Abstract
Eosinophils are critically dependent on IL-5 for their activation, differentiation, survival, and augmentation of cytotoxic activity. We previously showed that the cytoplasmic domain of the hematopoietic receptor, βc, which is shared by IL-5, IL-3, and GM-CSF, is directly ubiquitinated and degraded by the proteasomes in a JAK2-dependent manner. However, studies describing the spatial distribution, endocytic regulation, and trafficking of βc-sharing receptors in human eosinophils are currently lacking. Using deconvolution microscopy and biochemical methods, we clearly demonstrate that IL-5Rs reside in and are internalized by clathrin- and lipid raft-dependent endocytic pathways. Microscopy analyses in TF1 cells and human eosinophils revealed significant colocalization of βc, IL-5Rα, and Cy3-labeled IL-5 with transferrin- (clathrin) and cholera toxin-B- (lipid raft) positive vesicles. Moreover, whereas internalized IL-5Rs were detected in both clathrin- and lipid raft-positive vesicles, biochemical data revealed that tyrosine phosphorylated, ubiquitinated, and proteasome-degraded IL-5Rs partitioned to the soluble, nonraft fractions (clathrin-containing). Lastly, we show that optimal IL-5-induced signaling requires entry of activated IL-5Rs into the intracellular compartment, as coimmunoprecipitation of key signaling molecules with the IL-5R was completely blocked when either endocytic pathway was inhibited. These data provide the first evidence that IL-5Rs segregate and traffic into two distinct plasma membrane compartments, and they further establish that IL-5R endocytosis regulates signaling both positively and negatively.
Keywords: endocytosis, receptor trafficking, ubiquitin/proteasome degradation pathway, eosinophils, signal transduction
INTRODUCTION
Interleukin-5 (IL-5) is a hematopoietic cytokine made predominantly by activated T helper cells, eosinophils, and mast cells [1]. This cytokine specifically regulates eosinophilic differentiation, activation, and survival by signaling through its heteromeric receptor composed of IL-5Rα and a shared signaling chain, βc [2,3,4]. Accordingly, IL-5, IL-5Rα, and eosinophils have been implicated in the pathogenesis of certain diseases involving eosinophilic infiltration, such as allergic inflammatory disorders and hypereosinophilic syndromes [5,6,7,8,9,10,11]. Therefore, understanding the basic molecular mechanisms governing IL-5 receptor (IL-5R) down-regulation is crucial to gaining insight into eosinophil physiology.
Endocytosis of receptors is an important regulatory mechanism for controlling the level of cell surface receptor expression, compartmentalized signaling, and degradation in lysosomes [12,13,14,15,16,17,18,19]. Recent endocytic trafficking studies have revealed a high diversity of internalization routes into mammalian cells: phagocytosis, clathrin-mediated, caveolin (lipid raft)-dependent, and clathrin- and caveolin-independent endocytosis [12,13,14,15,16,17,18,19,20,21,22,23]. Clathrin-mediated endocytosis (CME) involves the assembly of a clathrin coat on the cytoplasmic face of the plasma membrane, which forms pits that invaginate to pinch off (scission) and become free clathrin-coated vesicles (CCVs). Clathrin-dependent endocytosis delivers internalized cell surface components and solutes to early endosomes. Here, molecules that need to be reutilized are recycled back to the plasma membrane or are transported to late endosomes for their degradation in the lysosomes [12,13,14,15,16,17,18,19].
A clathrin-independent mechanism for removing activated receptors from the cell surface is the lipid raft/caveolar pathway (also referred to as lipid raft membrane microdomains) [20,21,22,23]. Lipid rafts are heterogeneous patches of cell membranes that are rich in protein, cholesterol, glycosphingolipids, sphingelomyelin, and phospholipids. Biochemically, lipid rafts are characterized by their resistance to extraction with nonionic detergents such as Triton X-100 at low temperature. Proteins with saturated acyl chains (like those with GPI anchorage) favor partitioning to the tightly packed, highly ordered lipid environment of the rafts [20,21,22,23].
Some, but not all lipid raft microdomains contain caveolae which are flask-shaped invaginations that are rich in caveolin, a cholesterol-binding protein [20,21,22,23]. Lipid rafts and caveolae function mainly in vesicular and cholesterol trafficking; however, they also function in the internalization of toxins, SV40 virus, and glycosyl phosphatidylinositol (GPI)-anchored proteins [20,21,22,23]. Moreover, lipid rafts and caveolae have been shown to control a variety of signaling pathways, including those for IL-2, T cell receptor, IFN-γ, and TGF-β [24,25,26,27].
To date, very little is known about the spatial and temporal regulation of IL-5R endocytosis [28]. Herein, we provide the first evidence that IL-5Rs reside in and internalize into two distinct endocytic compartments in human TF1 cells and eosinophils. In addition, we establish that endocytosis of activated IL-5Rs serves to both amplify (initially) and terminate signaling.
MATERIALS AND METHODS
Cell culture, materials, and inhibitors
The human erythroleukemic cell line, TF-1-F11, was subcloned, as described previously [29], and endogenously expresses the IL-5, IL-3, and GM-CSF receptors. Recombinant human IL-5 was baculovirus-expressed and affinity-purified as described previously [29]. For all kinetic, flow cytometric, and fluorescence microscopy analyses in the presence or absence of inhibitors, TF1 cells were depleted of IL-5 for 24 h in RPMI 1640 containing 10% FBS (cytokine starvation). Cells were then stimulated with 10 ng/ml IL-5 for the indicated times, except for Cy3-IL-5 colocalization assays in which 250 ng/ml were used for visualization of the labeled ligand.
Filipin (FP) and nystatin (NYS) were purchased from Sigma (St. Louis, MO, USA), dissolved in 100% dimethyl sulfoxide, and used at 2.5 (FP) or 25-50 (NYS) μg/ml final concentration for 30 min before IL-5 stimulation. Cy3-IL-5 was prepared using a Fluorolink-Cy3 labeling kit, according to the manufacturer’s instructions (GE Healthcare, New York, NY, USA) and used at 250 ng/ml. Alexa Fluor-488-conjugated transferrin (Tfr) and cholera toxin subunit B (CTB) were purchased from Molecular Probes. Anti-transferrin receptor antibodies were purchased from BD Biosciences (San Jose, CA, USA).
Eosinophil isolation
Peripheral blood Leukopaks from normal healthy volunteers were obtained from the Gulf Coast Regional Blood Center (Houston, TX, USA). Briefly, blood was diluted 1:5 with HBSS, layered onto a Ficoll-Paque Plus gradient (density, 1.077 g/ml) (GE Healthcare), and centrifuged at 700 g for 30 min at 18°C. The supernatant and mononuclear cells (PBMCs) at the interface were carefully removed, and erythrocytes in the polymorphonuclear cell pellet (PMNs) were lysed in chilled distilled water. Eosinophils were isolated by negative selection using anti-CD16 magnetic beads (Miltenyi Biotec, Gladbach, Germany) to bind and remove the neutrophils with an Auto-MACS flow machine. Eosinophil purity was estimated by Giemsa stain and was usually 90-95% pure (n=4).
