Abstract
CD4+CD25+forkhead box p3 (Foxp3)+ regulatory T cells (Treg) control peripheral tolerance. Although Treg are anergic when stimulated through the TCR, mature bone marrow-derived, but not splenic, dendritic cells (DC) can induce their proliferation after TCR stimulation in the absence of IL-2. One possibility is that the DC produce proinflammatory cytokines such as IL-1 or IL-6 that function as growth factors for Treg. We have analyzed the costimulatory effects of IL-1 on the expansion of Foxp3+ Treg in vitro. When CD4+CD25+ T cells were cultured in the presence of splenic DC and IL-1, marked expansion of the Foxp3+ T cells was observed. The effects of IL-1 were mediated on CD4+CD25+Foxp3– T cells present in the starting population rather than on the DC or on the CD4+CD25+Foxp3+ T cells. In contrast, stimulation of CD4+CD25+ T cells with plate-bound anti-CD3 and IL-1 in the absence of DC resulted in the outgrowth of a CD4+CD25+Foxp3– T cell population composed of NKT cells and non-NKT, IL-17-producing cells. Foxp3+ Treg purified from mice expressing the reporter gene enhanced GFP in the Foxp3 locus failed to proliferate when costimulated with IL-1. These findings have important implications for the design of protocols for the expansion of CD4+CD25+ T cells for cellular biotherapy.
Keywords: rodent, dendritic cells, costimulation, cell proliferation
INTRODUCTION
CD4+CD25+forkhead box p3 (Foxp3)+ regulatory T cells (Treg) play an important role in modulation of immune responses. Treg were first characterized in mice, where they are generated in the thymus and constitute 5–10% of the CD4+ T cell pool in the periphery. In humans and mice, they have been shown to suppress CD4 and CD8 T cell responses in a cell contact-dependent, cytokine-independent manner in vitro and to require activation through the TCR to be functional [1, 2]. Accumulating data have shown that Foxp3+ Treg play a role in the control of autoimmune disorders, infections, tumors, allergy, and transplantation tolerance [3].
Adoptive cellular immunotherapy with CD4+CD25+Foxp3+ T cells has been proposed as a potential therapy for chronic autoimmune diseases and for the induction of tolerance to allografts (graft versus host disease, organ transplantation) [4]. A number of major problems exist with implementation of this approach. First, because of the lack of a cell surface antigen that is specific for Foxp3+ Treg, their purification requires the use of surrogate markers, such as CD25, which are also expressed on activated, conventional T cells. In general, even highly purified CD4+CD25+ T cells routinely contain 5–10% or more Foxp3– T cells. In addition to the lack of a specific marker, the in vitro expansion of Treg to numbers sufficient for use in cell-transfer studies is hampered by their “anergic” phenotype and hyporesponsiveness, even with strong TCR-based stimulation. Nevertheless, stimulation of mouse CD4+CD25+ T cells with immobilized anti-CD3 (with or without anti-CD28) in the presence of high concentrations of exogenous IL-2 or IL-4 and to a lesser extent, IL-7, leads to their significant expansion over 4–10 days of culture [5]. However, many of these early studies did not evaluate Foxp3 expression by the expanded cells, and significant outgrowth of CD25+Foxp3– T cells was likely. The expansion of human CD4+CD25+ T cells under similar conditions is complicated further by the observation that under certain stimulatory conditions, activated human CD4+Foxp3– T cells may be induced to express Foxp3 in the absence of Treg functions [6].
There is considerable controversy as to the potential role of accessory cells and their associated costimulatory signals in the expansion of Foxp3+ Treg. We [7] and others [8] have previously shown that mature, fully activated bone marrow-derived dendritic cells (BMDC), but not splenic DC, could induce the proliferation of mouse CD4+CD25+ Treg in the absence of exogenous IL-2; similar results were also reported in humans [9]. Although the mechanism by which activated BMDC stimulate the proliferation of Treg has not been defined, one likely possibility is that they produce significant amounts of proinflammatory cytokines such as IL-1 or IL-6, which function as growth factors for Treg expansion. Indeed, Kubo et al. [10] have recently shown that the addition of anti-IL-1 and anti-IL-6 antibodies would abrogate the expansion of CD4+CD25+ Treg cultured with mature BMDC.
