Abstract
HIV infection causes rapid and lasting defects in the population of Vγ2Vδ2 T cells. To fully describe the impact of HIV, we examined PBMC samples from HIV+ patients receiving highly active antiretroviral therapy, who had displayed prolonged viral control and CD4 counts above 300 cells/mm3. We observed lower frequencies of CD27–/CD45RA– Vγ2Vδ2 cells in HIV+ individuals when compared with controls, coupled with an increased proportion of CD45RA+ cells. These changes were common among 24 HIV+ patients and were not related to CD4 cell count or viral RNA burden. Vγ2 cells from HIV+ individuals had lower expression of Granzyme B and displayed reduced cytotoxicity against Daudi targets after in vitro stimulation. There was increased expression of FasR (CD95) on Vγ2 cells from HIV+ PBMC that may be a mechanism for depletion of Vγ2 cells during disease. In addition to the well-characterized defects in the Vγ2 repertoire and functional responses to phosphoantigen, the proportion of CD27–/CD45RA– Vγ2Vδ2 T cells after isopentenyl pyrophosphate stimulation was reduced sharply in HIV+ donors versus controls. Thus, HIV infection has multiple impacts on the circulating Vγ2Vδ2 T cell population that combine to reduce the potential effector activity in terms of tumor cytotoxicity. Changes in Vγ2Vδ2 T cells, along with concomitant effects on NK and NKT cells that also contribute to tumor surveillance, may be important factors for elevating the risk of malignancy during AIDS.
Keywords: natural immunity, gamma delta, effector memory, cytotoxicity
INTRODUCTION
Human γδ T cells account for 1–10% of CD3+ cells in peripheral blood of healthy adults [1]. The majority expresses a Vγ2-Jγ1.2Vδ2 TCR [2, 3] and is mostly negative for lineage markers CD4 and CD8 [4]. Antigen recognition by Vγ2-Jγ1.2Vδ2 T cells is TCR-dependent but not restricted by MHC [5, 6]. In vitro, Vγ2Vδ2 cells proliferate and release cytokines in response to low molecular weight alkylphosphate compounds that are intermediates of mammalian or microbial sterol biosynthesis [3, 7]. This subset responds to microbial or viral pathogens [8,9,10] and plays important roles in tumor immunity [11]. Vγ2Vδ2 T cells are cytotoxic for tumor cell lines [12], including but not limited to cancers of the lymphoid lineage [6, 13,14,15]. Importantly, Vγ2-Jγ1.2Vδ2+ cells are depleted after HIV infection [2], accounting for the AIDS-related decline in Vγ2Vδ2+ cell numbers and functional responses to phosphoantigen [16,17,18,19]. We propose that HIV-mediated depletion of Vγ2Vδ2 cells removes an important component of tumor immunity and contributes to the increased risk for AIDS-related malignant diseases.
Several recent publications described Vγ2Vδ2 phenotypes and the impact of infectious diseases including HIV [17, 20,21,22]. In these studies, cell surface expression of CD27 and CD45RA has been used to define naïve and memory subpopulations of γδ T cells [17, 20, 21, 23, 24]. CD27 is a 120-kDa transmembrane homodimer of the TNFR/growth factor receptor family that is expressed on a majority of B, T, and NK cells. The interaction of CD27 with its ligand CD70 is important for activating naïve (CD45RA+) T cells and promoting maturation concomitant with loss of CD27 [25]. CD45 is a transmembrane tyrosine phosphatase with several isoforms: The CD45RA form is found on naïve/resting [26, 27] and terminally differentiated T cells [20, 21]. When used together, CD27 and CD45RA expressions patterns can be used to define naïve (CD27+/CD45RA+), central memory (CD27+/CD45RA–), and effector (CD27–) subsets for γδ cells [17, 21]. CD27–/CD45RA– (effector memory) cells have been reported to be primary IFN-γ producers and CD27–/CD45RA+ cells were shown to have cytotoxic effector functions [21]. CD16 (Fcγ-RIII) was expressed mainly on CD27–/CD45RA+ cells [20]. Cross-linking Fcγ-RIII on CD27–/CD45RA+ but not CD27–/CD45RA– Vγ2 cells resulted in activation of the ERK pathway and NK-like functional responses that are usually associated with terminally differentiated T cells [20].
Recent studies have shown that declining levels of CD27–/CD45RA– Vγ2 cells and loss of their contribution to IFN-γ production promoted mycobacterial tuberculosis [17]. In the same study, a relative reduction in CD27–/CD45RA– cells was noted for a small group (n=7) of HIV+ individuals. A subsequent report demonstrated that the frequency of CD27–/CD45RA– Vγ2 cells increased with highly active antiretroviral therapy (HAART) but declined again during a structured treatment interruption [22]. We reported recently [2, 14] that the Vγ2 repertoire is highly stable in healthy controls, but specific losses of Jγ1.2+ cells are pronounced in HIV disease, even during 2–3 years of HAART. Here, we use CD27 and CD45RA expression to define subpopulations of Vγ2Vδ2 T cells in HIV+ individuals that differ from control donors and are related to defects in the TCR repertoire occuring after HIV infection. The conventional descriptions of naïve and memory cell types are problematic for Vγ2 T cells, where we expect exaggerated effector phenotypes and few truly naïve cells. However, the distribution of Vγ2Vδ2 T cells among phenotypic subsets during HIV revealed a major disruption in this T cell population and provided new clues about possible mechanisms for specific cell depletion.
MATERIALS AND METHODS
PBMC isolation and Vγ2Vδ2 stimulation
Heparinized blood (30 ml) was collected from each of 17 HIV– and 26 HIV+ volunteers with approval from the Institutional Review Board at the University of Maryland (Baltimore, MD, USA) and informed consent of the donors. The HIV+ donors (Table 1) were selected by the following criteria: CD4 count greater than 300/μl, HAART therapy with prolonged viral suppression and no AIDS-defining illness. The patients with undetectable viral load displayed viral suppression for at least 12 months before participating in this study. PBMC were isolated by centrifugation over Ficoll-Paque density gradients, as described by the manufacturer (GE Healthcare, Piscataway NJ, USA). Purified PBMC were stored at 107 cells/ml in a freezing mix consisting of 90% FBS (Invitrogen, Carlsbad, CA, USA) and 10% dimethyl sulfate (Sigma Chemical Co., St. Louis, MO, USA).