Cy3-IL-5, transferrin-488, and cholera toxin-B subunit-488 colocalization assays
Cytokine-starved TF1 cells (100,000 cells per tube) were serum starved for 60 min at 37°C and placed on ice for 20-30 min to stop basal endocytosis. For colocalization assays with anti-βc and anti-IL-5Rα antibodies, all cells were incubated with 10 ng/ml unlabeled IL-5, and either 10 μg/ml Tfr-488 or 10 μg/ml CT-B-488, on ice for 30-60 min. For colocalization with Cy3-IL-5, 10 μg/ml Tfr-488, or 10 μg/ml CT-B-Alexa 488 were added to the cells in the presence of 250 ng/ml Cy3-IL-5. After washing unbound label with serum-free media, cells were transferred to 37°C for 30 to 60 min for uptake of labeled markers. Endocytosis was terminated by washing with cold PBS. All cells were treated with acid wash (50 mM glycine, 150 mM NaCl, pH 2.5) for 5 min on ice to remove cell surface ligand, except for 0 min label in TF1 cells to show cell surface binding (Fig. 4, upper left panel).
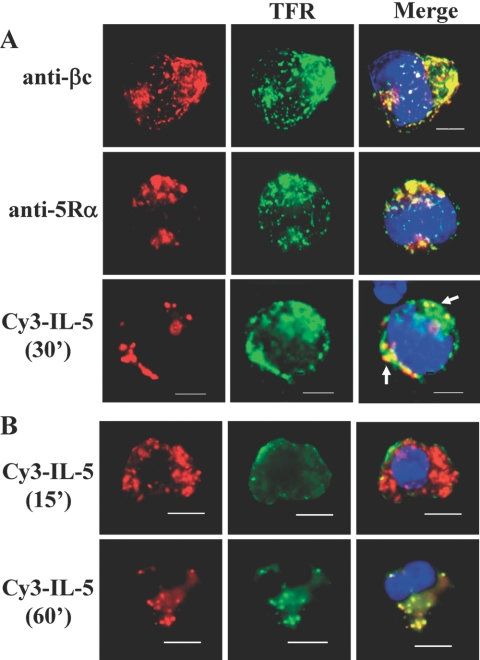
Fig. 1.
IL-5Rs colocalize with transferrin-positive vesicles (clathrin-containing). (A) Cytokine-starved TF1 cells were serum starved and incubated with cold transferrin-488 (10 μg/ml) and cold unlabeled IL-5 (10 ng/ml) for 30 min as described in Materials and Methods. For dual uptake assays, 250 ng/ml Cy3-IL-5 and 10 μg/ml Tfr-488 were added together. Cells were treated with acid wash, fixed, stained with anti-βc (upper panels) or anti-IL-5Rα (middle panels), and mounted with ProLong Gold reagent, which contains a DAPI stain (blue) for nuclear detection. Images were collected and analyzed by deconvolution microscopy. Note the colocalization (merge) of βc (upper right panel), 5Rα (middle right panel), and Cy3-IL-5 (lower right) with Tfr-488. Percentage of cells counted showing βc/Tfr co-localization was 77%; 67% for 5Rα/Tfr; and 35% for Cy3-IL-5/Tfr. Raw cell counts are listed in Table 1. Scale bar = 5 μm. (B) Cy3-IL-5 and Tfr-488 (clathrin-positive) colocalize in human eosinophils. After isolation from leukopaks, freshly isolated eosinophils were “synchronized” overnight in RPMI medium containing 10% FBS (no IL-5). Dual uptake assays with Cy3-IL-5 (250 ng/ml) (red) and Tfr-488 (green) were performed exactly as described for TF1 cells, and colocalization was visualized by deconvolution microscopy at 15 and 60 min after initiation of internalization at 37°C. Nuclei are visualized with DAPI stain. These results are representative of 4 independent experiments (n=4) with a minimum of 80-100 cells analyzed.
Fig. 2.
IL-5Rs colocalize with CTB-positive vesicles (lipid rafts). (A) Cytokine-starved TF1 cells were serum-starved for 60 min before the addition of cold cholera toxin-B (CTB)-488 (10 μg/ml, green) and unlabeled IL-5 (10 ng/ml) to chilled cells on ice for 30 min. The remaining procedure is exactly the same as in Fig. 1. Percentage of cells counted showing βc/CTB colocalization was 13%; 17% between 5Rα/CTB, and 45% between Cy3-IL-5/CTB. Raw cell counts are listed in Table 1. Scale bar = 5 μm. (B) Cy3-IL-5 and CTB-488 (lipid raft-positive) colocalize in human eosinophils. The procedure is exactly the same as in Fig. 1B, except cells were labeled with CTB-488 and Cy3-IL-5. Again, these results are representative of 4 independent experiments and reflect a minimum of 80-100 cells, which were analyzed.
Fig. 3.
CTB and transferrin receptors do not colocalize. (A) Chilled TF1 cells were simultaneously bound with CTB-488 (green) and anti-transferrin receptor antibodies on ice. Cells were moved to 37°C for dual uptake of both markers for 60 min. After fixation and permeabilization, internalized transferrin receptor antibodies were labeled with Alexa Fluor 594 (red) and visualized by deconvolution microscopy. Note how both biological markers stain separately. Nuclei are counterstained with DAPI (blue). (B) IL-5 binding does not interfere with antibody staining of IL-5 receptors. Chilled TF1 cells were incubated with (+IL-5, hatched line) or without 10 ng/ml IL-5 (–IL-5, solid black line) for 30 min on ice. Cell surface receptors were labeled with the same anti-βc and anti-IL-5Rα-PE-conjugated antibodies used in Figs. 1 and 2, and analyzed by flow cytometry. The shaded histogram represents cells labeled with an isotype-matched control antibody. Note the overlap of –IL-5 and +IL-5 histograms, indicating that the ligand does not interfere with antibody staining.
Fig. 4.
Internalized IL-5Rs colocalize with the lysosomal marker, LAMP-1. Cytokine-starved TF1 cells (upper panels) and eosinophils, which had been cultured in 1 ng/ml human IL-5 for 1 day and cytokine-starved for 1-2 days (lower panels), were processed as described in Figs. 1 and 2 with 250 ng/ml cold Cy3-IL-5 and either left on ice (0 min) or transferred to 37°C for 30 or 60 min. After acid washing, the 30- and 60-min time points only, all cells were fixed, cytospun, and stained with mouse anti-human LAMP-1 mAb (1:50 dilution, BD Biosciences), followed by incubation with 1:500 dilution of donkey anti-mouse IgG-Alexa-647, whose color was changed to green for visualization and colocalization analysis (for TF1 cells), or goat anti-mouse IgG-Alexa-488 for EOS. Nuclei are counterstained with DAPI (blue). Percentage of TF1 cells showing colocalization between Cy3-IL-5 and LAMP-1 was 18%; EOS had 10% colocalization (6+ cells/60; n=2).
Cells were then fixed with 4% formaldehyde for 20 min, cytospun (700 rpm, 5 min), and either mounted with Prolong Gold anti-fade reagent (which contains DAPI counterstain, Molecular Probes), or processed for antibody labeling as follows: cells were permeabilized with 0.2% Triton, blocked with 10% normal goat serum, and incubated with 4 μg/ml mouse-anti-human-βc (S-16) mAb conjugated to PE (Fig. 1A), 2 μg/ml goat-anti-human-5Rα polyclonal (R&D Systems, Minneapolis, MN, USA) (Fig. 1A), 2 μg mouse-anti-human βc mAb (Fig. 2A), 2 μg mouse-anti-human IL-5Rα mAb (BD Biosciences) (Fig. 2A), or 1:50 dilution of mouse anti-human LAMP-1 mAb (BD Biosciences) (Fig. 4) for 1 h at RT. After washing unbound βc, 5Rα and LAMP-1 antibodies (3 times with 1× PBS), cells were incubated with 1:1,000 dilution of rabbit anti-goat IgG-Alexa-594 (for anti-5Rα polyclonal Abs), 1:1,000 goat anti-mouse IgG-Alexa-594 (for anti-βc and anti-5Rα mAbs), and 1:500 donkey anti-mouse IgG-Alexa-647 or goat anti-mouse IgG-Alexa-488 (for anti-LAMP-1 mAb) (all 2°Abs were purchased from Molecular Probes, Eugene, OR, USA). After washing unbound 2°Abs (3 times with PBS), cells were mounted with Prolong Gold antifade reagent.
