Abstract
Although regulatory T cells (Tregs) are well described, identifying autoaggressive effector T cells has proven more difficult. However, we identified CD4loCD40+ (Th40) cells as being necessary and sufficient for diabetes in the NOD mouse model. Importantly, these cells are present in pancreata of prediabetic and diabetic NOD mice, and Th40 cells but not CD4+CD40– T cells transfer progressive insulitis and diabetes to NOD.scid recipients. Nonobese-resistant (NOR) mice have the identical T cell developmental background as NOD mice, yet they are diabetes-resistant. The seminal issue is how NOR mice remain tolerant to diabetogenic self-antigens. We show here that autoaggressive T cells develop in NOR mice and are confined to the Th40 subset. However, NOR mice maintain Treg numbers equivalent to their Th40 numbers. NOD mice have statistically equal numbers of CD4+CD25+forkhead box P3+intrinsic Tregs compared with NOR or nonautoimmune BALB/c mice, and NOD Tregs are equally as suppressive as NOR Tregs. A critical difference is that NOD mice develop expanded numbers of Th40 cells. We suggest that a determinant factor for autoimmunity includes the Th40:Treg ratio. Mechanistically, NOD Th40 cells have low susceptibility to Fas-induced cell death and unlike cells from NOR and BALB/c mice, have predominantly low Fas expression. CD40 engagement of Th40 cells induces Fas expression but further confers resistance to Fas-mediated cell death in NOD mice. A second fundamental difference is that NOD Th40 cells undergo much more rapid homeostatic expansion than Th40 cells from NOR mice.
Keywords: autoimmunity, homeostasis, Treg, tolerance, diabetes
INTRODUCTION
Autoimmune diseases are characterized by persistent, noninfection-related inflammation, leading to extensive tissue damage. In type 1 diabetes (T1D), this translates clinically as progressive lymphocyte infiltration into the pancreatic islets (insulitis), leading to loss of insulin production. In the NOD T1D model, mice spontaneously develop progressive insulitis beginning at 2 weeks of age, leading to diabetes onset by 16–20 weeks of age [1]. Although several genetic factors influence disease, a common feature of autoimmunity is the occurrence and persistence of autoaggressive T cells.
Although the existence of autoaggressive T cells is understood, identification of such T cells in the periphery has proven difficult. Although self-antigen-reactive, autoaggressive T cells were identified in pancreatic lymph nodes of human T1D subjects [2], only limited success identifying such T cells in the periphery has been achieved overall using tetramer technology [3, 4], which has serious limitations. Tetramers express only a small peptide portion, typically 15–21 amino acids, of a specific antigen; therefore, numerous tetramers would be necessary to study even a small subset of potentially autoaggressive T cells. Studies in T1D have focused on pre-pro insulin and glutamic acid decarboxylase proteins as driver self-antigens, including tetramer identification of such T cells [3, 5,6,7,8,9]. Focusing on a phenotypically descriptive, autoaggressive T cell subset would aid discovery of additional antigens for study.
We discovered that a panel of highly diabetogenic T cell clones, including the well-described BDC2.5 clone, expresses CD40 [10]. We further examined CD40 expression on primary T cells in NOD mice and identified a unique effector CD4+ T cell population, characterized as CD4loCD40+ (Th40) cells [10,11,12,13], which were detected in autoimmune and nonautoimmune mouse strains but occur at much greater percentages in autoimmunity [13]. Importantly, Th40 cells were isolated directly from the pancreata of prediabetic and diabetic NOD mice [10]. Highly purified Th40 cells, when isolated from spleens of diabetic or prediabetic NOD mice, transfer progressive insulitis and diabetes to NOD.scid recipients [10, 11]. Percentages of Th40 cells are low in young NOD mice but expand in the periphery concurrently with progressive insulitis [11]. These findings suggest that Th40 cells are capable of breaking tolerance in NOD mice.
The role of CD40 in autoimmunity is quite extensive. Importantly, blocking CD40–CD154 interactions in NOD mice at 3 weeks of age not only prevented development of diabetes [14] but also prevented expansion of Th40 autoaggressive T cells [11]. Consequently, following anti-CD154 blockade, the CD4+CD25+, potential regulatory T cell (Treg) subset occurred at percentages seen in nonautoimmune mice [11], suggesting that CD40 signals may affect homeostasis between regulatory and autoaggressive T cells. The ligand for CD40, CD154, is activation-induced on T cells [15] but also is expressed on platelets and some APCs [16]. Furthermore, CD154 occurs in cell surface and soluble forms [17]. Interestingly, CD154 is hyperexpressed in T1D and other autoimmune diseases [18, 19].
An important part of homeostatic control includes Tregs, which suppress effector T cells [20,21,22]. Although Tregs occur intrinsically, they also can be induced. Descriptive markers for Tregs include CD4+CD25+ [23, 24], CD62L+ [25, 26], CTLA-4+ [27, 28], α/β integrin-positive [29], the glucocorticoid-induced TNFR (GITR) [30], CD103 [30], and low expression of CD127, a component of the IL-7R [31]. The most consistent marker for intrinsic Tregs is the forkhead box P3 (FoxP3) transcription factor [21, 22, 32,33,34]. As FoxP3 cannot be surface-stained, studies have focused on the CD4+CD25+ subset [35, 36].
Here, we show that although nonobese-resistant (NOR) mice do not develop T1D, they harbor autoaggressive T cells, nevertheless with autoaggression confined to Th40 cells. Mechanistically, the potential autoaggressive:regulatory, defined here as Th40: CD4+CD25+FoxP3+, T cell ratio in NOR mice remains consistent. Prediabetic NOD mice on the other hand demonstrate a significantly greater Th40:Treg ratio as a prelude to diabetes onset. One mechanism for the homeostatic disruption lies in the cell death–cell survival and proliferation processes. Immediately ex vivo, Th40 cells from NOD mice have low Fas expression, and Th40 cells from NOR and BALB/c have high Fas expression. We show that engaging CD40 on Th40 cells from NOD mice induces greater Fas expression but ablates cell death. Th40 cells from NOD mice undergo more rapid homeostatic proliferation in vivo than Th40 cells from NOR mice. Collectively, these studies suggest that CD40 signals promote disruption of homeostasis in autoimmune-prone NOD mice to favor autoimmunity.