Cytokines of the IL-1 family have been shown to display pleiotropic effects and to be involved in host defense and the neuroimmune-endocrine network regulation. Polymorphism or absence of the IL-1R antagonist gene belonging to the IL-1 family cluster has thus been associated with the severity/susceptibility of inflammatory diseases such as ankylosing spondylitis or chronic inflammatory polyarthropathy [11, 12]. Similarly, IL-1β and -α, the most characterized members of this family, were shown with TNF-α to play a role in the pathogenesis and development of rheumatoid arthritis [13, 14]. The direct effects of the IL-1 family of cytokines on the survival, expansion, or function of Foxp3+ Treg have not yet been investigated. Here, we have analyzed in-depth the costimulatory effects of IL-1 on the expansion of Foxp3+ Treg in vitro. We demonstrate that in the presence of splenic DC and soluble anti-CD3, the addition of IL-1α,β results in the expansion of Foxp3+ T cells. However, the costimulatory effects of IL-1 on the expansion of Foxp3+ Treg were not mediated by acting on the splenic DC or on the Foxp3+ T cells themselves but on the few CD4+CD25+Foxp3– T cells that routinely contaminate cell sorter-purified CD4+CD25+ T cell preparations. Surprisingly, when CD4+CD25+ T cells were stimulated by immobilized anti-CD3 and IL-1α,β in the absence of DC, few Foxp3+ could be isolated, and a marked expansion of IL-17-producing NKT cells and conventional CD4+ T cells was observed. We conclude from these studies that IL-1 has pleiotropic effects on CD4+CD25+Foxp3– T cells. In the presence of DC and IL-1, CD4+CD25+Foxp3– T cells facilitate the selective expansion of Foxp3+ Treg. However, under DC-free conditions, IL-1 promotes the expansion of NKT and non-NKT, IL-17-producing T cells that may actually be pathogenic T cells. The implication of these findings for the design of protocols for the preparation of Foxp3+ Treg for therapeutic use will be discussed. In addition, these results suggest that a complex, fine-tuning mechanism might exist for the regulation of the expansion of Foxp3+ Treg in vivo, depending on the nature of the surrounding cytokine environment.
MATERIALS AND METHODS
Mice
BALB/c and C57BL/6 mice were obtained from the National Cancer Institute (Frederick, MD, USA). Type I IL-1R-deficient (−/−) mice (backcrossed on a C57BL/6 background) [15] were obtained from Taconic Farms (Germantown, MD, USA) and CD1(d)−/− mice (backcrossed 11 times on a BALB/cJ background) [16] from The Jackson Laboratories (Bar Harbor, ME, USA). Yasmine Belkaid [Mucosal Immunology Unit and Immunobiology Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD, USA] kindly provided enhanced GFP (eGFP) Foxp3 knock-in mice. All mice were bred at the NIH under specific pathogen-free conditions and used at 6–8 weeks old.
Splenic DC purification and in vitro activation
After isolation, spleens were fragmented and digested in the presence of liberase blendzyme II (Roche Molecular Biochemicals, Indianapolis, IN, USA) and DNase (2 μg/ml; Roche Applied Science, Indianapolis, IN, USA) for 30 min at 37°C in complete medium (modified RPMI 1640, supplemented by 10% FBS, 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 10 mM Hepes, 4×10−7M β-ME, 1 mM essential amino acids, 1 mM sodium pyruvate; all from Biofluids, Rockville, MD, USA). RBCs were removed using an ammonium chloride-potassium lysis buffer (Biosource, Camarillo, CA, USA). T and NK cells were depleted after addition of PE-conjugated anti-NK (DX5 clone) and anti-CD3 (2C-11 clone) antibodies (BD PharMingen, San Diego, CA, USA) and anti-PE magnetic beads on an autoMACS (Miltenyi Biotech, Auburn, CA, USA). CD11c+ cells were then isolated by positive selection on mass spectrometry magnetic columns (Miltenyi Biotech). The purity was 90–95%. For their activation in vitro, the CD11c+ cells were cultured overnight in complete medium supplemented with LPS (Escherichia coli strain 0111:B4, Sigma Chemical Co., St. Louis, MO, USA) at a concentration of 100 ng/ml. After activation, DC were collected and analyzed for their phenotype by flow cytometry. They were stained with the following antibodies (all from BD PharMingen): anti-CD11c-biotinylated (HL3 clone) and streptavidin-allophycocyanin, PE-conjugated anti-CD86 (GL1 clone), anti-CD80 (16-10A1 clone), anti-CD40 (3/23 clone), or anti-I-A/I-E (M5/114.15.2 clone).
CD4+CD25+ T cell purification
CD4+ CD25+ T cells were purified using an autoMACS as described previously [2]. For most of the experiments, CD4+CD25+ T cells were sorted after staining with FITC-conjugated anti-CD4 (RM4-5 clone) and PE-conjugated anti-CD25 (PC61 clone) after FcR blocking (2.4G2 clone; all purchased from BD PharMingen). Foxp3+ CD4+ CD25+ T cells were sorted according to eGFP expression from the eGFP knock-in mice, Tricolor-conjugated anti-CD4 (Caltag, Burlingame, CA, USA), and PE-conjugated anti-CD25.
CFSE labeling
CD4+CD25+ T cells were labeled with 1 μM CFSE for 8 min at room temperature and washed three times in FBS and complete medium before being seeded.
Analysis of the CD4+CD25+ T cell population
CD4+ T cells were purified by autoMACS from peripheral and mesenteric lymph node suspensions and stained with the following mAb: PE-conjugated anti-CD25 (PC61 clone, BD PharMingen) and FITC-conjugated anti-Foxp3 (eBiosciences, San Diego, CA, USA). NKT cells were detected using the allophycocyanin-conjugated CD1 tetramer provided by the NIH tetramer facility (Emory University, Atlanta, GA, USA). The cells were stained 1 h at 4°C with the CD1d tetramer.