Deconvolution fluorescence microscopy was performed on a Zeiss AxioImager Z1 epifluorescence microscope with a Plan Apochromat ×63/1.4 numerical aperture (NA) oil-immersion objective. Images were acquired with a Zeiss Axiocam charge-coupled device camera using Axiovision software. Z-series of optical sections were performed at 0.2- to 0.3-μm increments, followed by deconvolution analysis with AxioVision LE software. Three-dimensional reconstructions were created for all microscopy images shown using the 25 to 30 Z-series images captured. In addition, for colocalization assays, images were further analyzed with AV4 MOD Wide-field Multiunmixing software (Zeiss) to eliminate any cross-talk (bleed-through) between the 488-nm, 595-nm, and 647-nm channels. Scale bar on images is 5 μm. Representative profiles are shown in Fig. 1, 2, and 4 (n=4).
Percentage of cells showing positive colocalization (two or more colocalized vesicles per cell) was calculated by counting number of cells with yellow vesicles over number of cells showing intracellular staining of Cy3-IL-5, βc, or IL-5Rα X 100. Raw cell counts for eosinophils and TF1 cells are listed in Table 1.
Table 1.
Co-localization of IL-5 Receptors with Tfr-488, CTB-488, and LAMP1-488
| Labeled markers | # of cells examined | # of cells with + co-local | % positivea |
|---|---|---|---|
| TF1 cells | |||
| Tfr/Cy3-IL5 | 88 | 31 | 35 |
| Tfr/IL-5Rα | 24 | 16 | 67 |
| Tfr/βc | 27 | 21 | 78 |
| CTB/Cy3-IL5 | 268 | 121 | 45 |
| CTB/IL-5Rα | 41 | 7 | 17 |
| CTB/βc | 34 | 7 | 20 |
| LAMP/Cy3-IL5 | 157 | 28 | 18 |
| Eosinophils | |||
| Tfr/Cy3-IL5 | 61 | 29 | 47 |
| CTB/Cy3-IL5 | 53 | 16 | 30 |
| LAMP/Cy3-IL5 | 60 | 6 | 10 |
Percentage of cells with positive co-localization was calculated by counting number of cells with yellow vesicles (more than 2 vesicles per cell) over number of cells showing intracellular staining of Cy3-IL-5, βc, or IL-5Rα X 100. Cy3-IL-5 was used at 250 ng/ml.
Sucrose gradient fractionation
Cytokine-starved TF1 cells (2-3 1 × 107) were stimulated with 10 ng/ml IL-5 for the indicated time points, and lysed for 10 min on ice with 0.5 ml 0.1% Triton X-100 lysis buffer, as described previously [30]. Lysates were brought to 40% sucrose by adding 0.5 ml of 80% sucrose solution. One milliliter of this 40% lysate was then added to the bottom of a 13 × 51 mm Beckman polyallomer centrifuge tube, followed by successive layering with 2 ml of 30% sucrose and 1 ml of 5% sucrose on top of the lysate [30]. Samples were spun in an ultracentrifuge at 50,000 rpm for 18 h at 4°C. After fractionation, five, 800-μl fractions were collected, and 20 μl of each fraction were run on an LDS gel. The remaining fraction was diluted 1× with lysis buffer and immunoprecipitated with either 2 μg α-βc mAb (S-16) or negative control mAb anti-GAPDH (Sigma).
Inhibition of clathrin-mediated endocytosis
Cytokine-starved TF1 cells (1×106) were treated with either control RPMI media, or RPMI supplemented with 0.45 M sucrose for 30 min before IL-5 stimulation (10 ng/ml) [31,32,33]. Receptor internalization was measured by flow cytometry analysis as described below.
Inhibition of lipid raft-mediated endocytosis
Cytokine-starved TF1 cells (1×106) were pretreated with filipin (2.5 μg/ml) or nystatin (25 μg/ml) for 30 min before IL-5 stimulation (10 ng/ml) [34,35,36]. Receptor internalization was measured by flow cytometry analysis as described below.
Flow cytometry
βc cell surface expression was measured by incubating 1 × 106 unstimulated or IL-5 stimulated (30 min) TF1 cells in PBS + 2% FBS with PE-labeled anti-βc (BD Biosciences) for 30 min on ice according to standard protocols. The labeled proteins were analyzed immediately on a Beckman-Coulter XL flow cytometer. The flow data were analyzed using WinMDI software and graphed using Excel software. Mean fluorescence intensities (MFIs) and se are described in the text.
Immunoprecipitation and immunoblot assays
All immunoprecipitation and immunoblot (IP/IB) assays were done as described previously [29] with the following antibodies: βc was IP with anti-βc S-16 mAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA); IBs were done with anti-βc polyclonal [H-300 (C-terminal), Santa Cruz Biotechnology; anti-βc polyclonal antibodies (N-terminal for βIP detection), R&D Systems], anti-ubiquitin mAb (P4D1) and anti-Lyn (all from Santa Cruz Biotechnology), anti-clathrin heavy chain and anti-GAPDH mAb (Sigma), anti-caveolin and anti-EEA-1 (BD Biosciences), and anti-IL-5Rα (R&D Systems). Anti-pSTAT5 and anti-phosphotyrosine (mAb clone 4G10) were purchased from Upstate Biotechnology (Lake Placid, NY, USA). Anti-JAK2 was purchased from Cell Signaling. Proteins were visualized by incubation with enhanced chemiluminescence Plus reagents (GE Healthcare), and images were captured with a FluorChem 8000 imaging system (Alpha Innotech, San Leandro, CA, USA).
RESULTS
IL-5 receptors colocalize with transferrin-positive compartments
Preliminary data in our laboratory using endocytosis inhibitors of both the clathrin-dependent and lipid raft-dependent endocytic pathways suggested that IL-5Rs resided in and internalized into both endocytic pathways (Fig. 7A, 9A, and data not shown). We sought to confirm these initial observations, by performing colocalization studies of βc and 5Rα with well-established biological markers for clathrin- and lipid raft-positive vesicles using deconvolution microscopy [27, 37,38,39,40,41]. To remove false colocalization, all of our images were analyzed with wide-field-multiunmixing software (Zeiss) to eliminate any ‘emission crosstalk’ between the two fluorescent channels being used. Furthermore, the subcloned TF1-F11 cell line was used as our model system for two main reasons: 1) they endogenously express the IL-5R (as well as IL-3 and GM-CSF receptors), and 2) they permit the study of IL-5R endocytosis in a hematopoietic cell environment.
Fig. 5.
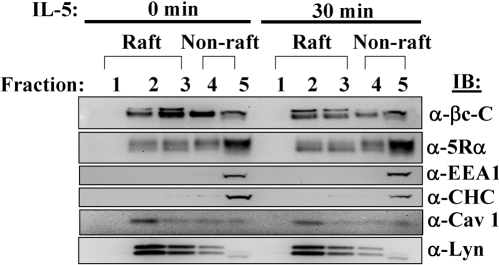
βc and IL-5Rα partition to both the lipid raft and soluble, nonraft fractions. Cytokine-starved TF1 cells (2 × 107) were either left unstimulated or stimulated with 10 ng/ml IL-5 for 30 min. Whole cell lysates (WCLs) were prepared with cold lysis buffer containing 0.1% Triton X-100 detergent on ice. Lysates were then processed for sucrose gradient fractionation as described in Materials and Methods. Twenty microliters of each fraction were loaded on an LDS-PAGE gel and analyzed by IB using antibodies listed on the right side of the panels. The lipid raft fractions are #1-3 and the soluble, nonraft fractions are #4-5.