MATERIALS AND METHODS
Animals
NOD, NOR, and BALB/c mice from Jackson Laboratories (Bar Harbor, ME, USA) were maintained and bred under Institutional Animal Care and Use Committee-approved conditions at the Webb-Waring Institute, University of Colorado Denver, School of Medicine, Animal Satellite Facility (Denver, CO, USA). All animals used were female, 9–12 weeks old.
Cell purification
T cells were isolated from spleens, peripheral lymph nodes (inguinal, brachial, axial, lingual, submaxillary, and popliteal), and pancreatic lymph nodes. Single cell suspensions of each tissue were made from individual mice for analysis and for cell death assays. CD4+ T cells were purified by passing cells through the lympholyte, and then CD8 and APCs were depleted. Lymphocytes were treated with Miltenyi Biotec (Auburn, CA, USA) anti-CD8 and anti-MHCII beads for 45 min. Cells were washed and passed through the Miltenyi Biotec AutoMacs set on Deplete S for sensitive depletion. Following depletion, cells were always >93% CD4+ and CD3+. All experiments were performed on purified CD4+ cells. Naïve CD4+ T cells were further purified by depleting CD69, CD25, and CD11c using the AutoMacs system.
Cell staining and flow cytometry
Purified CD4+ cells were stained using directly conjugated antibodies followed by three PBS–5% BSA washes. All samples were treated with 2.4.G2, anti-FcR antibody to prevent nonspecific binding. Staining antibodies included CyChrome™ and PE- or FITC-conjugated anti-CD4 (H129.19 or RM 4-4); PE-conjugated anti-Fas (JO2) and PE-conjugated anti-CD25 (7D4); FITC- or PE-conjugated FoxP3; and rat or mouse isotype control antibodies, all from BD-PharMingen (San Diego, CA, USA). FITC-or AlexaFluor-647 [fluoresces in fluorescent-4 (FL-4)] 1C10 [37] anti-CD40 was generated in-house. Staining strategies were constructed to prevent overlaps of fluors. Cells were assayed using a Becton Dickinson FACSCalibur, where 25,000 events were collected then analyzed by Cell-Quest™ software. Cells were gated on forwardhi- versus sidelo-scatters [38].
Cell death assays
T cells were assayed for cell death from individual mice. Ex vivo T cells were untreated or CD40-engaged using an anti-CD40 IgM (clone HM-40-3, BD-PharMingen) or isotype control, 10 μg/ml, for 1 h, washed, and then incubated overnight. For induced cell-death studies, T cells, 2 × 106, were further treated or not with biotinylated anti-Fas at 5 μg/ml (BD-PharMingen). Following antibody treatment for 1 h, T cells were washed, then treated with avidin at 2.5 μg/ml for 30 min, and washed. Cells were incubated for 18 h. For analysis, T cells were stained with FITC-conjugated anti-CD40 (1C10 recognizes a different epitope from HM-40-3). Levels of CD40 on T cells were determined to be the same before and after induction of cell death. Cells were washed with PBS-5% BSA followed by PBS, fixed using 2% paraformaldehyde for 5 min at room temperature, washed three times with PBS-5% BSA, treated with 1% saponin in PBS-5% BSA for 30 min, and then made 0.5% propidium iodide (PI) that fluoresces in FL-2 for 20 min. Cells were washed three times with PBS-5% BSA and then assayed by flow cytometry. PI intercalates DNA; therefore, by this method, apoptosis is measured as the first detected peak [39, 40].
Isolation/removal of CD25+ T cells
CD8- and MHCII-depleted T cells were treated further with biotinylated anti-CD25 (BD-PharMingen), washed, and incubated with streptavidin-magnetic beads (Miltenyi Biotec). Cells were washed with PBS and passed over an LD magnetic column or AutoMacs. After depletion, CD25+ cells could not be detected in the samples, even after 24 h in culture.
Cell proliferation assays
In vitro
T cells were isolated from lymph nodes and spleens of age-matched, female NOD and NOR mice. Cells were passed through lympholyte and then CD8-, CD69-, CD11c-, and MHC-class II-depleted using the Miltenyi Biotec AutoMacs system. Remaining cells were >93% CD3+ and CD4+. CD25+ cells were isolated from the naïve CD4+ population by treating with biotinylated anti-CD25 and then streptavidin-isolation beads (Miltenyi Biotec) and passed through AutoMacs. Cells demonstrated 98% purity. Remaining effector cells (CD4+, CD69-, and CD25-depleted) were CFSE-labeled as described [41] and then plated on anti-CD3- and anti-CD28-coated plates at 5 × 105 cells per well. Tregs, CD4+CD25+, were added at 0:1 (maximum proliferation control), 1:1, 2:1, and 4:1 ratios. Cells were incubated for 48 h. Proliferation was measured by loss of CFSE mean fluorescent intensity as described [41]. Experiments were done in triplicate and repeated two times.
In vivo
Naïve CD4 cells were purified by negative selection. Cells were positively selected for CD40 using biotinylated anti-CD40 (nonagonist 4F11) followed by iron-conjugated streptavidin beads and passing cells through the Automacs. Cells were >96% CD40+ when stained with a different (FGK-45) CD40 antibody. T cells at 2.5 × 106 were treated with anti-Fas and i.p.-injected into NOD.scid recipients. In an additional experiment, Th40 cells were not Fas-engaged. Six out of seven recipients of NOD T cells became diabetic, blood glucose >160 for 2 weeks, and four out of six NOR T cell recipients became diabetic. Cells were isolated from spleens and pancreatic lymph nodes and counted.
Adoptive transfers and histology
Th40 T cells were sorted by AutoMacs (Miltenyi Biotec) using biotinylated anti-CD40 (1C10+4F11) and shown to have 94% purity by flow cytometry. Th40 cells (2×106) isolated from NOD mice were transferred by i.p. injection [10] to 10-day-old NOD.scid recipients, six total in three separate experiments, which received NOR Th40 cells. CD40-depleted T cells (2×106) from NOR mice were transferred to NOD.scid recipients (six total in three separate experiments) at 10 days of age. In some experiments, CD40-depleted T cells were then CD25 (7D4, BD-PharMingen)-depleted, using the AutoMacs and found to have 99% purity. These T cells (2×106) were transferred to NOD.scid recipients, six total as before, and monitored for hyperglycemia. In some experiments, after CD40 and CD25 depletion, the remaining T cells were treated with 10 μg/ml biotinylated anti-CD3, 145.2C11, for 45 min, washed, and treated with 5 μg/ml streptavidin for 30 min. Cells were washed and incubated for 3 h. Activated T cells were i.p.-injected, 2 × 106, into six 10-day-old NOD.scid recipients. At sustained hyperglycemia, blood glucose >200 mg/dl for 3 consecutive days, animals were killed and pancreata removed, sectioned, and stained with aldehyde/fuchsin showing infiltrations and loss of insulin granules.
