann, K. W., Palmer, D. C., Klebanoff, C. A., Rosenberg, S. A., Leonard, W. J., Restifo, N. P. (2008) IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. BloodIn Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W J, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- Skak K, Kragh M, Hausman D, Smyth M J, Sivakumar P V. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard W J, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- Ma H L, Whitters M J, Konz R F, Senices M, Young D A, Grusby M J, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- Brady J, Hayakawa Y, Smyth M J, Nutt S L. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- Ugai S, Shimozato O, Yu L, Wang Y Q, Kawamura K, Yamamoto H, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and -independent antitumor effects. Cancer Gene Ther. 2003;10:771–778. doi: 10.1038/sj.cgt.7700630. [DOI] [PubMed] [Google Scholar]
- Ugai S, Shimozato O, Kawamura K, Wang Y Q, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther. 2003;10:187–192. doi: 10.1038/sj.cgt.7700552. [DOI] [PubMed] [Google Scholar]
- Davis I D, Skrumsager B K, Cebon J, Nicholaou T, Barlow J W, Moller N P, Skak K, Lundsgaard D, Frederiksen K S, Thygesen P. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, Devries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 50.Frederiksen, K. S., Lundsgaard, D., Freeman, J. A., Hughes, S. D., Holm, T. L., Skrumsager, B. K., Petri, A., Hansen, L. T., McArthur, G. A., Davis, I. D., et al. (2008) IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster A L, Rodgers V L, Satoskar A R, Whitters M J, Young D A, Collins M, Grusby M J. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom T B, Oukka M, Kuchroo V K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang X O, Martinez G, Zhang Y, Panopoulos A D, Ma L, Schluns K, Tian Q, Watowich S S, Jetten A M. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov I I, Spolski R, Min R, Shenderov K, Egawa T, Levy D E, Leonard W J, Littman D R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Frohlich A, Marsland B J, Sonderegger I, Kurrer M, Hodge M R, Harris N L, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- Weaver C T, Hatton R D, Mangan P R, Harrington L E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Sivori S, Cantoni C, Parolini S, Marcenaro E, Conte R, Moretta L, Moretta A. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol. 2003;33:3439–3447. doi: 10.1002/eji.200324533. [DOI] [PubMed] [Google Scholar]
- Vosshenrich C A, Ranson T, Samson S I, Corcuff E, Colucci F, Rosmaraki E E, Di Santo J P. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- Toomey J A, Gays F, Foster D, Brooks C G. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74:233–242. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179:7157–7165. doi: 10.4049/jimmunol.179.10.7157. [DOI] [PubMed] [Google Scholar]
- Kishida T, Hiromura Y, Shin-Ya M, Asada H, Kuriyama H, Sugai M, Shimizu A, Yokota Y, Hama T, Imanishi J. IL-21 induces inhibitor of differentiation 2 and leads to complete abrogation of anaphylaxis in mice. J Immunol. 2007;179:8554–8561. doi: 10.4049/jimmunol.179.12.8554. [DOI] [PubMed] [Google Scholar]