Fig. 6.
Signaling competent IL-5Rs partition mainly to the clathrin-containing fractions. Cytokine-starved TF1 cells (2×107) were either left unstimulated (0 min) or stimulated with 10 ng/ml IL-5 for 30 min. WCLs were prepared with cold lysis buffer containing 0.1% Triton X-100 detergent on ice and processed for sucrose gradient fractionation as described in Materials and Methods. Each fraction (800 μl) was IP with either 2 μg anti-βc mAb (S-16), or anti-GAPDH control mAb (first 5 lanes), loaded on an LDS-PAGE gel and analyzed by IB using antibodies listed on the right side of the panels. The lipid raft fractions are #1-3 and the soluble, nonraft fractions are #4-5. Note how the majority of tyrosine phosphorylated, ubiquitinated, and proteasome targeted (βIP) IL-5Rs localize to fraction #5.
Fig. 7.
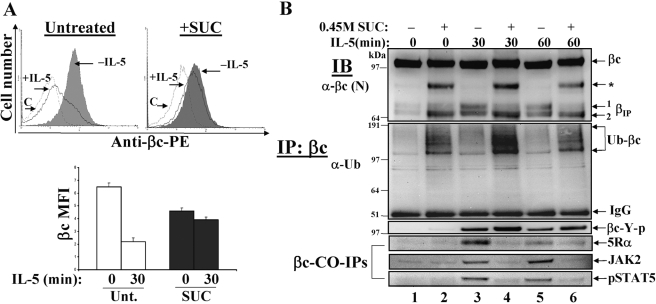
Reduction of clathrin-dependent endocytosis alters IL-5-mediated βIP generation and signaling. (A) Cytokine-starved TF1 cells (1×106 cells/tube) were either left untreated (upper left panel) or treated with 0.45 M sucrose (upper right panel) for 30 min to disrupt clathrin lattices. βc cell surface expression was measured by flow cytometry before (–IL-5, shaded histograms) and after 30’ IL-5 stimulation (+IL-5, solid black line). The hatched line represents cells labeled with an isotype-matched control antibody (C). Mean fluorescence intensities (MFI) are plotted and sem are listed in the text. βc: Untreated n = 3; n = 3 for SUC. (B) WCL were prepared from cells treated as described in 7A, IP with anti-βc mAb (S-16), and serially IB with anti-βc, anti-Ub, anti-phosphotyrosine (4G10), anti-IL-5Rα, anti-JAK2, and anti-pSTAT5. Note how the proteolytic processing of βIP is altered, and how ubiquitinated βc receptors accumulate in the presence of sucrose-treated cells (even-numbered lanes). Also, note that CO-IPs of 5Rα, JAK2, and pSTAT5 are blocked in the sucrose-treated lanes.
Fig. 9.
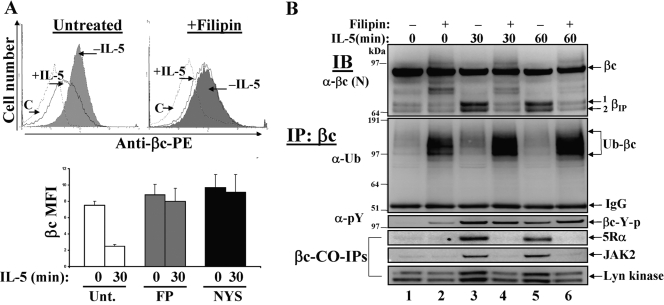
Inhibition of lipid raft-dependent endocytosis blocks βIP generation and IL-5-mediated signaling. (A) Cytokine-starved TF1 cells (1×106 cells/tube) were either left untreated (upper left panel) or treated with 2.5 μg/ml filipin (upper right panel) or 25 μg/ml NYS (lower panel) for 30 min to disrupt lipid raft microdomains. βc cell surface expression was measured by flow cytometry before (–IL-5, shaded histograms) and after 30′ IL-5 stimulation (+IL-5, solid black line). The hatched line represents cells labeled with an isotype-matched control antibody [C]. Mean fluorescence intensities (MFI) are plotted and sem are listed in the text. Untreated n = 5; FP n = 4. (B) TF1 cells were pretreated the same as in Fig. 9A, followed by an IL-5 (10 ng/ml) time course assay for 0, 30, and 60 min. WCLs were prepared, IP with anti-βc mAb (S-16), and serially IBed with the listed antibodies in the figure. Note how both βIP bands (βIP 1 and 2) are greatly reduced, with the simultaneous accumulation of ubiquitinated βc receptors in the presence of filipin (even-numbered lanes). Also, note how CO-IPs of 5Rα and JAK2 are blocked in the filipin-treated lanes (even-numbered lanes).
TF1 cells were allowed to internalize Alexa 488-coupled transferrin (Tfr-488, green) and subsequently immunolabeled with anti-βc (Fig. 1A, top left panel, red) or anti-5Rα antibodies (middle left panel, red). After 60 min of IL-5 stimulation at 37°C, the majority of βc and 5Rα receptor staining was localized predominantly in two distinct regions of the cell in large scattered vesicles. Moreover, transferrin labeling (green) of recycling endosomes had a similar distribution pattern as IL-5R staining, and thus merging of the respective red and green images revealed substantial overlap (yellow color) between the two molecules in these subcellular structures (Fig. 1A, merged panels, upper and middle; raw cell numbers in Table 1). Therefore, these data indicate that both IL-5R subunits traffic through transferrin-positive recycling endosomes and further support our previous study which demonstrated that ∼30% of basal βc receptors recycle back to the cell surface in TF1 cells [42].
To determine whether the ligated, thus signaling-competent, IL-5Rs trafficked through transferrin-positive vesicles, we fluorescently labeled IL-5 with Cy3 and performed dual uptake assays with Alexa 488-coupled transferrin and Cy3-IL-5. Ligated IL-5 receptors internalized efficiently into large vesicles at one pole of the cell (red), which overlapped with internalized transferrin (green), thus demonstrating colocalization between the two molecules (Fig. 1A, bottom three panels).
We next sought to confirm the presence of IL-5Rs in transferrin-positive vesicles in freshly isolated human eosinophils. In an effort to maintain the eosinophils in a quiescent, synchronous state, we cultured them overnight in RPMI with 10% serum (no IL-5) immediately after isolation. The trafficking of activated IL-5Rs was examined as described for TF1 cells using Cy3-IL-5 (red) and Tfr-488 (green) (Fig. 1B). After 15 min of internalization, Cy3-IL-5 was detected abundantly in large scattered vesicles throughout the eosinophil cytoplasm. Conversely, transferrin-positive vesicles were detected mainly along the plasma membrane. By 60 min, Cy3-IL-5-positive vesicles became punctate and fewer in number. However, some of these vesicles overlapped with the transferrin-positive vesicles, thus demonstrating their colocalization (Fig. 1B, yellow vesicles). The reduced level of Cy3-IL-5 at 60 min possibly reflects its degradation, as has been shown biochemically for the IL-5Rs [29, 42].
In summary, our data demonstrate that activated IL-5Rs are regulated by a clathrin-dependent endocytic pathway in TF1 cells and human eosinophils.