TABLE 1.
Profile of HIV+ Donors
| Sample ID | Sex | Age | CD4 T cell count (cells/μl) | Viral load (copies/ml) | Vγ2+ (%)a |
|---|---|---|---|---|---|
| PD001 | M | 45 | 475 | b | 2.3 |
| PD002 | M | 52 | 494 | <50 | 0.6 |
| PD003 | M | 50 | 453 | <50 | 0.2 |
| PD004 | F | 52 | 508 | b | 0.3 |
| PD005 | M | 49 | 681 | b | 0.5 |
| PD006 | M | 43 | 613 | <50 | 0.4 |
| PD007 | M | 51 | 524 | <50 | 0.9 |
| PD008 | M | 56 | 335 | <50 | 0.2 |
| PD009 | F | 44 | 418 | <50 | 0.2 |
| PD010 | F | 43 | 399 | <50 | 2.8 |
| PD011 | F | 46 | 463 | <50 | 0.5 |
| PD012 | M | 40 | 530 | <50 | 0.5 |
| PD013 | M | 47 | 316 | b | 0.0 |
| PD014 | M | 36 | 785 | 3524 | 1.3 |
| PD015 | M | 59 | 473 | 83 | 1.6 |
| PD017 | F | 41 | 510 | <50 | 1.0 |
| PD018 | M | 43 | 443 | 16,100 | 2.5 |
| PD019 | F | 48 | 630 | <50 | 0.6 |
| PD020 | M | 48 | 367 | 15,500 | 0.2 |
| PD021 | M | 46 | 496 | 8640 | 2.9 |
| PD022 | M | 34 | 431 | 465 | 3.9 |
| PD023 | F | 47 | 996 | <50 | 0.8 |
| PD024 | M | 41 | 550 | 78 | 3.2 |
| PD025 | M | 50 | 449 | 176,000 | 0.5 |
| PD026 | F | 56 | 472 | <50 | 0.7 |
| Summary | nM = 17 nF = 8 | 46.7 ± 6.0 | 512 ± 144 | – | 1.1 ± 1.1 |
The number of Vγ2+ T cells as a percentage of all lymphocytes.
No data available. PD, positive donor referring to HIV status.
For in vitro stimulation, cells were thawed and resuspended in RPMI-1640 medium (Invitrogen) supplemented with 10% FBS, 2 mM L-glutamine (Invitrogen), 1 U/ml penicillin/streptomycin (Invitrogen), and 100 U/ml recombinant human IL-2 (Tecin, Biological Resources Branch, National Institutes of Health, Bethesda, MD, USA). Isopentenyl pyrophosphate (IPP) or PHA (Sigma Chemical Co.) was added at concentrations of 15 μM or 2.5 μg/ml, respectively. Cultures were incubated for 14 days at 37°C and 6% CO2 and were replenished every 3 days by the addition of IL-2-supplemented R10 without further addition of IPP or PHA. Viable counts were performed using Trypan blue exclusion (Sigma Chemical Co.).
Antibody staining and flow cytometry
Cell surface markers were detected on 4 × 105 cells that were stained with labeled mAb for 15 min at 4°C. The cells were washed and fixed with 1% paraformaldehyde. Granzyme B (GrB) was detected by intracellular staining using the Cytofix/Cytoperm kit from BD Biosciences (San Diego, CA, USA). Flow cytometry data were collected on a FACSCalibur (BD Biosciences), where at least 3 × 104 lymphocyte events were acquired for each sample (lymphocytes were defined based on forward- and side-scatter profiles). Flow cytometry data were analyzed with FlowJo software (Tree Star, San Carlos, CA, USA).
The following antibodies were used in this procedure: FITC-conjugated anti-Vγ9, clone IMMU360 (Beckman Coulter, Somerset, NJ, USA); R-PE-conjugated anti-CD95, clone DX2 (BD Biosciences); PE-conjugated anti-GrB, clone GB12 (Caltag, Burlingame CA); PE:cyanine-5-conjugated anti-CD45RA, clone HI100 (BD Biosciences); allophycocyanin-conjugated anti-CD27, clone 0323 (eBioscience, San Diego, CA, USA); and the appropriate isotype controls (Becton Dickinson, San Jose, CA, USA). Note that Vγ4 and Vγ2 refer to the same molecule.
Stimulation index (SI) represents the proportional increase in Vγ2 cells following IPP stimulation compared with control with IL-2 but without IPP. SI is defined as the ratio of the absolute number of Vγ2 lymphocytes on Day 14 of the IPP expansion:absolute number of Vγ2 lymphocytes on Day 14 of controls (IL-2 alone).
RNA extraction and RT-PCR
Total RNA was extracted from at least 106 cells (RNeasy mini kit, Qiagen, Valencia, CA, USA). Total RNA (1 μg) was converted into cDNA using the RT system (Promega, Madison, WI, USA) in a reaction containing 500 ng oligonucleotide A (T15V), 1 mM deoxynucleotriphosphates, 5 mM MgCl2, 10 mM Tris-HCl, pH 8.8, 50 mM KCl, 0.1% Triton X-100, 18 units avian myeloblastosis virus RT, and 10 units RNasin RNase inhibitor. Each reaction was incubated at 42°C for 2 h, and then cDNA was diluted to 100 μL by adding 80 μL deionized water. PCR reactions were performed using 5 μL cDNA as template and 500 nM each of forward and reverse primers, 0.2 mM dNTPs, 2 mM MgCl2, 10 mM Tris-HCl, pH 8.8, 50 mM KCl, 0.1% Triton X-100, and 1 unit AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA). The following primers were used: oligo-Vγ2 (5′-ATCAACGCTGGCAGTCC-3′), oligo-Cγ1 (5′-GTTGCTCTTCTTTTCTTGCC-3′), 5′ β-actin (5′-GTGGGGCGCCCCAGGCACCA-3′), and 3′ β-actin (5′-CTCCTTAATGTCACGCACGATTTC-3′). PCR was run with the following profile: denaturation for 1 min at 94°C; 5 min at 68°C; 45 cycles (45 s at 94°C, 1 min at 60°C, 1 min at 72°C); extension for 10 min at 72°C. PCR products were separated on a 2% agarose/Tris-acetate-ethylenediaminetetraacetic acid buffer gel containing 0.5 μg/ml ethidium bromide.