CD4+CD25+ T lymphocyte cultures and proliferation assays
After activation, splenic DC were collected and washed twice to remove any cytokines. Viable cells were counted after exclusion of dead cells by trypan blue. eGFP+ CD4+CD25+ T cells and CFSE-labeled CD4+CD25+ were seeded at 3 × 104 cells/well in triplicates in 96-well plates at a DC:T cell ratio of 1:1. The cultures were grown for 72 h in the presence of soluble anti-CD3 (1 μg/ml, 2C-11 clone, BD PharMingen) at 37°C. IL-1α and IL-1β were added at a final concentration of 5 ng/ml (2.5 ng/ml each), IL-4 at 5 ng/ml, and IL-2 at 50 U/ml. In some experiments, the anti-IL-2 antibody (clone S4B6, BD PharMingen) was added at a concentration of 20 μg/ml. eGFP+CD4+CD25+ T cells and CD4+CD25+ were also seeded at 3 × 104 cells/well in triplicates in 96-well plates with plate-bound anti-CD3 (5 μg/ml) for 5 days, with or without IL-1α,β or IL-2.
For the 3H-TdR-based proliferation assays, the cells were pulsed for the last 18 h with 1 μCi 3H-TdR (Amersham Biosciences, Piscataway, NJ, USA) before being collected and assessed for radioactivity. All results were expressed as the mean cpm ± sd of triplicate cultures.
Intracellular cytokine and Foxp3 staining
For intracellular cytokine staining, the cells were harvested, pooled, and seeded in 24-well plates for 6 h in the presence of plate-bound anti-CD3 and anti-CD28 antibodies (3 μg/ml each). Monensin (2 μM; Calbiochem, La Jolla, CA, USA) was added in the last 2 h. The cells were then fixed in 4% paraformaldehyde, washed, permeabilized in PBS containing 0.1% saponin and 0.1% BSA, and stained with allophycocyanin-coupled anti-IL-2 (JES6-5H4 clone), allophycocyanin-anti-IL-13 (kindly provided by W. E. Paul, NIAID, NIH, Bethesda, MD, USA), allophycocyanin-anti-IFN-γ (XMG1.2 clone), PE-anti-IL-17 (TC11-18H10 clone), and PE-anti-IL-4 (11B11 clone). Foxp3 staining was performed according the manufacturer’s instructions (Foxp3 staining kit, eBiosciences).
Flow cytometry analysis
Flow cytometry acquisition of the samples was performed on a FACSCalibur (BD Immunocytometry Systems, Mountain View, CA, USA) and analyzed using CellQuest® and FlowJo® softwares.
RESULTS
IL-1α and IL-1β drive the proliferation of CD4+CD25+ T cells when cultured in the presence of mature splenic DC
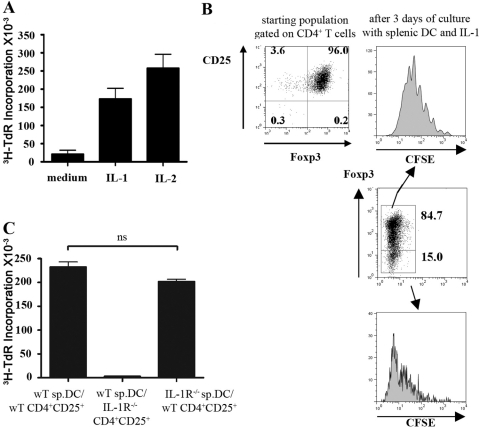
We [7] and others [8] have previously shown that TCR stimulation in the presence of mature BMDC, but not splenic DC, would drive the expansion of CD4+CD25+ T cells in the absence of IL-2. One study [10] suggested that the production of proinflammatory cytokines by activated BMDC played an important role in driving this response, as the addition of anti-IL-6 and anti-IL-1α,β antibodies to these cultures inhibited proliferation. However, in preliminary studies, we failed to see any effects of anti-IL-1 and/or anti-IL-6 on the proliferation of CD4+CD25+T cells in the presence of mature BMDC. To directly examine the role of IL-1 in the expansion of CD4+CD25+ Treg, we cultured mature splenic DC with cell sorter-purified CD4+CD25+ T cells in the presence or absence of exogenous IL-1α and -β. As previously reported [7], no proliferation was observed in the presence of soluble anti-CD3 and mature splenic DC, but a substantial response was seen when IL-1α and -β were added to the cultures; the addition of exogenous IL-2 resulted in a somewhat greater proliferative response (Fig. 1A). Although the starting population was ∼96% Foxp3+(Fig. 1B), analysis of Foxp3 expression after 3 days of culture indicated that ∼80% of the cells expanded in the presence of IL-1 still expressed high levels of Foxp3 but that some Foxp3–T cells (∼20%) were also present (Fig. 1B). Foxp3+ and Foxp3– subsets expanded, with an average of six rounds of division for the Foxp3+ subset (Fig. 1B, upper panels), and the Foxp3– subset proliferated faster and completely diluted CFSE (Fig. 1B, lower panels).