Statistical analysis included the Holm-Sidak and Mann-Whitney one-way ANOVA. The SigmaStat™ statistical package was used.
RESULTS
Diabetes-resistant NOR mice develop autoaggressive T cells
NOD and NOR mice have an identical T cell development background, carrying I-Ag7 MHC class II and possessing >90% of the same genome, but NOR mice do not develop diabetes [42]. This leads to the question of whether NOR mice develop autoaggressive T cells. In one report, NOR total splenic T cells that were CD25-depleted and Con A-stimulated caused insulitis in NOD.scid recipients [43]. When Tregs were present, only peri-insulitis occurred. We tested the hypothesis that purifying peripheral T cells to contain only the Th40 subset from NOR mice would induce insulitis and fulminant diabetes.
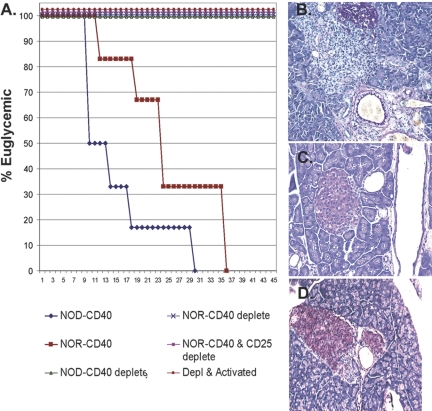
Th40 cells purified from NOR mice and adoptively transferred to NOD.scid recipients induced hyperglycemia (Fig. 1A), as we have shown with NOD Th40 cells [10, 11]. The rate of disease transfer kinetics was slower than transfer of T cells from NOD mice (Fig. 1A). Recipients of Th40-depleted cells from NOR or NOD mice remain euglycemic (Fig. 1A). Purification of CD40+ T cells could have selectively isolated Th40 cells away from the Treg population, which would functionally be the same as depleting Tregs. We addressed if Treg- and Th40-depleted T cells are capable of pathogenesis. Recipients of T cells that were Th40- and Treg-depleted remained euglycemic (Fig. 1A). Furthermore, even activation of the cells remaining after Th40 and Treg cell depletions did not transfer diabetes (Fig. 1A). Th40 cells purified from NOR mice and adoptively transferred to NOD.scid recipients induced rapid and destructive insulitis, demonstrated by loss of insulin granules (Fig. 1B). CD40-depleted T cells did not induce pancreatic pathology, i.e., no T cell infiltration and normal insulin production (Fig. 1C). Th40-depleted and CD25+ depleted T cells only allowed for some peri-insulitis with insulin granules remaining intact (Fig. 1D).
Fig. 1.
Th40 cells from diabetes-resistant NOR mice harbor autoaggressive T cells. CD40+ T cells isolated from NOR mice compared with NOD mice were adoptively transferred (2×106) to NOD.scid recipients. Controls included CD40-depleted T cells and CD40-depleted and then CD25-depleted T cells that were adoptively transferred with identical numbers. Animals were monitored for glucose levels >200 mg/dl. (A) Graph representing hyperglycemia incidence. NOD.scid recipients of NOD or NOR Th40 cells rapidly become hyperglycemic, but CD40-depleted followed by CD25-depleted T cell recipients do not exhibit hyperglycemia through 45 days. If CD40- and CD25-depleted T cells were activated with anti-CD3 cross-linking for 3 h and then adoptively transferred, no hyperglycemia occurred through 45 days. Pancreata were sectioned and stained by aldehyde/fuchsin to measure insulin granules. (B) Pancreatic histology from Th40 recipients demonstrates massive islet infiltration and few insulin granules; (C) histology from CD40-depleted pancreas of T cell recipients demonstrates no infiltration and normal insulin granule levels; (D) histology from recipients of CD40-depleted followed by CD25-depleted T cells shows peri-insulitis but normal insulin granule production. Each histology figure is representative of the six recipient mice for each treatment. Experiments included two mice for each treatment and were done three separate times.
Th40 and Tregs in pancreatic lymph nodes
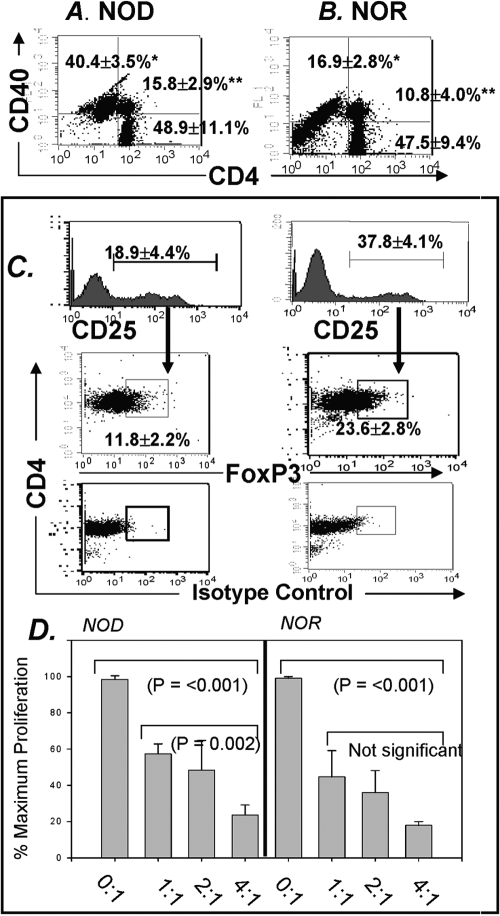
As demonstrated in Figure 1, Th40 cells isolated from NOR mice can be highly autoaggressive [10,11,12, 44]. Given that diabetogenic T cells are initially detected in draining pancreatic lymph nodes [2, 22, 45], we compared percentages of Th40 cells within purified CD4+ cells from pancreatic lymph nodes of prediabetic NOD mice with that of age-matched, diabetes-resistant NOR mice. Pancreatic lymph nodes of 9-week-old NOD mice contain a substantial Th40 population (Fig. 2A). Although NOR mice have the identical T cell developmental background as NOD mice, pancreatic lymph nodes from NOR mice maintain a significantly (P<0.001) lower percentage of Th40 cells (Fig. 2B)
Fig. 2.