Spectratype analysis
Primer extension reactions were performed as described previously [3]. Each reaction contained 1 μL PCR product, 3 mM MgCl2, 0.2 mM dNTP, 0.2 unit Taq DNA polymerase (Promega), 10 mM Tris-HCl, pH 8.8, 50 mM KCl, and 0.1 μM 6-carboxyfluorescein (6-FAM)-labeled primer (Cγ6-FAM for Vγ2 chains: 5′-AATAGTGGGCTTGGGGGAAAC-3′; Cδ16-FAM for Vδ2 chains: 5′-ACGGATGGTTTGGTATGAGG-3′). Run-off products (4 μL) were diluted in deionized formamide and 1 μL N,N,N′,N′-trimethyl-6-rhodamine-labeled molecular size standard was added to each sample. After a denaturation step (5 min at 95°C, followed by immediate quenching on ice), products were loaded on an Applied Biosystem microcapillary genetic analyzer (Perkin-Elmer, Foster City, CA, USA) and run for 27 (Vγ2 chains) or 24 (Vδ2 chains) min on a performance-optimized polymer-4. Molecular size and relative frequency of extension products were determined using Genescan software (Perkin-Elmer). To standardize the data, irrespective of the run-off primer position, CDR3 length variation was expressed in terms of the total Vγ2 or Vδ2 coding region lengths. Run-off product lengths were corrected by adding the length of the known Vγ2 or Vδ2 mRNA coding regions outside the run-off product. According to this calculation, the major peak for Vγ2 chains is 996 nt based on a corresponding run-off product length of 447 nt.
Daudi cell line and calcein-release cytotoxicity assay
Daudi B cells (CCL-213, American Type Culture Collection, Manassas, VA, USA) were cultured in R10 supplemented with 4.5 g/L glucose, 1.5 g/L NaHCO3, 10 mM HEPES, and 1 mM sodium pyruvate. To quantify the cytotoxic capacity of Vγ2Vδ2 T cells against these Daudi B cell targets, we used a nonradioactive, fluorometric cytotoxicity assay involving the dye calcein-acetoxymethyl (AM) [28]. Tumor targets were stained for 15 min at 37°C in 5.9% CO2 with 2 μM calcein-AM (Molecular Probes, Eugene, OR, USA) at a concentration of 106 cells/ml. The cells were washed once in PBS and combined at various E:T ratios in 96-well, round-bottom microtiter plates (Corning Inc., Corning, NY, USA) and incubated at 37°C in 5.9% CO2 for 4 h; assays were performed in triplicate. For maximal release, 2× final concentration of Triton-X (Sigma Chemical Co.) was added to the appropriate target wells. Following incubation, supernatants were transferred to 96-well, flat-bottomed microtiter plates; the calcein content was measured using a Wallac Victor2 1420 multichannel counter (λ485/535 mm). Percent specific lysis is calculated as: [(test release–spontaneous release)/(maximum release–spontaneous release)] × 100.
RESULTS
CD27/CD45RA expression in HIV+ and control donors
The distribution of Vγ2 cells among naïve/memory/effector subsets was evaluated in PBMC from HIV+ (n=24) or control (n=17) individuals (Tables 1and 2). HIV+ donors were screened to ensure that we could detect the Vγ2 marker on at least 0.2% of peripheral blood lymphocytes; reliable measurements were not possible below this level. This screen eliminated only one donor (PD013) from further analysis. A significant difference was observed for Vγ2 frequency between donor groups (control 4.2±3.6% vs. HIV+ 1.1±1.1%; P=0.0004; Table 3), similar to what was reported previously [14, 22, 29].
TABLE 2.
Profile of Control Donors
| Sample ID | Sex | Age | Vγ2+ (%)a |
|---|---|---|---|
| ND001 | M | 53 | 3.4 |
| ND003 | M | 27 | 2.9 |
| ND004 | M | 56 | 3.8 |
| ND005 | M | 37 | 2.1 |
| ND006 | F | 28 | 1.8 |
| ND007 | F | 27 | 1.7 |
| ND008 | M | 28 | 14.1 |
| ND012 | M | 29 | 2.9 |
| ND013 | M | 34 | 7.3 |
| ND014 | M | 33 | 2.5 |
| ND015 | F | 26 | 5.4 |
| ND016 | F | 57 | 1.4 |
| ND017 | M | 32 | 3.9 |
| ND018 | F | 26 | 1.1 |
| ND019 | F | 28 | 10.2 |
| ND020 | F | 28 | 1.0 |
| ND021 | F | 26 | 6.3 |
| Summary | nM = 9 nF = 8 | 33.8 ± 10.7 | 4.2 ± 3.6 |
The number of Vγ2+ T cells as a percentage of all lymphocytes. ND, normal donors.
TABLE 3.
Vγ2 Frequency and CD27/CD45RA Phenotype in HIV+ and Control Donors
| Sample set | n | Vγ2 frequency | CD27/CD45RA quadrant frequencies
|
|||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | −/+ | |||
| HIV− (ND) | 17 | 4.2 ± 3.6 | 14.5 ± 13.7 | 39.6 ± 21.6 | 24.8 ± 19.3 | 21.1 ± 22.4 |
| HIV+ (PD) | 24 | 1.1 ± 1.1a | 28.8 ± 13.6a | 28.4 ± 18.7 | 5.7 ± 5.8a | 37.1 ± 21.7b |
P < 0.005;
P < 0.05.
Control and HIV+ donors had differences in CD27 and CD45RA expression on Vγ2 cells. Control individuals had higher average frequencies of CD27–/CD45RA– Vγ2 cells compared with HIV+ individuals (Table 3), confirming a previous report with a smaller patient group [17]. Additionally, the Vγ2+CD45RA+ phenotype was proportionally more abundant in HIV+ donors than controls; this was true for CD27– and CD27+ subsets (Table 3). Only the proportion of CD27+/CD45RA– cells was not statistically different between control and HIV+ groups (P=0.09). These observed differences did not correlate with CD4 counts or viral loads.