Fig. 1.
IL-1α,β drive the proliferation of CD4+CD25+T cells in the presence of mature splenic DC. (A) FACS-purified CD4+CD25+T cells were stimulated with soluble anti-CD3 (1 μg/ml), mature splenic DC, in the presence or absence of IL-1α,β (5 ng/ml) or IL-2 (50 U/ml). 3H-TdR incorporation was measured after 72 h of culture, and values are represented as the mean cpm of triplicate culture wells ± sd. (B) FACS-purified CD4+CD25+T cells were stained for the Foxp3 expression. The sorted cells were CFSE-labeled and stimulated with soluble anti-CD3 and mature splenic DC in the presence of IL-1α,β for 3 days. The cells were then analyzed for their CFSE dilution profile and Foxp3 expression. The histograms represent the CFSE divisions of the gated Foxp3– and Foxp3+ populations. (C) FACS-purified CD4+CD25+ T cells sorted from wild-type (wT) or IL-1R−/− mice were cultured with mature splenic DC (sp.DC) isolated from wild-type or IL-1R−/− mice and anti-CD3 (1 μg/ml) in the presence or absence of IL-1α,β (5 ng/ml). 3H-TdR incorporation was measured after 72 h of culture. These data are representative of four separate experiments. ns, Not significant.
To determine if the effect of IL-1 was mediated directly on the responder CD4+CD25+ Treg or indirectly on the splenic DC via the induction of soluble factors or costimulatory molecules, we purified splenic DC and CD4+CD25+ T cells from IL-1R−/− mice. Although we observed a reduced percentage and number of CD4+ and CD8+ T cells in the lymph nodes from IL-1R−/− mice, the percentage of CD25+Foxp3+ cells in the CD4+ population was similar to that seen in wild-type mice (data not shown). CD4+CD25+ T cells isolated from wild-type mice demonstrated a significant, proliferative response when cultured with splenic DC from IL-1R−/− mice. CD4+CD25+ T cells isolated from IL-1R−/− mice failed to proliferate when cultured in the presence of IL-1 and splenic DC from wild-type mice (Fig. 1C). These results indicate that in the presence of mature splenic DC, IL-1 exerts its costimulatory effects by acting directly on CD4+CD25+ T cells and not on the DC.
The costimulatory effects of IL-1 on the expansion of CD4+CD25+Foxp3+ cells are mediated indirectly via CD4+CD25+Foxp3– T cells
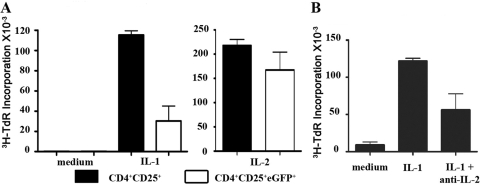
Although the results above strongly suggested that IL-1 was acting directly on the CD4+CD25+ population, it remained possible that the target for the action of IL-1 was the minor population of CD4+CD25+Foxp3– T cells that always contaminate the CD4+CD25+Foxp3+ cells (3–10% present in sorted CD4+CD25+ T cells). To address this issue, we made use of the recently engineered mice expressing eGFP in the Foxp3 locus, sorted the CD4+CD25+eGFP+ cells [99.3% eGFP+(Foxp3+); data not shown], and cultured them with mature splenic DC and IL-1 (Fig. 2A). In the presence of IL-1, the proliferative responses of the CD4+CD25+eGFP+ T cells were three- to fourfold lower than the CD4+CD25+ T cells from wild-type mice but similar in the presence of exogenous IL-2. In addition, the recovery of the CD4+CD25+eGFP+ cells (less than or equal to onefold) was much lower than that of CD4+CD25+ T cells (three- to fourfold increase) after 3 days of culture (data not shown).
Fig. 2.
CD4+CD25+Foxp3– T cells modulate the proliferation of CD4+CD25+ Foxp3+ T cells. (A) FACS-sorted CD4+CD25+ and CD4+CD25+eGFP+ T cells were stimulated with soluble anti-CD3 (1 μg/ml), mature splenic DC in the presence or absence of IL-1α,β (5 ng/ml) or IL-2 (50 U/ml). 3H-TdR incorporation was measured after 72 h of culture. (B) FACS-purified CD4+CD25+T cells were stimulated with soluble anti-CD3 (1 μg/ml) and mature splenic DC in the presence of IL-1α,β (5 ng/ml). The anti-IL-2 antibody (clone S4B6) was added at 20 μg/ml.