IL-5 receptors colocalize with lipid raft endocytic markers
To confirm the presence of IL-5Rs in lipid raft-positive compartments, TF1 cells were incubated with a commonly used biological marker for labeling lipid rafts, Alexa 488-coupled cholera toxin-B subunit (CTB) [39,40,41]. CTB binds to GM-1 gangliosides, which are enriched in lipid raft microdomains, and thus enters most cells through lipid raft-mediated endocytosis. Figure 2A demonstrates the presence of internalized CTB in large, aggregated subcellular compartments throughout the cells (middle panels, green). However, the merging of immunostained (anti-βc and anti-5Rα) and Cy3-labeled IL-5 images (red) with those of CTB (green) revealed significant colocalization between the two molecules (Fig. 2A, right panels, yellow color; raw cell numbers in Table 1).
Next, we set out to investigate whether IL-5Rs could be detected in CTB-positive structures in human eosinophils by using the same dual uptake assay (Fig. 2B). After 15 min of internalization, Cy3-IL-5-positive vesicles (red) appeared in large granular vesicles densely scattered throughout the cytoplasm (Fig. 2B). Similarly, CTB staining (green) was detected in large scattered vesicles, some of which colocalized with Cy3-IL-5 (Fig. 2B, yellow). Furthermore, colocalization between the two markers was still detected even after 60 min of internalization, (Fig. 2B, bottom right panel).
As an internal control, we wanted to confirm that CTB- and transferrin-labeled markers compartmentalize separately in TF1 cells. We, thus, performed a similar colocalization experiment with these molecules (Fig. 3A). Chilled cells were bound with both CTB-488 (green) and anti-transferrin receptor antibodies simultaneously. After internalization of both markers at 37°C, residual cell surface label was removed by acid washing. Detection of internalized anti-transferrin receptor antibodies was performed by labeling with a red secondary antibody (Alexa Fluor 594). As seen in Fig. 3A, CTB-positive vesicles (green) did not colocalize with transferrin receptor-positive vesicles (red), which thus indicates that these markers compartmentalize separately after 60 min of internalization. This result supports our hypothesis that IL-5Rs reside in and traffic through separate endocytic compartments.
Lastly, to rule out the possibility that IL-5 binding to cell surface IL-5Rs interfered with the antibody staining (Figs. 1A and 2A) (which could lead to detection of only unoccupied receptors), we performed a simple flow cytometry assay (Fig. 3B). The logic behind this assay was that if IL-5 binding to IL-5Rs blocked the epitopes recognized by our antibodies; then the mean fluorescence intensity (MFI) of IL-5-bound cells would decrease in the presence of IL-5. Chilled TF1 cells were preincubated with (+IL-5) or without IL-5 (–IL-5) for 30 min and then labeled for βc and IL-5Rα cell surface receptors with the same antibodies used in Figs. 1A and 2A (cells were not transferred to 37°C for endocytosis). Flow cytometry analysis revealed that IL-5 binding to cell surface receptors prior to antibody labeling did not interfere with epitope recognition by these antibodies, as evidenced by overlap of histograms between –IL-5 cells (solid line) and +IL-5 cells (hatched line).
Collectively, these data clearly demonstrate the presence of IL-5Rs in lipid raft-positive vesicles and show for the first time that IL-5Rs traffic through separate endocytic pathways in TF1 cells and human eosinophils.
Detection of IL-5 receptors in the lysosomes
To confirm earlier biochemical analyses, which demonstrated that activated IL-5Rs were degraded in the lysosomes [29], we performed a colocalization assay in TF1 cells and eosinophils with Cy3-IL-5 and antibodies against the lysosomal marker, LAMP-1 (Fig. 4). In unstimulated TF1 cells (0′), Cy3-IL-5 (red) staining localized to a “capped” region of the plasma membrane, whereas LAMP-1-staining (green) was detected in scattered intracellular vesicles. After 60 min of internalization, Cy3-IL-5 was detected intracellularly in punctate vesicles (red), some of which overlapped with lysosomes (yellow color). The colocalization between these two molecules was also detected in eosinophils, albeit not as frequently as in TF1 cells (10% for EOS vs. 18% for TF1 cells, see Table 1). These data confirm our previous biochemical observations and thus show that the final destination for some of the internalized IL-5Rs is the lysosome.
βc and IL-5Rα partition to both lipid raft and nonraft fractions
An additional method was used to further substantiate that IL-5Rs localized to both of these plasma membrane microenvironments. To this end, we analyzed the subcellular localization of IL-5R subunits by sucrose density gradient fractionation as described by Young et al. [30] (Fig. 5). Unstimulated and IL-5 stimulated (10 ng/ml) TF1 cells were lysed in lysis buffer containing Triton X-100 at 4°C and centrifuged at high speed to separate the low buoyancy, Triton X-100 insoluble material (lipid rafts, top fractions #1-3) from the detergent soluble material (nonraft, bottom fractions #4-5). Fractions (FRs) of 0.8 ml were collected from the top of the centrifuge tube and analyzed for the partitioning of βc by IB with anti-βc C-terminal antibodies (H300). As expected, full-length βc receptors partitioned to both the lipid raft (FRs #2,3) and the soluble, nonraft FRs (FRs #4,5) at both time points examined (Fig. 5, upper panel). Moreover, consistent with previous observations in our laboratory, βc protein levels decreased in FRs #2-4 following 30 min of IL-5 stimulation (Fig. 5, upper panel) [29, 42]. Interestingly, we detected a βc doublet in the lipid raft fractions, which was not present in the nonraft fractions. Whether these βc doublets represent lipid-modified versions of the receptor remains to be determined.
Similarly, IB with anti-5Rα antibodies revealed that, like βc, IL-5Rα localized to both the raft and soluble nonraft fractions, with the majority of the protein located in soluble FR #5 (Fig. 5, second panel). To control for the quality of the sucrose fractionation, IB analysis with antibodies against two nonraft markers, early endosomes antigen 1 (EEA1), and clathrin heavy chain (CHC) was performed [12,13,14,15,16,17,18,19]. As expected, both markers localized to the nonraft fractions (Fig. 5, third and fourth panels). Location of lipid rafts was confirmed by IB analyses with antibodies against lipid raft markers such as the Src-kinase, Lyn, and caveolin (Fig. 5, two lower panels) [43]. As expected, a prominent band corresponding to Cav-1 was localized to FR#2, while fainter bands could still be detected in FRs#3-5. Moreover, while Lyn kinase was detected mainly in FRs #2-3, a small amount of protein partitioned to fractions #4-5. Similarly, other studies have shown that small amounts of Lyn kinase partition to the nonraft fractions [43]. Lastly, the presence of Lyn kinase in the lipid raft fractions confirms a previous report, which used freshly isolated eosinophils for their studies [44].
Taken together, these data demonstrate that IL-5Rs can be detected biochemically in both lipid raft and nonraft plasma membrane microenvironments and thus, confirm our microscopy results.
Signaling-competent and proteasome-targeted IL-5Rs localize mainly to the soluble, nonraft fractions
To investigate whether signaling IL-5Rs internalize into the clathrin- or lipid-raft-mediated endocytic pathways, we subjected the sucrose fractions from Fig. 5 to IP with anti-βc or negative control (anti-GAPDH) mAbs, followed by IB with various antibodies (Fig. 6). We first examined the partitioning of proteasome-generated βIP by IB with anti-βc antibodies against its N terminus [29]. This approach revealed the presence of βIP in the soluble nonraft FRs #4-5, and not in the lipid raft FRs (Fig. 6, first panel, anti-βc-N). IB with antiphosphotyrosine (mAb clone 4G10) antibodies revealed high levels of tyrosine phosphorylated βc receptors in soluble, nonraft FR#5, although weak tyrosine phosphorylation was seen in the lipid raft FRs #2-3 (Fig. 6, second panel, 30’ IL-5). Moreover, IB with anti-5Rα antibodies revealed its association with βc immune complexes mainly in IL-stimulated FR#5, the same location where tyrosine phosphorylation of βc was abundant (Fig. 6, third panel). However, weak interactions between βc and 5Rα were also detected in FRs #2-4 (Fig. 6, third panel).