Th40 and Tregs in draining pancreatic lymph nodes of NOD and NOR mice. Dot-plots representing CD4 versus CD40 in pancreatic lymph nodes from (A) eight individual NOD and (B) six individual age-matched NOR, female mice. Gates were set from isotype controls. The Th40 population (*) and the CD4hiCD40+ (**) are increased significantly in NOD (*, P<0.001; **, P=0.011; statistics performed by Holm-Sidak one-way ANOVA). (C) Histograms representing CD25 in purified CD4+ T cells from draining pancreatic lymph nodes of NOD and NOR mice. CD25 is increased significantly (P<0.001; Holm-Sidak, ANOVA) in NOR mice. From gated CD25+ cells, FoxP3 versus CD4 was examined. Isotype controls are shown. NOR mice demonstrate a significantly (P<0.001; Holm-Sidak ANOVA) higher percentage of FoxP3+ cells. Data represent analysis from individual mice, two of each strain per experiment, and experiments were performed three separate times. Percentages are reported as means ± sd. (D) Cell proliferation assays were performed as described in Materials and Methods. Proliferation of NOD Th40 cells was suppressed by NOD Tregs. NOR Th40 cells were suppressed by NOR Th40 cells. NOD Tregs were equally effective as NOR Tregs at controlling induced proliferation of Th40 cells. Ratios are Treg:Th40 concentrations. Data are from single NOD and NOR mice done three times, and experiments were repeated two times.
To address the potential tolerance mechanism preventing diabetes onset in NOR mice, we compared percentages of intrinsic Tregs between NOR and age-matched NOD mice in pancreatic draining lymph nodes. Studies have shown that peripheral Tregs from NOD mice express normal levels of GITR and CTLA-4 and are equally as suppressive as Tregs from other mouse strains [46]. From purified CD4+ T cells, levels of CD25+ cells were measured. Interestingly, diabetes-resistant NOR mice have almost a twofold higher percentage of CD25+ cells within the CD4+ population from the pancreatic lymph nodes (Fig. 2C). Within gated CD25+ cells, FoxP3+ cells are present in pancreatic lymph nodes of NOD and NOR mice (Fig. 2C). Pancreatic lymph nodes from NOR mice, however, have a greater percentage of FoxP3+ cells (P<0.001). Although the overall percentage of CD25+FoxP3+ cells is lower in NOD mice pancreatic lymph nodes, Tregs from NOD mice are fully functional (Fig. 2D). In fact, we confirmed that intrinsic Tregs, within the CD4+CD25+ population, from NOD lymph nodes have suppressive function equivalent to Tregs from NOR mice (Fig. 2D). These data are the first to directly demonstrate that Th40 cells are regulated by Tregs.
Ratio of autoaggressive and Tregs
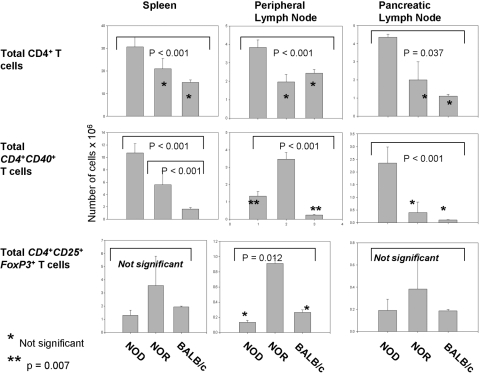
To gain better understanding of the relationship between intrinsic Tregs and the now-described, autoaggressive T cells, we determined the actual numbers of these different cell populations. Total CD4+ T cell numbers in spleen, peripheral lymph nodes, and draining pancreatic lymph nodes of NOD, NOR, and BALB/c mice were compared. Unlike previous studies focusing only on CD4hi, we included CD4lo T cells. NOD mice exhibit significantly (P<0.001) more overall CD4 T cells in spleen than NOR or BALB/c mice (Fig. 3). Total numbers of CD4+ T cells are not significantly different between NOR and BALB/c mice. Not surprisingly, NOD mice have expanded CD4+ T cell levels in pancreatic lymph nodes but also in peripheral lymph nodes.
Fig. 3.
Total number of CD4, Th40, and Tregs in spleen and peripheral and pancreatic lymph nodes of NOD, NOR, and BALB/c mice. CD4+ T cells were isolated from individual, 9- to 12-week-old female NOD, NOR, and BALB/c mice (>90% CD3+). T cells from spleen and peripheral and pancreatic lymph nodes were counted by trypan blue exclusion from six individual mice and averaged. Lymph node data are total cells per lymph node. Total number of Th40 cells was determined by staining total CD4+ T cells from each mouse and from each site with anti-CD40 and analysis by FACS to yield a percentage and then calculating the actual number. Total number of Tregs was determined from each mouse and each site by determining the percentage of CD4+CD25+FoxP3+ cells within the total number of counted cells and then calculating the actual number. Data represent analysis from individual mice, two of each strain per experiment, and experiments were performed three separate times.
Consistent with Th40 harboring autoaggressive cells, NOD mice have significantly (P<0.001) more Th40 cells in spleen and pancreatic lymph nodes (Fig. 3). NOD mice have more Th40 cells per peripheral lymph node than BALB/c mice. NOR mice have more Th40 cells than NOD or BALB/c mice only in the peripheral lymph node. The numbers of Th40 cells in pancreatic lymph node from NOR mice are not significantly different from those of BALB/c mice (Fig. 3). We examined the absolute number of CD4+CD25+FoxP3+ in spleen and peripheral and pancreatic lymph nodes. Although the percentages of Tregs differs, the absolute numbers of classically described Tregs, CD4+CD25+FoxP3+, in NOD mice spleen and per pancreatic lymph node were statistically not different than that of NOR and BALB/c mice (Fig. 3). Interestingly, NOR mice that are diabetes-resistant statistically have more Tregs than NOD or BALB/c per peripheral lymph node (Fig. 3).