One possible explanation for the costimulatory effects of the CD4+CD25+Foxp3– subset on the proliferation of the CD4+CD25+Foxp3+ population is that the former produces IL-2 that drives the proliferation of the Foxp3+ T cells. To assess this possibility, we cultured CD4+CD25+ T cells with mature splenic DC and IL-1 in the presence or absence of anti-IL-2. The proliferation of CD4+CD25+ T cells was reduced by ∼50% (Fig. 2B), indicating that the CD4+CD25+Foxp3– T cells were capable of producing IL-2. Taken together, these results suggest that IL-1 is primarily mediating its costimulatory effects on the expansion of CD4+CD25+Foxp3+ T cells by acting indirectly on the CD4+CD25+Foxp3– population to induce the production of IL-2, as well as potentially other stimulatory cytokines, or by inducing the expression of costimulatory molecules on the surface of the Foxp3–cells that augment the proliferation of the Foxp3+ cells.
IL-1 induces the expansion of CD4+CD25+Foxp3– T cells in the absence of DC
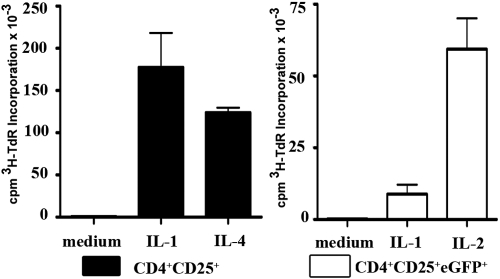
To more directly analyze the effects of IL-1 in the activation of CD4+CD25+ T cells, we stimulated highly purified CD4+CD25+ T cells from peripheral lymph nodes of C57BL/6 mice (90.5–96.8% Foxp3+; data not shown) with plate-bound anti-CD3 in the presence or absence of IL-1. Surprisingly, CD4+CD25+ T cells proliferated as well or better in the presence of IL-1 as the CD4+CD25+ T cells stimulated with exogenous IL-4 (Fig. 3, left panel; or IL-2; data not shown). The extent of cellular expansion on Day 5 was comparable in cultures stimulated with IL-1 or IL-4 (mean of 3.3±1.0 in IL-1 and 4.2±1.3 in IL-4). Consistent with the data presented in Figure 2, sorted CD4+CD25+eGFP+ cells failed to proliferate when cultured in the presence of IL-1 but readily proliferated in the presence of exogenous IL-2 (Fig. 3, right panel).
Fig. 3.
IL-1α,β costimulate the expansion of CD4+CD25+Foxp3– T cells when CD4+CD25+T cells are stimulated with plate-bound anti-CD3. FACS-sorted CD4+CD25+T cells were stimulated with plate-bound anti-CD3 (5 μg/ml) in the presence of medium alone, IL-1α,β (5 ng/ml), or IL-4 (5 ng/ml). 3H-TdR incorporation was determined after 5 days of culture. CD4+CD25+eGFP+ T cells were stimulated with plate-bound anti-CD3 (5 μg/ml) in the presence of medium, IL-1α,β, or IL-2 (50 U/ml). 3H-TdR incorporation was measured after 5 days of culture. This experiment was performed three times with similar results.
IL-1 selectively induces the expansion of Foxp3– NKT cells and IL-17-producing cells
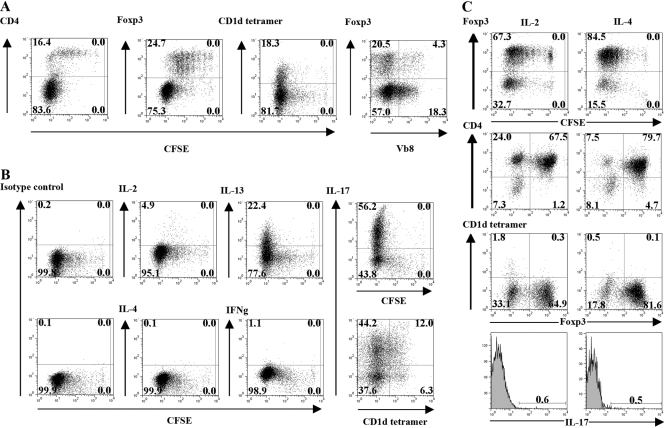
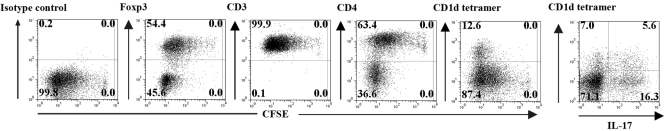
As IL-1 appeared to exert a potent, costimulatory effect on the expansion of CD4+CD25+Foxp3– T cells rather than the Foxp3+ cells, it was important to analyze in detail the phenotype of the expanded cells. Seventy-five percent of the recovered cells were Foxp3–, expressed low levels of CD4, and were enriched in Vβ8+, CD1d-tetramer+ NKT cells (∼25% CD1d-tetramer+; ∼25% Vβ8+; Fig. 4A). In contrast, the Foxp3+ cells were uniformly CD4high and were ∼20% Vβ8+. The Foxp3–CD1d tetramer+ cells also expressed CD94 (25.2% of the CD1d tetramer+ cells) and NKG2A,C,E (43.8% of the CD1d tetramer+ cells) receptors but were negative for the NK1.1 marker (data not shown). In addition to their unique pattern of cell surface antigen expression, intracellular cytokine staining of the Foxp3– cells, expanded in the presence of IL-1 (Fig. 4B), revealed that they were markedly enriched in IL-17 (56.2% of total cells representing ∼75% of the Foxp3– cells) and IL-13 producers (22.4% of total cells representing ∼30% of the Foxp3–cells). Approximately 20% of the Th17 cells were CD1d tetramer+ cells. The capacity of IL-1 to expand the CD4+CD25+Foxp3– cells was unique, as CD4+CD25+ T cells expanded in IL-2 or IL-4 (Fig. 4C) remained predominantly Foxp3+ and contained few CD1d tetramer+ cells (<2%) or IL-17 producers (<1%). Taken together, these results indicate that the polyclonal activation of the CD4+CD25+ population in the absence of DC but in the presence of IL-1 results in the outgrowth of several distinct Foxp3– T cells subsets including NKT cells capable of producing IL-17 and IL-13, as well as a subset of non-NKT, IL-17-producing cells.