Vγ2 cells from HIV-infected individuals have less stored GrB
We next evaluated differences in cytoplasmic GrB that might reveal a reduced effector function in HIV disease. GrB is an aspartyl serine protease found in cytoplasmic granules of cytotoxic T cells and NK cells [30]. The presence of intracellular granzyme identifies potential cytotoxic cells in PBMC [31, 32]. We used intracellular staining for GrB as a functional marker for effector cells within the Vγ2 population. PBMC from healthy donors (n=16) were stained for Vγ2, GrB, CD45RA, and CD27. When we excluded Vγ2 cells and looked at the remaining lymphocyte populations (Vγ2–, mostly αβ T cells), the majority of GrB+ cells was CD27–/CD45RA+ (Fig. 1). However, a high proportion of all Vγ2+ cells was GrB+, including all but the CD27+/CD45RA– subset (Fig. 1B and Supplemental Table 1). The highest frequency of GrB+ cells was in the CD27–/CD45RA+ fraction, consistent with the cytotoxic potential of CD45RA single-positive cells [20, 21].
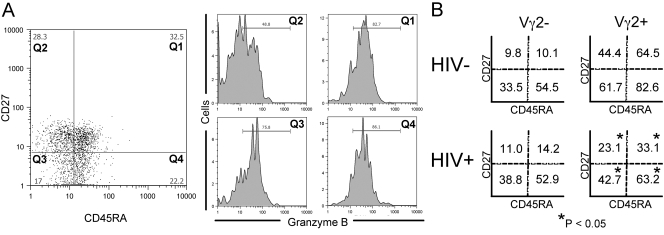
Fig. 1.
Frequencies of GrB+ Vγ2 cell subpopulations from HIV+ and control donors. PBMC from 24 HIV+ and 16 control donors were stained for flow cytometry with Vγ2, GrB, CD27, and CD45RA mAb. (A) Left panel: CD27 versus CD45RA stain of donor ND001 Vγ2+ cells showing four populations labeled Q1–Q4. The frequency of GrB+ cells in each quadrant was determined and is depicted in the four panels on the right. The mean frequency of GrB+ cells in each quadrant for both populations of cells and for both donor groups is represented in B. Lymphocytes were defined by forward- and side-scatter profiles and were further divided into Vγ2+ and Vγ2– fractions. Values represent average frequencies of GrB+ cells in a specified, CD27/CD45RA-defined fraction. Significant decreases were observed for HIV+ donors in each Vγ2+ fraction (P<0.025); no differences were observed in Vγ2– fractions. P values result from Student’s t-tests.
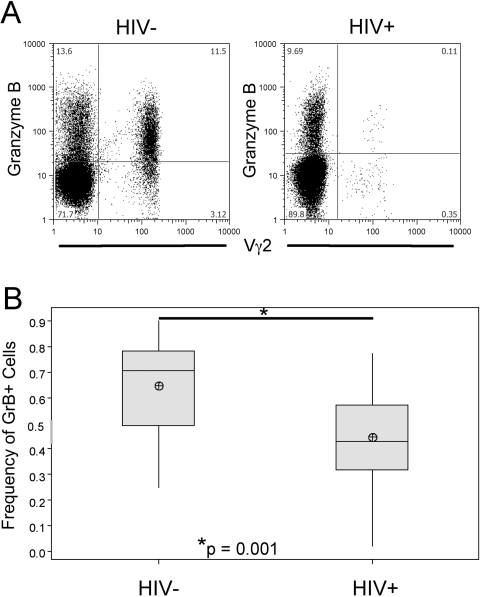
We compared the frequencies of GrB+ Vγ2 cells in HIV+ and control donors. PBMC from 24 HIV+ donors were stained for Vγ2, GrB, CD45RA, and CD27. Despite observing that CD27–/CD45RA+ Vγ2 cells were proportionally higher in HIV+ samples (Table 3), the overall frequency of GrB+ Vγ2 cells was lower (P=0.001; Fig. 2). The CD27–/CD45RA+ population from HIV+ donors still had the highest frequency of GrB+ cells, although the GrB+ fraction was smaller in each of the CD27/CD45RA quadrants when compared with controls; P < 0.025 (Fig. 1B and Supplemental Table 1). These data suggested that Vγ2 cells in HIV+ individuals have a reduced cytotoxic capacity. We detected no changes in GrB expression among Vγ2– cells from HIV+ individuals.
Fig. 2.
Frequency of GrB+ cells from HIV+ and control donors. (A) Representative samples of HIV+ (PD011) and control (ND008) donors showing GrB versus Vγ2. (B) Average frequencies of GrB+ cells in total Vγ2+ population from HIV+ (n=24) or control donors (n=16). Shaded boxes represent interquartile range (IQR) of distribution, horizontal line represents the median, and crossed circles represent sample mean; whiskers mark distance from IQR to the furthest point that is not an outlier, and outliers are defined as >1.5 × IQR from the edge of the box. P value is a result of Student’s t-test.
CD95 expression is higher on Vγ2 cells from HIV+ donors
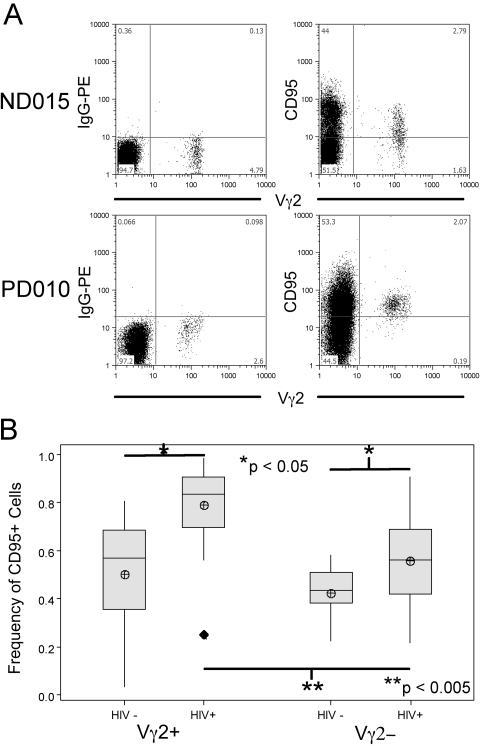
We next measured expression of CD95 (FasR) in the CD27/CD45RA-defined fractions of Vγ2+ and Vγ2– cells from HIV+ donors (n=24) and controls (n=11). CD95 is a marker for cell differentiation and may be important for indirect cell depletion mechanisms in SIV infection of macaques [33]. We found no significant differences in CD95+ frequencies between the Vγ2– and Vγ2+ lymphocytes among control donors (0.419±0.121 vs. 0.487±0.269, respectively; Fig. 3B), although there was a trend for higher CD95 expression in the Vγ2+ population. The proportion of CD95+ Vγ2 cells was increased significantly among HIV+ individuals (0.788±0.171 vs. 0.487±0.269 for control donors; Fig. 3B). Moreover, the frequency of CD95+ cells was higher between Vγ2+ cells compared with Vγ2– lymphocytes in HIV+ donors (Fig. 3B; P<0.005). Vγ2 cells in HIV+ individuals are mostly positive for CD95, a phenotype normally associated with memory T cells, despite the fact that HIV+ donors only have a small proportion of CD27–/CD45RA– Vγ2 cells.