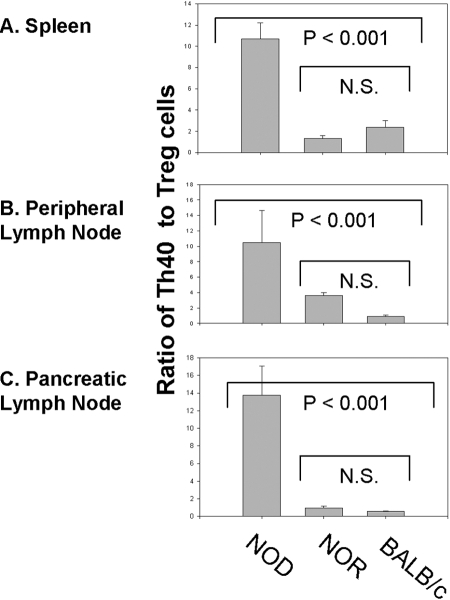
We directly compared the ratio of Th40:Treg cells. Tissues examined include spleen (Fig. 4A), peripheral lymph nodes (Fig. 4B), and pancreatic lymph nodes (Fig. 4C). In all tissues, the ratio of Th40:Treg cells is significantly (P<0.001) higher in NOD mice than in NOR or BALB/c mice, thus suggesting homeostatic dysregulation in NOD mice. When this ratio is compared, there is no significant difference between NOR and BALB/c mice. The peripheral lymph node ratio of Th40:Treg cells in NOR mice is not significantly (P=0.951) higher than that of BALB/c mice (Fig. 4B). Although Th40 cell numbers are elevated in NOR peripheral lymph nodes, the elevated numbers of Tregs compensate, as reflected by the Th40:Treg ratio. As might be expected, NOD mice have the highest ratio of Th40:Treg cells in pancreatic lymph nodes (Fig. 4C).
Fig. 4.
Ratio of Th40:Treg cells in spleen and peripheral and pancreatic lymph nodes. The ratio of actual numbers of Th40 and Treg, CD4+CD25+FoxP3+, was determined. Data represent analysis from individual mice, two of each strain per experiment, and experiments were performed three separate times. N.S., Not significant.
CD40-engaged Th40 T cells resist Fas-mediated cell death
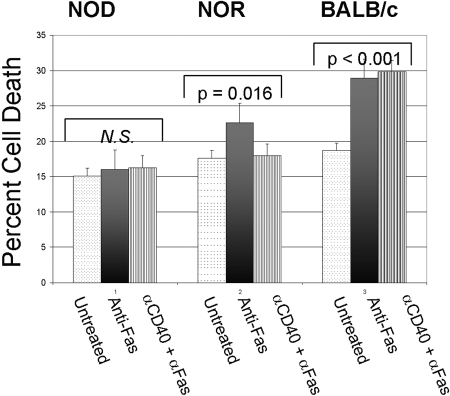
Although several factors can affect homeostatic dysregulation, we examined the effect of CD40 engagement on induced cell death. A major mechanism for lymphocyte cell death involves the Fas cell death pathway [47]. Fas is an important component of lymphocyte clearance through the activation-induced cell death mechanism [48]. However, depending on T cell naïve or memory phenotypes, Fas can induce cell death or cell proliferation [49]. Th40 cells from NOD mice treated with anti-Fas at 5 μg/ml were resistant to cell death above background levels (Fig. 5). NOR Th40 cells, however, were significantly (P=0.016), although not robustly, susceptible to Fas-mediated cell death (Fig. 5). CD40 engagement ablated Fas-mediated cell death of Th40 cells from NOR mice (Fig. 5). Th40 cells from BALB/c mice were susceptible to Fas-mediated cell death above basal levels, but CD40 engagement did not rescue the induced cell death (Fig. 5).
Fig. 5.
Fas-induced cell death in Th40 cells. Purified CD4+ T cells from three individual, 9- to 12-week-old female NOD, NOR, and BALB/c mice were untreated or CD40-oligomerized for 18 h. Following this treatment, cells were treated with 5 μg/ml anti-Fas for 18 h. Cell death was determined using PI to intercalate DNA. Percentages are the average of percent apoptotic cells. Cell death is verified by TUNEL assay measuring fragmented DNA [38]. Data reported are averaged from three individual mice with experiments performed three separate times.
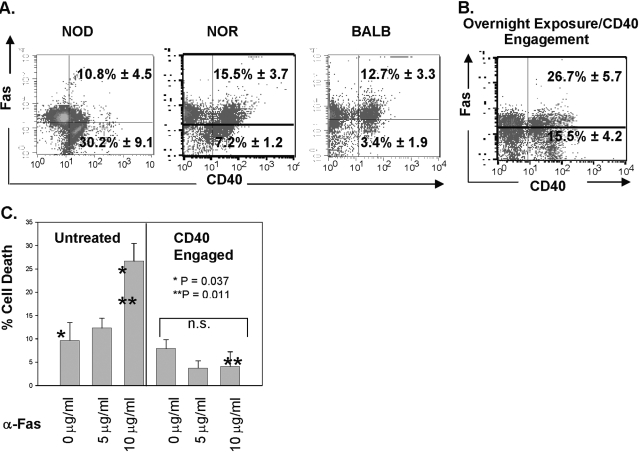
As NOD Th40 cells appear resistant to Fas-mediated cell death, we examined Fas levels on Th40 cells from the different mouse strains. The majority (92.8%) of immediately ex vivo Th40 cells from NOD mice is Fas– or Faslo (Fig. 6A). In contrast to this, immediately ex vivo, Th40 cells from NOR or BALB/c backgrounds are predominantly Fashi (Fig. 6A). Th40 cells from NOD mice that were CD40-engaged overnight had drastic increases (up to 52%) in Fas expression (Fig. 6B). Given this finding, we determined if increasing the concentration of anti-Fas antibody affects induced cell death of NOD Th40 cells. As before, lower concentrations (5 μg/ml) of anti-Fas had no significant effect on NOD Th40 cells (Fig. 6C). However, NOD Th40 cells were susceptible to Fas-mediated cell death when a super-saturating, 10 μg/ml, concentration of anti-Fas was used (Fig. 6C). When NOD Th40 cells were pretreated (18 h) with anti-CD40 and then treated with anti-Fas, even at the higher concentration, cells remained Fas cell death-resistant, although the majority of those T cells now expresses Fas (Fig. 6B).
Fig. 6.
Fas expression on Th40 cells. Immediately ex vivo T cells from spleens of NOD, NOR, or BALB/c mice were examined. (A) CD4+ T cells were purified and stained for CD40 versus Fas expression. Dot-plots are from individual mice and represent an average of nine NOD, six NOR, and six BALB/c mice. (B) Purified CD4+ T cells were incubated overnight with anti-CD40. Cells were stained for CD40 and Fas. Quadrants are identical for A and B, and gates were set from appropriate isotype controls. Data represent eight individual NOD mice. (C) Purified CD4+ T cells were incubated overnight without treatment or were CD40-oligomerized for 18 h. Cells from each subset were treated with anti-Fas at the concentrations shown for an additional 18 h. Cell death was determined by PI intercalation into DNA and flow cytometry as described in Figure 5. Data are from the averages of three individual mice per experiment with experiments performed three separate times. Percentages are reported as means ± sd.