Similarly, IB analysis of unstimulated cells with anti-ubiquitin antibodies revealed an abundance of basal βc ubiquitination in nonraft FR #5, which was still present after 30 min of IL-5 stimulation in the same fraction (Fig. 6, fourth panel), although low levels of βc ubiquitination were also detected in the lipid raft fractions under both conditions (Fig. 6, fourth panel). Lastly, to determine the subcellular localization of βc/JAK2 interactions, the membrane was blotted with anti-JAK2 antibodies, which revealed a pre-existing interaction between these two molecules in nonraft fractions #4-5, but not in the lipid rafts, prior to and after IL-5 stimulation. These results confirm previous observations showing a pre-existing association between βc and JAK2 [45, 46].
Taken together, these data demonstrate that although βc and 5Rα partition to both the nonraft and lipid raft fractions, the activated, ubiquitinated, and proteasome-degraded IL-5Rs localized to the soluble, clathrin-containing fractions. This suggests that signaling-competent IL-5Rs internalize and traffic mainly through the clathrin-dependent endocytic pathway and is consistent with our previous data showing that βIP/5Rα are degraded in the lysosomes [29]. Furthermore, a molecular link between proteasomes (α-subunit XAPC7) and the late endocytic sorting machinery was recently reported and thus provides a mechanistic explanation supporting our observation that proteasome-generated βIP traffics through the CME pathway [47].
Lastly, our biochemical findings demonstrate the association of JAK2 with βc in the soluble, but not the lipid raft compartment. This result suggests that JAK-STAT-mediated signals activated by IL-5 traffic through the clathrin-dependent pathway.
Reduced clathrin-mediated endocytosis alters βIP generation and blocks intracellular signaling
To examine the biological consequences of blocking entry of activated IL-5Rs into the clathrin-dependent endocytic pathway, IL-5-induced signal transduction events were analyzed in TF1 cells in the presence or absence of hypertonic medium (0.45 M sucrose) (Fig. 7). Treatment of cells with 0.45 M sucrose interferes with clathrin lattice assembly by depleting the cytosol of free clathrin and thus, is a commonly used technique for inhibiting clathrin-mediated endocytosis [31,32,33]. We first examined whether βc cell surface removal after IL-5 stimulation was inhibited in the presence of 0.45 M sucrose (Fig. 7A).
To this end, TF1 cells were pretreated with or without sucrose for 30 min, followed by flow cytometry analysis of βc cell surface expression before and after IL-5 stimulation (Fig. 7A, upper panels). As seen before in untreated TF1 cells [42], IL-5 stimulation reduced the cell surface expression of βc by 66% (Fig. 7A, upper left panel; quantified in lower panel, mean MFI±sem: Untreated, 0 min = 6.5±0.3 vs. 30 min =2.2±0.2, n=3). By contrast, treatment with 0.45 M sucrose resulted in a 30% decrease of basal βc cell surface expression in unstimulated cells, which was reduced by only 15% after IL-5 stimulation (Fig. 7A, upper right panel; quantified in lower panel +SUC 0 min = 4.6±0.2 vs. 30 min = 3.9±0.2). Together, these results indicate that treating cells with hyperosmolar sucrose reduces βc internalization by 21% (66-45%). Reduction in basal βc receptors in the presence of sucrose suggests that the presence of clathrin is required for stability of βc cell surface expression.
We next performed a time course assay of sucrose-treated TF1 cells and analyzed whole cell lysates (WCL) by IP with anti-βc antibodies and serial IBs with various antibodies (Fig. 7B). In untreated cells, IB with anti-βc antibodies demonstrated that IL-5 stimulation resulted in ‘classical βIP’ generation [29, 42], wherein cytoplasmically truncated βc products migrate as two hazy bands between 65 and 70 kDa (Fig. 7B, upper panel, lanes 3 and 5, two bottom arrows denoting βIP 1 and βIP 2). By contrast, treatment with 0.45 M sucrose of unstimulated and IL-5-stimulated cells (Fig. 7B, even-numbered lanes) resulted in the appearance of the bottom βIP band at its expected size (βIP 2), but absence of the upper βIP band (βIP 1). Consequently, a previously undetected truncated βc band was observed at a much higher molecular weight in the same lanes (Fig. 7B, upper panel, arrow with asterisk), leading us to suspect that the proteolytic processing of the upper βIP band (βIP 1) was blocked under these conditions.
For the majority of proteins degraded by the proteasome, the covalent attachment of at least one ubiquitin chain precedes their degradation [48,49,50,51]. Since IL-5 stimulation leads to the direct ubiquitination of βc [42], we wanted to examine how βc ubiquitination was affected when clathrin-dependent trafficking was blocked. IB with anti-Ub antibodies revealed a marked accumulation of ubiquitinated βc smears in the 0.45 M sucrose-treated lanes (Fig. 7B, second panel, even-numbered lanes), as compared with untreated cells (Fig. 7B, second panel, odd-numbered lanes).
Similarly, blotting the membrane with anti-phosphotyrosine mAb (clone 4G10) revealed that, compared with untreated cells, βc tyrosine phosphorylation was equivalent (if not stronger) in the 0.45 M sucrose treated lanes (Fig. 7B, third panel). Moreover, CO-IP assays with anti-5Rα, anti-JAK2, and anti-pSTAT5 revealed significant impairment of βc protein/protein interactions with these signaling molecules in the presence of sucrose (Fig. 7B, bottom 3 panels, compare lanes 3-6). Lastly, to confirm that treatment of TF1 cells with 0.45 M sucrose specifically blocked the clathrin-dependent pathway, and not the lipid raft pathway, a dual uptake assay was performed with TFR-488 and CTB-488 in the presence of this inhibitor (Fig. 8). As compared with untreated cells (Fig. 8, upper panels), sucrose-treated cells showed impaired internalization of TFR, whereas CTB internalization was not significantly affected (Fig. 8, middle panels).
Fig. 8.
Specificity of endocytosis inhibitors. Cytokine-starved TF1 cells (100,000 cells per tube) were serum-starved for 60 min and either left untreated (upper panels), treated with 0.45 M sucrose (middle panels), or 50 μg/ml NYS (lower panels) for 30 min to block each endocytic pathway. Tfr-488 and CTB-488 (both at 10 μg/ml) uptake was performed as described in the Materials and Methods. After acid washing the cells, we visualized the internalized transferrin and CTB by deconvolution microscopy.
In aggregate, these data strongly demonstrate that partial proteolytic processing of βc occurs in the clathrin-dependent pathway, and thus, further support our working model of IL-5R down-regulation, which shows that βIP generation occurs after receptor internalization [42]. Moreover, our data clearly demonstrate two significant findings about βc post-translational modifications: 1) full-length βc receptors are ubiquitinated and tyrosine phosphorylated at the plasma membrane; and 2) endocytic trafficking through the clathrin-dependent pathway regulates the deubiquitination and dephosphorylation of βc receptors (since both modifications persist in the presence of sucrose). Lastly, and importantly, our data also show that activated IL-5R internalization into the clathrin-dependent pathway is required for amplification of IL-5-induced signaling, since blocking entry into this endosomal pathway inhibited the CO-IP of key signaling molecules with the receptors. The same observation has been reported for the growth hormone and epidermal growth factor receptors [52, 53].