Fig. 3.
Frequencies of CD95+ cells from HIV+ and control donors. PBMC from HIV+ (n=24) and control (n=11) donors were stained for flow cytometry with Vγ2 and CD95 mAb. Lymphocytes were gated by forward- and side-scatter profiles; Vγ2+ cells and Vγ2– (αβ) cells were then examined for CD95 expression. (A) Representative samples of CD95 versus Vγ2 stains for one HIV+ and one control donor, as well as related isotype stains. (B) Averaged frequencies of CD95+ Vγ2 cells from HIV+ and control donors. Shaded boxes represent IQR of distribution, horizontal line represents the median, and crossed circles represent sample mean; whiskers mark distance from IQR to the furthest point that is not an outlier, and outliers are >1.5 × IQR from the edge of the box and are represented by filled diamonds. Significant differences are highlighted; P values are results of Student’s t-tests.
IPP expansion does not restore the CD27–/CD45RA– subset in HIV+ PBMC
To ascertain whether the HIV-associated loss of effector memory phenotype (CD27–/CD45RA–) was reversible, we stimulated PBMC with IPP and stained for Vγ2, CD27, and CD45RA after 14 days in culture. With control donors (n=9), we observed significant increases in the fraction of CD27–/CD45RA– cells (Fig. 4, upper panels, for a representative sample). When the cultures were maintained up to 35 days, the distribution among subsets did not change (data not shown).
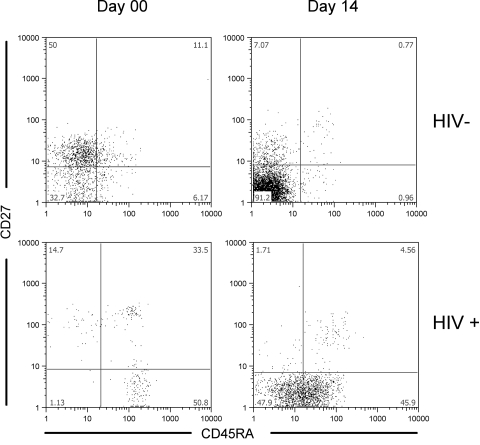
Fig. 4.
Vγ2 cells differ in expression of CD27 and CD45RA between HIV+ and control donors before and after IPP stimulation. PBMC from HIV+ (n=24) and control (n=9) donors were stained with Vγ2, CD27, and CD45RA antibodies before and after stimulation with 100 U IL-2, IL-2 and 15 μM IPP, or IL-2 and 0.25 μg/ml PHA. Shown are representative plots for HIV+ and control donors on Day 00 and after 14 days in culture post-15 μM IPP stimulation. The average frequency of CD27–/CD45RA– Vγ2 cells is higher among control donors than HIV+ donors after IPP stimulation (78.4±10.3 vs. 47.4±25.2, respectively; P<0.001). Conversely, CD27–/CD45RA+ Vγ2 cells are more frequent after IPP stimulation among HIV+ donors than controls (20.1±19.4 vs. 1.1±0.7, respectively; P<0.005). Similar trends were observed after stimulation with IL-2 alone. P values are results of Student’s t-tests.
We stimulated HIV+ PBMC with IPP and analyzed the subset distributions. A previous study [21] reported that CD27+/CD45RA– cells were the main subset that responded to phosphoantigen stimulation. The proportions of CD27+/CD45RA– cells among HIV+ donors were similar to controls (Table 3), yet the HIV+ samples (n=24) had lower IPP proliferative responses (SI=2.8±4.8 for the HIV+ compared with 7.1±5.3 for controls; P=0.027), which were likely a result of specific depletion of Vγ2-Jγ1.2+ cells [2] that are mainly responsible for IPP responsiveness [3]. After phosphoantigen stimulation, Vγ2 cells from HIV+ donors (n=24) were mostly CD27–/CD45RA+ (Fig. 4, lower panels, for a representative sample). After expansion, the ratio of CD27–/CD45RA– to CD27–/CD45RA+ Vγ2 cells in HIV+ PBMC was 10 times lower than the ratio in control donors (11.8±16.8 vs. 118.4± 98.5, respectively; P<0.001). Vγ2Vδ2 cells from HIV+ donors accumulated in the CD27–/CD45RA+ compartment after antigen stimulation in contrast to control cells that were found in the double-negative fraction.
Longer Vγ2 chains encode the phosphoantigen response in HIV+ and control PBMC
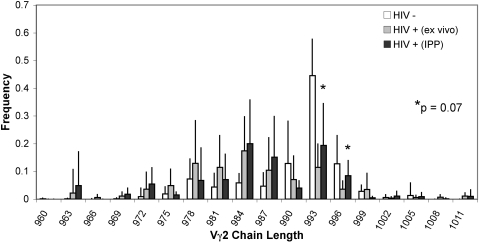
We asked whether the low-level phosphoantigen responses in HIV+ individuals still depended on Vγ2-Jγ1.2 T cells, as is the case in control donors. Spectratype analyses [2, 3, 14] were used to assess the Vγ2 repertoire in PBMC and again 14 days after IPP stimulation for HIV+ (n=24) and control (n=14) PBMC. Figure 5 illustrates differences in the distribution of Vγ2 chain lengths between HIV+ donors (gray bars) and controls (white bars) ex vivo. Among control donors, we noted a familiar bias in the distribution of chain lengths with accumulation of Vγ2 chains between 987 and 996 nt in length that mainly use the Jγ1.2-joining segment [3, 14]. Among HIV+ donors, the distribution of Vγ2 chain lengths in unstimulated PBMC centered on 984 nt (Fig. 5 and ref. [2]). We then selected seven HIV+ PBMC specimens that started with low (<0.10) frequencies of TCR Vγ2 chains between 990 and 996 nt. After IPP stimulation, there were small increases (Fig. 5, black bars) in Vγ2 chains at 993 and 996 (P=0.07), the lengths commonly associated with phosphoantigen responses [3]. Additionally, IPP stimulation of HIV+ PBMC expanded a small fraction of Vγ2+ cells, which had a frequency of GrB expression that was not different from controls (Supplemental Table 2 and Supplemental Fig. 1). Although proliferative responses and CD27/CD45RA phenotypes were quantitatively distinct for HIV+ and control donors, the phosphoantigen responses in both groups depended on the same subset of cells expressing the longer Vγ2 chains and resulted in up-regulation of GrB.