Th40 cells from NOD mice proliferate more readily than Th40 cells from NOR mice
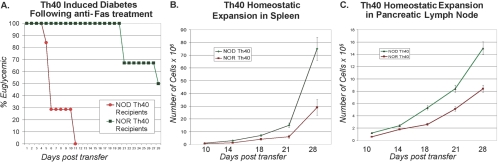
The amount of cell death induced by anti-Fas in NOR Th40 cells is significant but not robust, and a distinct difference in Th40 cell numbers occurs between NOD and NOR mice. We examined the effects of treating Th40 cells from NOD and from NOR mice with anti-Fas, followed by adoptive transfers of these cells to NOD.scid recipients (Fig. 7A). Mice receiving anti-Fas-treated Th40 cells from NOD donors exhibited rapid hyperglycemia (Fig. 7A). Mice receiving anti-Fas-treated Th40 cells from NOR donors exhibited delayed hyperglycemia, as compared with Th40 cells that were not Fas-engaged (compare Figs. 7A with 1A). Th40 cells were not killed by Fas treatment, as cells were recovered from spleen and pancreatic lymph nodes.
Fig. 7.
Th40 homeostatic proliferation. Equal numbers, 2.5 × 106, of naïve Th40 cells that were Fas-cross-linked from NOD (circles) or NOR (squares) mice were injected into NOD.scid recipients. (A) Six out of seven recipients of NOD T cells became hyperglycemic at 6 days and seven out of seven by Day 11 post-transfer, blood glucose >180 mg/dl for at least 4 continuous, separate days. Three out of six NOR T cell recipients became hyperglycemic after 28 days. For cell recovery and proliferation, two NOD and two NOR mice were killed at 10, 14, 18, and 21 days post-transfer. Numbers of T cells in spleen (B) and pancreatic lymph nodes (C) were determined. Anti-Fas engagement had no effect on Th40 expansion. Th40 cells from NOD and NOR that were not anti-Fas-engaged gave the same expansion rates. Cells were stained and found to be >98% CD4+ and CD40+.
The potential for induced cell death alone would not account for the differences in Th40 numbers between NOD and NOR mice. We considered that the homeostatic proliferation rate may be greater in Th40 cells from NOD mice. Th40 cells were isolated from age- and gender-matched NOD and NOR mice. Activated cells and importantly, Tregs were removed, and then naïve Th40 cells were injected into NOD.scid recipients to allow comparison of homeostatic proliferation [50]. After injecting equal numbers of NOD or NOR Th40 cells, greater numbers of Th40 cells were detected in spleen (Fig. 7B) and pancreatic lymph nodes (Fig. 7C) in recipients of NOD Th40 cells after 10, 14, 18, 21, and 28 days. Th40 cells from NOR mice did expand in number over time in spleen and pancreatic lymph nodes, demonstrating that these T cells are not static. By 21 days post-T cell transfer, the numbers of NOD Th40 cells were 2.5-times greater than the numbers of NOR Th40 cells (Fig. 7). These data demonstrate that in addition to lower Fas-mediated cell death susceptibility, Th40 cells from NOD mice undergo more rapid homeostatic proliferation.
DISCUSSION
Autoimmune diseases, including T1D, are characterized by persistence of autoaggressive T cells. These rogue T cells have broken peripheral tolerance to promote inflammation, provide “help” for B cells to produce autoantibodies, and in many cases, directly attack self-tissue leading to tissue destruction [51,52,53]. Mechanisms controlling effector, autoaggressive T cells include induced anergy, activation-induced cell death, and suppression mediated by Tregs. Reports indicate that in the NOD mouse model of T1D, Tregs are fully functional [46, 54], and studies further indicate that Tregs in NOD mice do not undergo preferential cell death, but rather, levels of Tregs remain constant [54]. Nonetheless, NOD mice develop T1D. A recent study suggests that NOD mice do not have a global defect in the generation or maintenance of Tregs [55]. As we show in this report, when the actual number of Tregs is measured, NOD mice statistically are not different than diabetes-resistant NOR mice and nonautoimmune BALB/c mice. Importantly, the Tregs from NOD mice are equally functional as Tregs from NOR mice. Why then do NOD mice develop T1D?
The existence of autoaggressive T cells, even in nonautoimmune individuals, is understood. A critical problem, however, has been defining a reliable cell surface marker for these effector T cells. We have shown that CD40, although typically associated with APCs, demarcates diabetogenic T cells in the NOD mouse model [10,11,12,13, 44], and recently, we showed this to be true in human T1D [56]. We demonstrated that Th40 cells are found directly in the pancreata of prediabetic and diabetic mice, and Th40 cells transfer T1D to NOD.scid recipients [10]. Th40 cells occur at normal numbers in 3-week-old, preinsulitis NOD mice but expand concurrently with progressive insulitis [11]. We show here that in pancreatic draining lymph nodes, even well prior to onset of hyperglycemia, Th40 cells are expanded significantly in percentage and actual cell numbers in NOD mice. In fact, we speculate that it is within this T cell population that an abnormality in autoimmune diabetes occurs. NOR mice have an identical T cell developmental background as NOD mice [42] and as we confirmed here, develop autoaggressive T cells, yet the levels of Th40 cells remain contained such that NOR mice do not develop diabetes. This suggests a tolerogenic mechanism that is effective in NOR mice and deficient in NOD mice and that may involve CD40.
There are several possible reasons for the exaggerated imbalance in NOD mice that results in the disruption of homeostasis leading to breach of tolerance and fulminate disease. Peripheral mechanisms to control T cell function include those imposed by Tregs, e.g., cell-to-cell contact-mediated TGF-β inhibition, TGF-β secretion, competition for antigen and competition for homeostatic space, and other control mechanisms include activation-induced and Fas-mediated cell death and homeostatic regulation of T cells. Disrupting any of these control mechanisms ultimately would result in the survival and accumulation of self-antigen-reactive, in this case, autoaggressive T cells. Mechanistically one fundamental difference between NOD and NOR or BALB/c Th40 cells includes immediately ex vivo expression levels of Fas and response of these T cells to Fas engagement. CD40 engagement protects NOD Th40 cells from Fas-mediated cell death, even at super-saturating concentrations of anti-Fas. CD40 engagement protects NOR Th40 cells from Fas-mediated cell death, but we demonstrated that NOR Th40 cells can be autoaggressive. Using a nonautoimmune control, BALB/c mice, Th40 cells are not protected from Fas-mediated cell death by CD40 engagement.