Fig. 4.
CD4+CD25+T cells expanded in the presence of IL-1α,β are enriched in Foxp3–CD1d tetramer+ NKT cells and IL-17-producing T cells. (A) CFSE profile, phenotype (CD4, Foxp3, CD1d tetramer, Vβ8 expression), and (B) cytokine intracellular staining of cells recovered 5 days after culture of the CD4+CD25+T cells with plate-bound anti-CD3 (5 μg/ml) and IL-1α,β. (C) CFSE profile, phenotype (Foxp3, CD1d tetramer), and cytokine intracellular staining of cells recovered 5 days after culture of CD4+CD25+T cells with plate-bound anti-CD3 (5 μg/ml) and IL-2 (50 U/ml) or IL-4 (5 ng/ml). The results are shown as a representative experiment from three separate experiments.
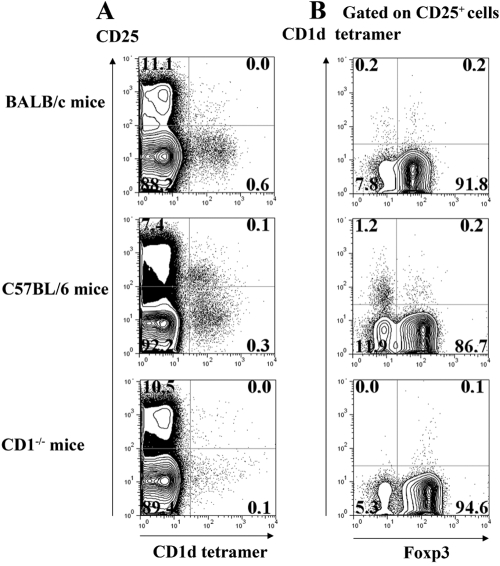
CD4+CD25+Foxp3–NKT cells can be detected in normal lymph nodes
CD4+CD25+ NKT cells have not been described previously in the mouse, although present in human peripheral blood [17]. We next determined whether small numbers of CD4+CD25+Foxp3–CD1d tetramer+ cells were present in the starting populations of CD4+CD25+ T cells used in our studies. We purified CD4+ T cells from peripheral lymph nodes of BALB/c, C57BL/6, and CD1d−/− mice and analyzed them for expression of CD25, invariant Vα14Vβ8 TCR (with the CD1d tetramer), and Foxp3 (Fig. 5). Eight to 11% of the CD4+ population in all strains expressed CD25 (Fig. 5A). When the CD4+CD25+ subset was analyzed (Fig. 5B), 1.2% of the cells from C57BL/6 mice bound the CD1d tetramer (Fig. 5B, middle panel). Few CD4+CD25+Foxp3–CD1d tetramer+ cells were detected in BALB/c (Fig. 5B, top panel), and none was present in CD1d−/− mice (Fig. 5B, bottom panel) or β2-microglobulin−/− mice (data not shown). Although NKT cells represent, at the most, only 1% of the starting CD4+CD25+ population, they appear to expand selectively during TCR stimulation in the presence of IL-1.
Fig. 5.
CD4+CD25+ NKT cells can be detected in mouse peripheral lymph nodes, which (A) from BALB/c, C57BL/6, or CD1(d)−/− mice (on a BALB/c background) were stained with anti-CD25 and CD1d tetramers. (B) Foxp3 expression and CD1d tetramer binding on gated CD25+ T cells. Data are representative of three different experiments with similar results.
Mature splenic DC facilitate the growth of CD4+CD25+Foxp3+ T cells
Although stimulation of CD4+CD25+ T cells by plate-bound anti-CD3 in the presence of IL-1 promotes the expansion of NKT cells and Th17 cells rather than Foxp3+ Treg, stimulation of the same population with soluble anti-CD3 in the presence of IL-1 and mature splenic DC facilitated the expansion of CD4+CD25+Foxp3+ Treg (Fig. 1, A and B). To resolve this paradox, we stimulated CD4+CD25+ T (90–95% Foxp3+) cells with plate-bound anti-CD3 in the presence of IL-1 and mature splenic DC for 5 days (Fig. 6). Under these stimulatory conditions, ∼60% of the recovered cells were still Foxp3+, and ∼40% were Foxp3–. About 25% of the Foxp3– cells were CD1d tetramer+ NKT cells, and ∼50% were IL-17-producers. Thus, mature splenic DC favor the growth of Foxp3+ Treg over Foxp3– T cells, but the latter remains necessary for the expansion of Foxp3+ cells.