Fig. 5.
CDR3 length distribution of Vγ2 TCR chains in control and HIV+ donors. cDNA was originated from control donors (n=9) and HIV+ donors (n=7) ex vivo and HIV+ samples (n=7) after IPP-generated expansion; these cDNA were used to amplify Vγ2 TCR chains using appropriate primer sets. PCR-amplified TCR chains were spectratyped using fluorophore-conjugated primers specific for the Cγ region. For each group of samples, relative frequency values for each chain length are represented by the averaged relative frequency ± sd. Increases in frequency of chains at 993 and 996 nt in length are observed in HIV+ samples after IPP stimulation; P = 0.07.
Vγ2 effectors from HIV+ donors display reduced capacity to lyse Daudi B cells after in vitro stimulation
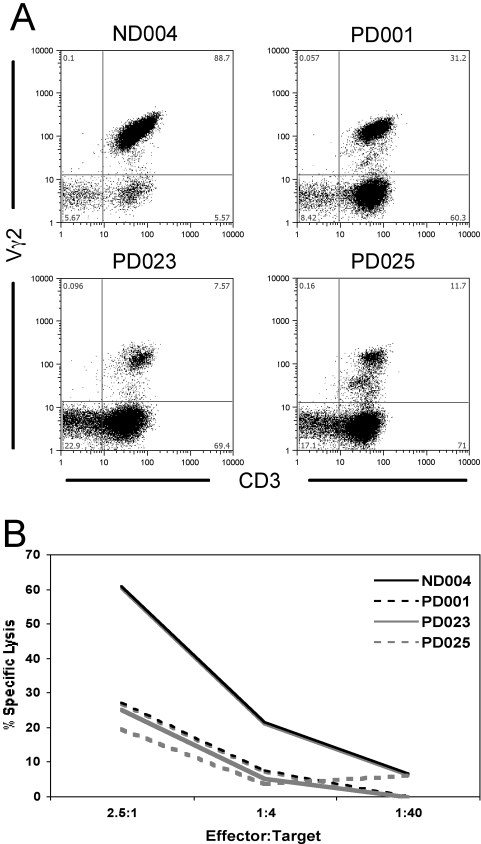
Finally, we expanded Vγ2Vδ2 cells out of PBMC from three HIV+ (PD001, PD023, and PD025) and one control donor (ND004) with IL-2 and IPP. The HIV+ donors were chosen on the basis of post-expansion CD27/CD45RA ratios; Vγ2 cells from PD001 are primarily double-negative for CD27/CD45RA, as is the case with control donors (including ND004). Contrastingly, Vγ2 cells from donors PD023 and PD025 are primarily CD45RA single-positive after IPP expansion (data not shown). After 14 days of expansion with high (100 U/ml) IL-2 and 7 days rest with low IL-2 (10 U/ml), we performed colorimetric cytotoxicity assays [28] against the classical Vγ2 target Daudi [14, 34, 35]. These expanded cell fractions contained varying levels of other lymphocyte subsets, such as NK cells, which are cytotoxic for Daudi B cells [36, 37]. Flow cytometry analysis on Day 21 allowed us to quantify the population of Vγ2 cells (Fig. 6A) apart from the CD3–/CD56+ NK cells (data not shown). Despite increased concentrations of NK cells (PD001=9.03% NK cells among all lymphocytes; PD023=21.5%; PD025=15.3%, compared with ND004 with 6.08%), HIV+ donor cells displayed much lower toxicity against the Daudi targets when compared with control donors (Fig. 6B), indicating a severe loss of Vγ2Vδ2 cytotoxic T cells.
Fig. 6.
Vγ2 cytotoxicity against Daudi B cell targets. Vγ2 effectors were generated by expanding PBMC (three HIV+ donors; one control) with IPP and IL-2 for 14 days and resting the cultures for 7 days prior to the cytotoxicity setup. (A) Vγ2 versus CD3 stains of the lymphocyte gates are shown for each of the four donors on the day of the toxicity experiment. (B) Vγ2 effectors are titrated over Daudi cell targets labeled intracellularly with 2 μM calcein-AM. Cytotoxicity was determined by assessing the presence of released fluorescent calcein in reaction supernatants.
DISCUSSION
We showed that HIV infection interferes with the control of Vγ2 cell activation, resulting in reduced Vγ2 cell numbers and altered distribution among subsets defined by CD27 and CD45RA expression. The Vγ2 population in HIV+ donors generally lacks the CD27–/CD45RA– subset but has an increased proportion of CD27–/CD45RA+ effectors. Despite the increased numbers of these terminally differentiated effectors, Vγ2 cells from HIV patients had lower overall expression of GrB. The decrease in GrB+ Vγ2 effectors in HIV disease results in a reduced capacity for tumor cytotoxicity, as we have demonstrated in our killing assays targeting Daudi B cells, an EBV lymphoma similar to common AIDS-related malignancies. Taken together, these data suggest that the HIV-induced alterations in frequency and function of Vγ2 cells may be related to the increased risk of malignant disease associated with HIV infection [38,39,40,41]. Additionally, we demonstrated that Vγ2 cells from HIV+ donors have a reduced capacity to generate CD27–/CD45RA– cells in vitro, showing that the response to stimulation differs from controls and that Vγ2+ cells have increased expression of CD95 when compared with Vγ2– cells in HIV+ and control donors. Vγ2+ cells are likely more susceptible to Fas-mediated deletion, a factor that might promote their loss during HIV disease, when FasL is elevated [42]. These features of Vγ2Vδ2 T cells reveal the complex impact of HIV infection. Combined with the known defect in the Vγ2 repertoire [2], these alterations in the γδ phenotype demonstrate how HIV limits the potential for γδ T cells to participate in normal tumor immune responses.