Inhibition of lipid raft-dependent endocytosis blocks βIP generation and IL-5-mediated signaling
Experimentally, lipid raft-dependent endocytic pathways can be inhibited by destabilizing membrane microdomains using cholesterol-sequestering/depleting agents such as filipin, nystatin, or methyl-β-cyclodextrin (MβC) [27, 34,35,36]. We confirmed inhibition of IL-5-induced βc internalization in the presence of filipin and nystatin by flow cytometry (Fig. 9A). Compared with untreated TF1 cells, which had a 67% reduction in βc MFI after 30 min of IL-5 stimulation (Fig. 9A, untreated, 0 min=7.5±0.5 vs. 30 min=2.5±0.2), removal of ligated βc from the cell surface was inhibited in the presence of both inhibitors (Fig. 9A, +FP 0 min=8.8±1.0 vs. FP 30 min=8.0±3.1; NYS 0 min=9.7±1.6 vs. 30 min=9.1±2.2). Moreover, compared with untreated cells, βc MFIs in filipin- and nystatin-treated cells increased slightly over baseline before IL-5 stimulation, indicating an accumulation of cell surface βc receptors when cholesterol was removed (Fig. 9A, compare 0 min time points). It is important to point out that accumulation of βc cell surface receptors in the presence of filipin and nystatin is completely opposite of what we observed with 0.45 M sucrose (Fig. 7A), demonstrating a degree of specificity between these endocytosis inhibitors at the cell surface.
To investigate the biochemical consequences of inhibiting lipid raft-dependent IL-5R endocytosis, the same IP/IB approach was used in the absence or presence of filipin in TF1 cells (Fig. 9B). As opposed to blocking the generation of only one βIP band, filipin-treated cells showed a block in both βIP bands. In addition, a marked accumulation of ubiquitinated βc receptors and normal levels of βc tyrosine phosphorylation was observed in filipin-treated cells, as compared with untreated cells (Fig. 9B, second and third panels). Furthermore, the association of IL-5Rα and JAK2 in βc immune complexes was completely inhibited in the presence of filipin, (Fig. 9B, fourth and fifth panels), whereas Lyn interactions were only slightly reduced (Fig. 9B, lower panel). The exact results were seen with nystatin-treated cells (data not shown).
Lastly, to confirm the specificity of cholesterol depletion on lipid raft-mediated endocytosis, the same dual uptake assay described above was performed with CTB and TFR in the presence of nystatin (Fig. 8, upper and lower panels). As expected, internalization of CTB was significantly blocked in the presence of nystatin, whereas TFR internalization was unaffected (Fig. 8, lower two panels).
Taken together, these results show that the proteolytic processing of both βIP bands (classical βIP) requires an intact lipid raft-dependent endocytic pathway. This observation suggests that the lipid raft endocytic pathway lies upstream of the clathrin-dependent pathway, as both βIP bands were blocked with filipin, but only the upper βIP band was affected by 0.45 M sucrose. If this is the case, then the possibility exists that IL-5Rs in lipid-raft-derived endosomes merge with the clathrin-dependent pathway, long after internalization, which supports previously reported observations (reviewed in 23).
Furthermore, these data confirm our results with 0.45 M sucrose-treated cells, which showed that βc ubiquitination and tyrosine phosphorylation occurred at the plasma membrane. And lastly, our data clearly show that activated IL-5R entry into the intracellular compartment is required for efficient IL-5-induced signal transduction, as βc CO-IPs with critical signaling molecules were blocked when either endocytic pathway was inhibited.
Overall, this study indicates that endocytosis of activated IL-5Rs serves a dual regulatory function in IL-5 signal transduction: 1) initially, it is required for signal amplification; and 2) it is required for signal termination (deubiquitination, dephosphorylation, and degradation of βc).
DISCUSSION
In general, ligand binding of membrane receptors promotes endocytosis of the ligand-receptor complex, leading first to signal propagation in endosomes, then to extinction of the activated signal [12,13,14,15,16,17,18,19]. We previously showed that JAK kinase activity was required for IL-5-induced IL-5R internalization in TF1 cells and freshly isolated human eosinophils [42]. Herein, we demonstrate by using three separate approaches that IL-5Rs reside in both clathrin and lipid raft membrane microdomains and are internalized by both endocytic pathways in both cell types. Moreover, we provide the first evidence that signaling-competent IL-5Rs partition to the nonraft fractions (clathrin-containing), where proteasome-generated βIP was mainly detected. Lastly, we show that endocytosis of activated IL-5Rs is required, first, for the efficient association of signaling molecules with membrane-trafficking receptors, and second, for down-regulating activated IL-5Rs in the lysosomes.
One key question raised by this study is whether lipid-raft-derived endosomes carrying activated IL-5Rs merge with endosomes in the clathrin-dependent pathway. In the sucrose fractionation studies (Fig. 6), βIP was detected only in the soluble, nonraft fractions (clathrin-containing) after IL-5 stimulation. However, our biochemical analyses with WCL treated with endocytosis inhibitors demonstrated that filipin-treated cells (lipid raft inhibitor) blocked the generation of both βIP bands, whereas sucrose-treated cells (CME inhibitor) blocked the generation of only the upper βIP band. The latter observation suggests that both pathways contribute to the proteolytic processing of βIP. A possible scenario that might explain these somewhat disparate results is that processing of the βc cytoplasmic domain begins in the lipid raft compartment where a small piece of the βc tail is removed. Once this happens, the partially truncated βc receptor translocates to the clathrin compartment for final proteasomal processing before its delivery to the lysosome. An alternative explanation for the results seen with filipin might be that cholesterol depletion affects both endocytic pathways, as we and others have observed with methyl-β-cyclodextrin (MβC), a cholesterol-sequestering drug [data not shown and 36]. However, this possibility seems unlikely as βc cell surface expression was altered very differently in clathrin-inhibited and lipid raft-inhibited endocytosis assayed by flow cytometry (Figs. 7A and 9A). Future colocalization studies with markers from each pathway should prove or disprove this proposed trafficking model.
CO-IP data in this study confirm previous observations that showed a pre-existing association of IL-5Rs with JAK2 in unstimulated cells [45, 46]. Our study, however, actually defined the plasma membrane compartments where these interactions take place. Together, these results suggest that, prior to ligand stimulation, IL-5Rs are spatially organized in two separate cell surface compartments and are equipped with their distinct signaling “arsenal” for rapid signal transduction. Recently, a seminal study described a critical role for membrane compartmentalization and endocytosis of the interferon receptors (IFN-α and IFN-γ) [54]. Namely, compartmentalization of IFN-Rs at the plasma membrane, through clathrin-dependent endocytosis and lipid-based microdomains, determined the biological responses induced by IFNs and contributed to the establishment of specificity within the JAK/STAT signaling pathway. Whether the sorting and partitioning of IL-5Rs into separate compartments is important for producing distinct biological responses in eosinophils will be an important area of investigation.
Furthermore, our observation demonstrating that reduction of IL-5R internalization blocks downstream signaling events in the presence of clathrin or lipid raft endocytosis inhibitors is also very exciting. For example, a recently published report demonstrated that the serum cholesterol-lowering drug, Simvastatin, effectively suppressed acute eosinophilic airway inflammation and Th2 cytokine secretion in a murine model of asthma [55]. The study further showed that the anti-inflammatory effect of this statin is at least in part mediated through a suppressive action on T lymphocytes; however, a clear mechanistic explanation of how this inhibition occurred was lacking. Perhaps our current results with the cholesterol-depleting drugs, filipin and nystatin, might shed some light on this mechanism. It is conceivable that Simvastatin elicits its inhibitory effects on T lymphocytes by blocking internalization of activated receptors, and thus inhibits the activation of signaling pathways, which up-regulate the expression of GATA3, a transcription factor critical for the up-regulation of IL-4 and IL-5 (TH2-skewing cytokines). Of course, this speculation remains to be tested in vivo but could potentially provide some insight into the anti-inflammatory effects of Simvastatin.