Fig. 6.
The expansion of Foxp3+ T cells stimulated with plate-bound anti-CD3 and IL-1 is dependent on the presence of mature DC and CD4+CD25+Foxp3– T cells. FACS-purified CD4+CD25+ T cells were cultured with mature splenic DC in the presence of plate-bound anti-CD3 (5 μg/ml) and IL-1α,β (5 ng/ml). The cells were harvested after 5 days of culture and stimulated for 6 h with anti-CD3 and anti-CD28 (3 μg/ml each). Their phenotype, Foxp3 expression, and intracellular cytokine production were analyzed.
DISCUSSION
The initial goal of this work was to determine the mechanism by which highly activated BMDC were capable of inducing the expansion of Foxp3+ Treg by TCR stimulation in the absence of exogenous cytokines. As the study of Kubo et al. [10] raised the possibility that IL-1 and/or IL-6 production by the activated DC played a role in the stimulatory activity of the DC, we tested whether the addition of IL-1 to cultures of nonstimulatory splenic DC and CD4+CD25+ T cells would lead to expansion of the Treg. We observed marked stimulation of the Foxp3+ cells, but some outgrowth of Foxp3– T cells was also observed. To determine the cellular target of the IL-1, we used criss-cross combinations of IL-1R−/− DC and CD4+CD25+ T cells. These studies directly demonstrated that the activity of the IL-1 was mediated on the CD4+CD25+ population. Surprisingly, when we purified Treg from mice that were transgenic for the eGFP reporter gene in the Foxp3 locus rather than on the basis of CD25 expression, much less proliferation of the Foxp3+ population was observed. This strongly suggested that CD25+Foxp3– T cells were the cellular targets for IL-1. Similar results were observed when we stimulated eGFP+ Treg cells with plate-bound anti-CD3 and IL-1 in the absence of splenic DC.
These results are in agreement with a recent study in which it was shown that IL-1β would activate CD4+CD25+Foxp3– T cells in the presence of splenic DC by directly acting on the T cells and not the DC [18]. This study also suggested that Foxp3+ T cells were not able to suppress IFN-γ production by CD4+CD25–Foxp3– T cells in the presence of IL-1β and that IL-1β rendered the CD25+Foxp3–cells resistant to suppression. However, when we measured IL-2 production by the CD4+CD25+Foxp3–cells using the cytokine capture assay and by intracellular staining, we observed suppression in the presence of Foxp3+ T cells (data not shown). Moreover, no IFN-γ production in the CD4+CD25+Foxp3– subset was detected by intracellular staining after 3 days of culture (data not shown). The discrepancy between the two studies is most likely secondary to the percent of Foxp3+ T cells in the CD4+CD25+ population at the beginning of the cultures (95–97% in our study versus 74% in the study by O'Sullivan et al. [18]). It is possible that in the presence of a large number of CD4+CD25+ effector cells or different subsets of effector cells, Foxp3+ Treg may only inefficiently control cytokine production. We have not yet completely analyzed the mechanisms by which CD4+CD25+Foxp3– effector T cells costimulate the proliferation/survival of CD4+CD25+Foxp3+ Treg in the presence of mature splenic DC and IL-1. As anti-IL-2 inhibited ∼50% of the proliferative response, it is likely that IL-1-induced IL-2 production by the CD4+CD25+Foxp3– T cells plays an important role. The induction of other cytokines or costimulatory signals that act directly on the Foxp3+ cells or function indirectly by acting via the DC is also possible.
We observed different results when we stimulated CD4+CD25+ T cells with plate-bound anti-CD3 and IL-1 in the absence of DC. We used plate-bound anti-CD3 to mimic the conditions that are used in the clinic with anti-CD3/anti-CD28 beads to expand human Treg. In short-term cultures, stimulation of cell sorter-purified CD4+CD25+ T cells in the presence of IL-4 and to a lesser extent, IL-2 led to a preferential outgrowth of Foxp3+ T cells. However, it should be noted that significant growth of contaminating Foxp3– cells was seen. Although several papers have claimed marked expansion of human or mouse CD4+CD25+ T cells in the presence of high concentration of IL-2, few of these studies have carefully examined Foxp3 expression in the expanded populations [19, 20]. Some studies have used a mAb to human Foxp3 that has now been shown to stain activated human T cells nonspecifically [6]. Although cell expansion was seen in our studies in the presence of IL-1, the expanded population consisted primarily of Foxp3– cells. Detailed phenotypic studies of these expanded cells demonstrated that they were composed of a mixture of NKT cells and conventional T cells, and both populations contained a high percentage of IL-17-producing cells. It appears that the expanded NKT cells were generated from a small number of CD4+CD25+ NKT cells that we identified in normal lymph node populations of C57BL/6 mice and that have also been described in man [17]. An IL-17-producing NK1.1– NKT cell population similar to the one we have expanded in the presence of IL-1 has recently been shown to be present in the lung and the liver of normal mice and to potentially play a role in neutrophil recruitment [21].