Several reports described the impact of HIV-1 on γδ T cells [2, 19, 43, 44]. Reduced frequency [2], loss of the reactive Jγ1.2-encoding subset [2], reduced IFN-γ production [17], and alterations in TCR repertoire specificity (Andrew M. Hebbeler, Nadia Papp, Cristiana Cairo, Haishan Li, Jean-Saville Cummings, Lisa P. Jacobson, Joseph B. Margolick, C. David Pauza; in press) are known impacts of HIV infection on γδ T cells. One of these studies described a loss of activated Vγ2 cells in patients infected with Mycobacterium tuberculosis and/or HIV [17]. These authors demonstrated a specific depletion of Vγ2+ cells with the CD27–/CD45RA– phenotype and later showed that the frequency of this population could be regulated by HAART [17, 22]. Our data indicate, however, that there is a prevailing loss of the CD27–CD45RA– phenotype in HIV disease, and this loss is not reversed by HAART, despite prolonged control of virus and absence of AIDS symptoms. These reports are consistent in identifying important phenotypic alterations to Vγ2+ cells in HIV-infected individuals but differ in the impact of antiretroviral therapy.
It was proposed that γδ T cells differentiate from naïve (CD27+/CD45RA+) to central memory (CD27+/CD45RA–) to effector memory (CD27–/CD45RA–) to cytotoxic effector populations (CD27–/CD45RA+) [20, 21]. Our studies raise questions about the validity of applying terms such as naïve, central, and effector memory to the circulating Vγ2Vδ2 T cell subsets. Specifically, there are concerns about the existence of truly naïve Vγ2Vδ2 cells in adults, where the mature populations are products of extensive positive selection [2, 3, 14, 45], resulting in a repertoire that is highly skewed toward expression of the Vγ2-Jγ1.2 rearrangement [3]. This skewing moves the distribution of Vγ2 chains from the median 984-nt length found in cord blood (C. Cairo, unpublished data) to the median 993-nt length found in healthy adults (Fig. 5). Further, just three lengths (990, 993, and 996 nt) represent more than 75% of all Vγ2 chains in healthy adults, and nearly all are Vγ2-Jγ1.2 [3, 14]. With a mature repertoire that was skewed by selection of mostly one V–J rearrangement, few cells are likely to be naïve in the sense that they have not seen cognate antigen or undergone cycles of cell division in the periphery.
Our data about other functional markers also raise doubts about the conventional compartment descriptions and how they apply to Vγ2Vδ2 populations. We have shown for control donors that 64.5% of Vγ2Vδ2 cells in the CD27+/CD45RA+ compartment express GrB (Fig. 1B and Supplemental Table 1), which is a marker for cytotoxic potential [30]. The presence of GrB argues that these cells have already been exposed to antigen and have returned to this compartment by regaining expression of CD27, perhaps triggered by a reduction of IL-2 [46]. However, it is important to note that CD45RA and CD27 expression levels are generally low in Vγ2Vδ2 T cells as compared with the remaining T cells that mostly express the α β TCR. We can define double-positive, single-positive, and double-negative subsets based on CD45RA and CD27 expression, but the distinction between γδ compartments is not as evident as with other T cells.
Among HIV+ donors, we observed specific differences in the distribution of phenotypic subsets and in the response to antigen stimulation. Overall, HIV+ donors had lower levels of GrB expression. This may be a result of a repertoire defect: Specific loss of Vγ2-Jγ1.2+ cells [2] renders them less responsive to antigen and less likely to express markers of cytotoxic potential. We noted the low levels of CD27–/CD45RA– cells in PBMC from HIV+ donors compared with the abundance of these cells in control donors. In vitro, it was difficult to produce double-negative cells with HIV+ PBMC. Instead, stimulated Vγ2 cells from HIV+ donors accumulated in the CD45RA single-positive fraction. This functional defect is important for understanding the implication of Vγ2Vδ2 T cell responses to antigen and their potential for tumor surveillance. Cytotoxicity data presented in this study (Fig. 6) demonstrated the failure of HIV+ Vγ2Vδ2 cells to respond to stimuli and lyse Daudi cells in vitro. As a population, Vγ2 cells in HIV patients have reduced function as tumor effectors, even among patients who are receiving antiretroviral therapy with effective control of viremia (Table 1). Although HAART reduces viral loads and restores the CD4 compartment, treatment does not reconstitute the Vγ2Vδ2 population and fails to restore tumor immunity.
We postulated that alterations in the Vγ2 phenotype are consequences of the extensive immune activation that occurs after HIV infection. The abundance of highly activated CD27–/CD45RA+ cells within the Vγ2 compartment and the high frequency of CD95+ cells suggest a broad activation of Vγ2+ cells similar to other lymphocyte subsets [47, 48], and activation-induced cell death is the likely mechanism for depletion. Vγ2Vδ2 T cells are only one subset of lymphocytes that is believed to be impacted by this mechanism of HIV-induced indirect killing. NK [49] and NKT [50] populations are also altered in HIV disease and like γδ cells, are refractory to infection in vitro. The combined effect of losing these vital effector subsets will have a significant impact on the risk for malignancy in HIV disease.
Supplementary Material
Acknowledgments
This work was supported by Public Health Services Grant CA113261 and supplementary funding from the National Cancer Institute and AI051212 from the National Institute of Allergy and Infectious Diseases. We are grateful to Drs. Ferenc Livak and Maria Salvato for critical comments. We also thank Shannon Berg and Rebecca Boyce for help with accumulating clinical specimens, and we are grateful to our patients and staff for participating in this research.