Understanding the basic molecular mechanisms governing the spatio-temporal regulation of IL-5R endocytosis and signal termination has importance for the development of novel approaches to modulate IL-5-mediated inflammation associated with asthma and hypereosinophilic syndromes. Furthermore, lessons learned from IL-5R down-regulation can be translated to the other βc-sharing receptors, GM-CSFR and IL-3R, and inflammatory disorders associated with these cytokines.
Acknowledgments
This work was supported by grants from the National Institutes of Health AI 063178 and the American Lung Association (to M. M.). We thank Dale S. Smith for excellent technical assistance with the LAMP-1/Cy3-IL-5 co-localization studies, Dr. Ryan Shanks for assistance with eosinophil isolation, as well as C. Jeanny Laurent, Wei Zhang, and Risheng Xu for technical assistance during various phases of the project and for helpful lab discussions.
References
- Arai K I, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Kitamura T, Harada N, Yokota T, Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Geijsen N, Koenderman L, Coffer P J. Specificity in cytokine signal transduction: lessons learned from the IL-3/IL-5 GM-CSF receptor family. Cytokine Growth Factor Rev. 2001;12:19–25. doi: 10.1016/s1359-6101(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston D P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–665. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kawada N, Yamada T, Kawada K, Takatsu K, Nagai H. Allergen-induced airway inflammation and bronchial responsiveness in interleukin-5 receptor a chain-deficient mice. Clin Exp Allergy. 2000;30:874–881. doi: 10.1046/j.1365-2222.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A, Robinson D S. Eosinophils, eosinophilic cytokines (interleukin-5), and antieosinophilic therapy in asthma. Curr Opin Pulm Med. 2002;8:33–38. doi: 10.1097/00063198-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Zabeau L, Gevaert P, Bachert C, Tavernier J. Interleukin-5, eosinophilic diseases and therapeutic intervention. Curr Drug Targets Inflamm Allergy. 2003;2:319–328. doi: 10.2174/1568010033484043. [DOI] [PubMed] [Google Scholar]
- Lee J J, Dimina D, Macias M P, Ochkur S I, McGarry M P, O'Neill K R, Protheroe C, Pero R, Nguyen T, Cormier S A. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- Humbles A A, Lloyd C M, McMillan S J, Friend D S, Xanthou G, McKenna E E, Ghiran S, Gerard N P, Yu C, Orkin S H. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Rothenberg M E, Hogan S P. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Bonifacino J S, Weissman A M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon H T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Ceresa B P, Schmid S L. Regulation of signal transduction by endocytosis. Curr Opin Cell Biol. 2000;12:204–210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Di Fiore P P, De Camilli P. Endocytosis and signaling. An inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Vons Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Conner S D, Schmid S L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4:213–224. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- Johannes L, Lamaze C. Clathrin-dependent or not: is it still the question? Traffic. 2002;3:443–451. doi: 10.1034/j.1600-0854.2002.30701.x. [DOI] [PubMed] [Google Scholar]
- Parton R G, Richards A A. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nabi I R, Le P U. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano R E. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo C G, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk M J, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- Subramaniam P S, Johnson H M. Lipid microdomains are required sites for the selective endocytosis and nuclear translocation of IFN-gamma, its receptor chain IFN-gamma receptor-1, and the phosphorylation and nuclear translocation of STAT1alpha. J Immunol. 2002;169:1959–1969. doi: 10.4049/jimmunol.169.4.1959. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G M, Le Roy C, Goodfellow A F, Wrana J L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Mita S, Takaki S, Tominaga A, Takatsu K. Comparative analysis of the kinetics of binding and internalization of IL-5 in murine IL-5 receptors of high and low affinity. J Immunol. 1993;151:6924–6932. [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston D P. Proteasome regulation of βc signaling reveals a novel mechanism for heterotypic desensitization. J Clin Invest. 2001;108:1797–1806. doi: 10.1172/JCI13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R M, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- Govers R, van Kerkhof P, Schwartz A L, Strous G L. Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. EMBO J. 1997;16:4851–4858. doi: 10.1093/emboj/16.16.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S H, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhof P, Sachse M, Klumperman J, Strous G J. Growth hormone receptor ubiquitination coincides with recruitment to clathrin-coated membrane domains. J Biol Chem. 2001;276:3778–3784. doi: 10.1074/jbc.M007326200. [DOI] [PubMed] [Google Scholar]
- Stang E, Johannessen L E, Knardal S L, Madshus I H. Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J Biol Chem. 2000;275:13940–13947. doi: 10.1074/jbc.275.18.13940. [DOI] [PubMed] [Google Scholar]
- Orlandi P A, Fishman P H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S K, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003;22:537–547. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff D R, Daro E A, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy A K, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B J. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- Nichols B J. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol. 2003;13:686–690. doi: 10.1016/s0960-9822(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston D H, Lei J T. JAK kinases control IL-5 receptor ubiquitination, degradation, and internalization. J Leukoc Biol. 2007;81:1137–1148. doi: 10.1189/jlb.0706465. [DOI] [PubMed] [Google Scholar]
- Stenberg P E, Pestina T I, Barrie R J, Jackson C W. The Src family kinases, Fgr, Fyn, Lck, and Lyn, colocalize with coated membranes in platelets. Blood. 1997;89:2384–2393. [PubMed] [Google Scholar]
- Bates M E, Busse W W, Bertics P J. Interleukin 5 signals through Shc and Grb2 in human eosinophils. Am J Respir Cell Mol Biol. 1998;18:75–83. doi: 10.1165/ajrcmb.18.1.2766. [DOI] [PubMed] [Google Scholar]
- Ogata N, Kouro T, Yamada A, Koike M, Hanai N, Ishikawa T, Takatsu K. JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and betac subunit, respectively, and are activated upon IL-5 stimulation. Blood. 1998;91:2264–2271. [PubMed] [Google Scholar]
- Gorska M M, Cen O, Liang Q, Stafford S, Alam R. Differential regulation of interleukin 5-stimulated signaling pathways by dynamin. J Biol Chem. 2006;281:14429–14439. doi: 10.1074/jbc.M512718200. [DOI] [PubMed] [Google Scholar]
- Dong J, Chen W, Welford A, Wadinger-Ness A. The proteasome alpha-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J Biol Chem. 2004;279:21334–21342. doi: 10.1074/jbc.M401022200. [DOI] [PubMed] [Google Scholar]
- Hicke L. Getting down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson K D. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol. 2000;11:141–148. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- Pickart C M. Ubiquitin in chains. Trends Biochem Sci. 2000;25:544–548. doi: 10.1016/s0968-0004(00)01681-9. [DOI] [PubMed] [Google Scholar]
- Glickman M H, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Alves dos Santos C M, van Kerkhopf P, Strous G J. The signal transduction of the growth hormone receptor is regulated by the ubiquitin/proteasome system and continues after endocytosis. J Biol Chem. 2001;276:10839–10846. doi: 10.1074/jbc.M003635200. [DOI] [PubMed] [Google Scholar]
- Burke P, Schooler K, Wiley H S. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M, Monier M-N, Fradagrada A, Mitchell K, Baychelier F, Eid P, Johannes L, Lamaze C. Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol Biol Cell. 2006;17:2896–2909. doi: 10.1091/mbc.E06-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay A, Leung B P, McInnes I B, Thomson N C, Liew F Y. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]