Taken together, our studies confirm and extend the findings of O'Sullivan et al. [18] that IL-1 is a selective, costimulatory factor for CD4+CD25+Foxp3– T cells. The function of this population of activated effector T cells in normal lymphoid tissue is unknown. It has been hypothesized that IL-2 produced by this population is required for the maintenance of Foxp3+ Treg under homeostatic conditions in normal mice [22]. In cultures containing splenic DC, IL-1 stimulation of CD4+CD25+Foxp3– T cells resulted in expansion and/or survival of Foxp3+ T cells. One signal provided by the splenic DC was the induction of IL-2 production by the CD4+CD25+Foxp3– T cells. Previous studies [7, 8] with BMDC also demonstrated that CD86 expression on the DC played a major role in the stimulation of the growth of CD4+CD25+ T cells, and this may have been secondary to costimulation of IL-2 production by the CD4+CD25+Foxp3– T cells or to a direct effect of CD86 on the CD4+CD25+Foxp3+ T cells. In the absence of splenic DC, the major effects of IL-1 appear to be expansion/differentiation of IL-17-producing NKT and conventional T cells. It is difficult to extrapolate these findings from in vitro studies to the in vivo situation. During an inflammatory response in vivo, one might expect to have activated DC and CD4+CD25+Foxp3+ and CD4+CD25+Foxp3–T cells in the same microenvironment (e.g., an active rheumatoid joint) that might contain high levels of proinflammatory cytokines such as IL-1. Depending on the ratios of the different cell populations and cytokines, the end result might be expansion of Treg or expansion of pathogenic, IL-17-producing T cells [23]. Lastly, a large number of protocols have been proposed for the expansion of Foxp3+ Treg for use in cellular biotherapy. Our results strongly suggest that appropriate caution must be exercised when these protocols involve the use of DC that produce IL-1 or complex mixtures of growth factors that might contain IL-1, resulting in activation of CD4+CD25+Foxp3– effector cells leading to expansion of pathogenic, autoreactive T cells rather than tolerogenic Treg. Careful analysis of cytokine production, particularly IL-17, by the expanded “Treg populations” should be included in every study. Such measures should ensure the production of bona fide Treg cells for use in cellular immunotherapy.
Acknowledgments
These studies were supported by funds from the Intramural Program of NIAID. The authors thank Mohammed Oukka and Yasmine Belkaid for providing eGFP Foxp3 knock-in mice. We also thank Sarah Tanskley, Thomas Moyer, and Carol Henry for cell sorting and Stéphane Caucheteux for helpful discussions.
References
- Piccirillo C A, Shevach E M. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- Thornton A M, Shevach E M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo C A, Shevach E M. Naturally-occuring CD4+CD25+ immuno-regulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Kang S M, Tang Q, Bluestone J A. CD4+CD25+ T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- Thornton A M, Piccirillo C A, Shevach E M. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- Tran D Q, Ramsey H, Shevach E M. Induction of Foxp3 expression in naive human CD4+Foxp3– T cells by T cell receptor stimulation is TGF β-dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster C, Shevach E M. Bone marrow-derived dendritic cells reverse the anergic state of CD4+CD25+ T cells without reversing their suppressive function. J Immunol. 2005;175:7332–7340. doi: 10.4049/jimmunol.175.11.7332. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman R M. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D K, Dhodapkar M V, Matayeva E, Steinman R M, Dhodapkar K M. Expansion of Foxp3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Hatton R D, Oliver J, Liu X, Elson C O, Weaver C T. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms A E, Crane A M, Sims A M, Cordell H J, Bradbury L A, Abbott A, Coyne M R, Beynon O, Herzberg I, Duff G W, Calin A, Cardon L R, Wordsworth B P, Brown M A. The interleukin 1 gene cluster contains a major susceptibility locus for ankylosing spondylitis. Am J Hum Genet. 2004;75:587–595. doi: 10.1086/424695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor α in antibody-induced arthritis. J Exp Med. 2002;196:77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- Smiley S T, Kaplan M H, Grusby M J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- Lee P T, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan B J, Thomas H E, Pai S, Santamaria P, Iwakura Y, Steptoe R J, Kay T W H, Thomas R. IL-1β breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–7287. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart L A, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Godfrey W R, Ge D J, Spoden D J, Levine B L, June C H, Blazar B R, Porter S B. In vitro-expanded human CD4+CD25+ T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- Michel M-L, Keller A C, Paget C, Fujio M, Trottein F, Savage P B, Wong C-H, Schneider E, Dy M, Leite-de-Moraes M C. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]