When T cells are activated, specifically through the TCR, there are two thresholds of activation: one that occurs with lower antigen dose to induce CD154, CD25, CD69, and other activation molecules [57]; the second that induces Fas ligand (FasL) and requires high antigen dose and long-lasting TCR engagement [57]. A plausible hypothesis is that during autoimmune conditions, autoaggressive T cell interaction with self-antigens is sufficient to induce CD154 and ultimately, relatively high FasL expression. As CD154 is induced at a lower antigen dose, the potential signal through CD40 would come first, with potential signals from Fas occurring later. As shown here, pre-engagement of CD40 on Th40 cells in NOD but not in T cells from a nonautoimmune background protects from Fas-mediated cell death. Although self-antigens eventually induce FasL, the induction of CD154 initially overrides the Fas signals in Th40 cells. Only when FasL is excessively high, and CD40 signals have been exhausted, perhaps after long-term antigen exposure, would Th40 cells be able to undergo cell death.
Importantly, Tregs from NOD mice are functional and occur at actual numbers equivalent to NOR and nonautoimmune BALB/c mice. Tregs are capable of controlling Th40 cells, even in NOD mice. However, some fundamental tolerance dysregulation occurs, leading to drastic disruptions in the balance between potentially pathogenic Th40 cells and Tregs. Such a fundamental difference between NOD and NOR mice includes that Th40 cells from NOD mice undergo more rapid homeostatic expansion than do Th40 cells from NOR mice. Throughout the experimental period and notably, by 28 days, Th40 cells from NOD mice had drastically outpaced Th40 cells from NOR. The hypothesis would be that although Tregs are capable of suppressing Th40 cells, as Th40 cells from NOD mice can homeostatically expand much more rapidly than Th40 cells from NOR, NOD Th40 cells overwhelm the Treg control, leading to the pathogenic T cell imbalance.
Mechanisms explaining the tolerance disruption leading to autoimmunity include many different pathways. The studies presented here collectively represent the delicate balance that must be maintained between identifiable, autoaggressive Th40 and Treg cells and the emerging role of CD40 in that homeostatic dance.
Acknowledgments
This work was supported by grants from the National Institutes of Health DK-07501, American Diabetes Association, Juvenile Diabetes Research Foundation, and Kleberg Foundation to D. H. W.
References
- Szanya V, Ermann J, Taylor C, Holness C, Fathman C G. The subpopulation of CD4(+)CD25(+) splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- Kent S C, Chen Y, Bregoli L, Clemmings S M, Kenyon N S, Ricordi C, Hering B J, Hafler D A. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- Oling V, Marttila J, Ilonen J, Kwok W W, Nepom G, Knip M, Simell O, Reijonen H. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun. 2005;25:235–243. doi: 10.1016/j.jaut.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Reijonen H, Novak E J, Kochik S, Heninger A, Liu A W, Kwok W W, Nepom G T. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- Gottlieb P A, Eisenbarth G S. Insulin-specific tolerance in diabetes. Clin Immunol. 2002;102:2–11. doi: 10.1006/clim.2001.5142. [DOI] [PubMed] [Google Scholar]
- Barker J M, Barriga K J, Yu L, Miao D, Erlich H A, Norris J M, Eisenbarth G S, Rewers M. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann D R, Hutton J C, Elliott J F, Eisenbarth G S. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski J M, Yu L, Nakayama M, Li M M, Lipes M A, Eisenbarth G S, Liu E. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- Ott P A, Dittrich M T, Herzog B A, Guerkov R, Gottlieb P A, Putnam A L, Durinovic-Bello I, Boehm B O, Tary-Lehmann M, Lehmann P V. T cells recognize multiple GAD65 and proinsulin epitopes in human type 1 diabetes, suggesting determinant spreading. J Clin Immunol. 2004;24:327–339. doi: 10.1023/B:JOCI.0000029120.77824.41. [DOI] [PubMed] [Google Scholar]
- Wagner D H, Jr, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci USA. 2002;99:3782–3787. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waid D M, Vaitaitis G M, Wagner D H., Jr Peripheral expansion of CD4loCD40+ auto-aggressive T cells during insulin-dependent diabetes mellitus. Eur J Immunol. 2004;34:1488–1497. doi: 10.1002/eji.200324703. [DOI] [PubMed] [Google Scholar]
- Vaitaitis G M, Poulin M, Sanderson R J, Haskins K J, Wagner D H., Jr Cutting edge: CD40-induced expression of recombination activating gene (RAG) 1 and RAG2: a mechanism for the generation of autoaggressive T cells in the periphery. J Immunol. 2003;170:3455–3459. doi: 10.4049/jimmunol.170.7.3455. [DOI] [PubMed] [Google Scholar]
- Wagner D H, Jr, Newell E, Sanderson R, Freed J H, Newell M K. Increased expression of CD40 on thymocytes and peripheral T cells in autoimmunity: a mechanism for acquiring changes in the peripheral T cell receptor repertoire. Int J Mol Med. 1999;4:231–242. doi: 10.3892/ijmm.4.3.231. [DOI] [PubMed] [Google Scholar]
- Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt H O, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–4627. [PubMed] [Google Scholar]
- Yellin M J, Sinning J, Covey L R, Sherman W, Lee J J, Glickman-Nir E, Sippel K C, Rogers J, Cleary A M, Parker M, Chess L, Lederman S. T lymphocytes T cell-B cell activating molecule/CD40-L molecules induce normal B cells or chronic lymphocytic leukemia B cells to express CD80(B7/BB1) and enhance their costimulatory activity. J Immunol. 1994;153:666–674. [PubMed] [Google Scholar]
- Stumpf C, Lehner C, Eskafi S, Raaz D, Yilmaz A, Ropers S, Schmeisser A, Ludwig J, Daniel W G, Garlichs C D. Enhanced levels of CD154 (CD40 ligand) on platelets in patients with chronic heart failure. Eur J Heart Fail. 2003;5:629–637. doi: 10.1016/s1388-9842(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Chiarelli F, Davi G, Ferri C, Desideri G, Fazia M, Iezzi A, Santilli F, Pini B, Cuccurullo C, Tumini S, Del Ponte A, Santucci A, Cuccurullo F, Mezzetti A. Enhanced soluble CD40 ligand contributes to endothelial cell dysfunction in vitro and monocyte activation in patients with diabetes mellitus: effect of improved metabolic control. Diabetologia. 2005;48:1216–1224. doi: 10.1007/s00125-005-1750-2. [DOI] [PubMed] [Google Scholar]
- Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–464. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- Harding S A, Sommerfield A J, Sarma J, Twomey P J, Newby D E, Frier B M, Fox K A. Increased CD40 ligand and platelet-monocyte aggregates in patients with type 1 diabetes mellitus. Atherosclerosis. 2004;176:321–325. doi: 10.1016/j.atherosclerosis.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Robinson D S. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5:136–141. doi: 10.1007/s11882-005-0087-8. [DOI] [PubMed] [Google Scholar]
- Fontenot J D, Rasmussen J P, Williams L M, Dooley J L, Farr A G, Rudensky A Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Pop S M, Wong C P, Culton D A, Clarke S H, Tisch R. Single cell analysis shows decreasing FoxP3 and TGF{β}1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R S, Whitters M J, Piccirillo C A, Young D A, Shevach E M, Collins M, Byrne M C. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow D, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Bach J F, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–161. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- Lepault F, Gagnerault M C. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- Eggena M P, Walker L S, Nagabhushanam V, Barron L, Chodos A, Abbas A K. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Bluestone J A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- Stassen M, Fondel S, Bopp T, Richter C, Muller C, Kubach J, Becker C, Knop J, Enk A H, Schmitt S, Schmitt E, Jonuleit H. Human CD25+ regulatory T cells: two subsets defined by the integrins α4β7 or α4β1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303–1311. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- Every A L, Kramer D R, Mannering S I, Lew A M, Harrison L C. Intranasal vaccination with proinsulin DNA induces regulatory CD4+ T cells that prevent experimental autoimmune diabetes. J Immunol. 2006;176:4608–4615. doi: 10.4049/jimmunol.176.8.4608. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam A L, Xu-Yu Z, Szot G L, Lee M R, Zhu S, Gottlieb P A, Kapranov P, Gingeras T R, Fazekas de St Groth B, Clayberger C, Soper D M, Ziegler S F, Bluestone J A. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, Kato T, Tawara I, Saito K, Ikeda H, Kuribayashi K, Allen P M, Schreiber R D, Sakaguchi S, Old L J, Shiku H. Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med. 2005;201:681–686. doi: 10.1084/jem.20041959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- Bluestone J A, Abbas A K. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Heath A W, Wu W W, Howard M C. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur J Immunol. 1994;24:1828–1834. doi: 10.1002/eji.1830240816. [DOI] [PubMed] [Google Scholar]
- Wagner D H, Jr, Hagman J, Linsley P S, Hodsdon W, Freed J H, Newell M K. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7–2. J Exp Med. 1996;184:1631–1637. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim K A, Hwang D Y, Lee T H, Kayagaki N, Yagita H, Lee M S. Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved. J Immunol. 2000;164:2931–2936. doi: 10.4049/jimmunol.164.6.2931. [DOI] [PubMed] [Google Scholar]
- Reparon-Schuijt C C, van Esch W J, van Kooten C, Rozier B C, Levarht E W, Breedveld F C, Verweij C L. Regulation of synovial B cell survival in rheumatoid arthritis by vascular cell adhesion molecule 1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000;43:1115–1121. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Quah B J, Warren H S, Parish C R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Serreze D V. Autoimmune diabetes results from genetic defects manifest by antigen presenting cells. FASEB J. 1993;7:1092–1096. doi: 10.1096/fasebj.7.11.8370480. [DOI] [PubMed] [Google Scholar]
- Ott P A, Anderson M R, Tary-Lehmann M, Lehmann P V. CD4+CD25+ regulatory T cells control the progression from periinsulitis to destructive insulitis in murine autoimmune diabetes. Cell Immunol. 2005;235:1–11. doi: 10.1016/j.cellimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wagner D H., Jr Re-shaping the T cell repertoire: TCR editing and TCR revision for good and for bad. Clin Immunol. 2007;123:1–6. doi: 10.1016/j.clim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alard P, Manirarora J N, Parnell S A, Hudkins J L, Clark S L, Kosiewicz M M. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55:2098–2105. doi: 10.2337/db05-0810. [DOI] [PubMed] [Google Scholar]
- Ju S T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumor counterattack. Immunol Cell Biol. 2002;80:131–137. doi: 10.1046/j.1440-1711.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Wade T, Wade W F, Newell M K. Dichotomy between naive and memory CD4(+) T cell responses to Fas engagement. Proc Natl Acad Sci USA. 1999;96:8104–8109. doi: 10.1073/pnas.96.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses C T, Thorstenson K M, Jameson S C, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci USA. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J J. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Elson C J, Barker R N. Helper T cells in antibody-mediated, organ-specific autoimmunity. Curr Opin Immunol. 2000;12:664–669. doi: 10.1016/s0952-7915(00)00160-6. [DOI] [PubMed] [Google Scholar]
- Sabelko-Downes K A, Russell J H, Cross A H. Role of Fas–FasL interactions in the pathogenesis and regulation of autoimmune demyelinating disease. J Neuroimmunol. 1999;100:42–52. doi: 10.1016/s0165-5728(99)00191-5. [DOI] [PubMed] [Google Scholar]
- Mellanby R J, Thomas D, Phillips J M, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Jiang W, Holler P D, Satpathy A, Campbell C, Bogue M, Mathis D, Benoist C. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci USA. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waid D M, Wagner R J, Putnam A, Vaitaitis G M, Pennock N D, Calverley D C, Gottlieb P, Wagner D H., Jr A unique T cell subset described as CD4loCD40+ T cells (TCD40) in human type 1 diabetes. Clin Immunol. 2007;124:138–148. doi: 10.1016/j.clim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jodo S, Xiao S, Ju S T. Hypothesis: a recurrent, moderate activation fosters systemic autoimmunity—the apoptotic roles of TCR, IL-2 and Fas ligand. J Biomed Sci. 1999;6:306–313. doi: 10.1007/BF02253519. [DOI] [PubMed] [Google Scholar]