References
- Parker C M, Groh V, Band H, Porcelli S A, Morita C, Fabbi M, Glass D, Strominger J L, Brenner M B. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders P J, Yin C, Martini F, Evans P S, Propp N, Poccia F, Pauza C D. HIV-mediated γδT cell depletion is specific for Vγ2+ cells expressing the Jγ1.2 segment. AIDS Res Hum Retroviruses. 2003;19:21–29. doi: 10.1089/08892220360473934. [DOI] [PubMed] [Google Scholar]
- Evans P S, Enders P J, Yin C, Ruckwardt T J, Malkovsky M, Pauza C D. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1./Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M B, McLean J, Scheft H, Riberdy J, Ang S L, Seidman J G, Devlin P, Krangel M S. Two forms of the T-cell receptor γ protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987;325:689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J Exp Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P, Malkovsky M, Braakman E, Sturm E, Bolhuis R L, Prieve A, Sosman J A, Lam V A, Sondel P M. γ/δ T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171:1567–1579. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C T, Lee H K, Leslie D S, Tanaka Y, Bukowski J F, Marker-Hermann E. Recognition of nonpeptide prenyl pyrophosphate antigens by human γδ T cells. Microbes Infect. 1999;1:175–186. [PubMed] [Google Scholar]
- Chen Z W, Letvin N L. Adaptive immune response of Vγ2Vδ2 T cells: a new paradigm. Trends Immunol. 2003;24:213–219. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges J F, Buckner D L, Rask K M, Kerns H M, Jackiw L O, Trunkle T C, Pascual D W, Jutila M A. Mucosal lymphatic-derived γδ T cells respond early to experimental Salmonella enterocolitis by increasing expression of IL-2R α. Cell Immunol. 2007;246:8–16. doi: 10.1016/j.cellimm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Das H, Kamath A, Bukowski J F. Human V γ 2V δ 2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi M R. Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14–18. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier J F, Scotet E, Bonneville M, Jotereau F. V γ 9V δ 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- Burns J, Lobo S, Bartholomew B. Requirement for CD4+ T cells in the γδ T cell proliferative response to Daudi Burkitt’s lymphoma. Cell Immunol. 1996;174:19–24. doi: 10.1006/cimm.1996.0289. [DOI] [PubMed] [Google Scholar]
- Hebbeler A M, Cairo C, Cummings J S, Pauza C D. Individual Vγ2-Jγ1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56:819–829. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard H, Al Saati T, Delsol G, Fournie J J. Synthetic phosphoantigens enhance human Vγ9Vδ2 T lymphocytes killing of non-Hodgkin’s B lymphoma. Mol Med. 2001;7:711–722. [PMC free article] [PubMed] [Google Scholar]
- Gan Y H, Pauza C D, Malkovsky M. γ δ T cells in rhesus monkeys and their response to simian immunodeficiency virus (SIV) infection. Clin Exp Immunol. 1995;102:251–255. doi: 10.1111/j.1365-2249.1995.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia C, Agrati C, Casetti R, Cairo C, Borsellino G, Battistini L, Mancino G, Goletti D, Colizzi V, Pucillo L P, Poccia F. Lack of CD27–CD45RA–V γ 9V δ 2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168:1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- Poccia F, Boullier S, Lecoeur H, Cochet M, Poquet Y, Colizzi V, Fournie J J, Gougeon M L. Peripheral V γ 9/V δ 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449–461. [PubMed] [Google Scholar]
- Wallace M, Scharko A M, Pauza C D, Fisch P, Imaoka K, Kawabata S, Fujihashi K, Kiyono H, Tanaka Y, Bloom B R, Malkovsky M. Functional γ δ T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med. 1997;3:60–71. [PMC free article] [PubMed] [Google Scholar]
- Angelini D F, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, Poccia F, Fournie J J, Battistini L. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–1807. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini F, Poccia F, Goletti D, Carrara S, Vincenti D, D'Offizi G, Agrati C, Ippolito G, Colizzi V, Pucillo L P, Montesano C. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vγ9Vδ2 T cells in chronically infected patients undergoing structured treatment interruption. J Infect Dis. 2002;186:847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vγ9/Vδ2 T cells. J Leukoc Biol. 2006;79:663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- Sicard H, Ingoure S, Luciani B, Serraz C, Fournie J J, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of V γ 9V δ 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- Kobata T, Agematsu K, Kameoka J, Schlossman S F, Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- Holmes N. CD45: all is not yet crystal clear. Immunology. 2006;117:145–155. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S C, Mitra D K, Watanabe N, Herzenberg L A, Herzenberg L A, Roederer M. Vδ1 and Vδ2 γδ T cells express distinct surface markers and might be developmentally distinct lineages. J Leukoc Biol. 2001;70:518–526. [PubMed] [Google Scholar]
- Alimonti J B, Shi L, Baijal P K, Greenberg A H. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J Biol Chem. 2001;276:6974–6982. doi: 10.1074/jbc.M008444200. [DOI] [PubMed] [Google Scholar]
- Kim W J, Kim H, Suk K, Lee W H. Macrophages express Granzyme B in the lesion areas of atherosclerosis and rheumatoid arthritis. Immunol Lett. 2007;111:57–65. doi: 10.1016/j.imlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Pallikkuth S, Wanchu A, Bhatnagar A, Sachdeva R K, Sharma M. Human immunodeficiency virus (HIV) gag antigen-specific T-helper and granule-dependent CD8 T-cell activities in exposed but uninfected heterosexual partners of HIV type 1-infected individuals in North India. Clin Vaccine Immunol. 2007;14:1196–1202. doi: 10.1128/CVI.00488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M S, Yin C C, Yagita H, Maeda T, Okumura K, Tikhonov I, Pauza C D. Attenuated disease in SIV-infected macaques treated with a monoclonal antibody against FasL. Clin Dev Immunol. 2007;2007:93462. doi: 10.1155/2007/93462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensussan A, Lagabrielle J F, Castaigne S, Boisson N, Miclea J M, Benbunan M, Degos L. Human CD3 γ δ + activated lymphocytes exhibit killer activity in vitro against autologous leukemic cells. Nouv Rev Fr Hematol. 1989;31:129–132. [PubMed] [Google Scholar]
- Li H, Deetz C O, Zapata J C, Cairo C, Hebbeler A M, Propp N, Salvato M S, Shao Y, Pauza C D. Vaccinia virus inhibits T cell receptor-dependent responses by human γδ T cells. J Infect Dis. 2007;195:37–45. doi: 10.1086/509823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggetta M P, Lanzilli G, Cottarelli A, Ravagnan G, Carteni M, De Maria S, Metafora B M, Metafora V, Metafora S. Anti-apoptotic seminal vesicle protein IV inhibits cell-mediated immunity. J Reprod Immunol. 2008 doi: 10.1016/j.jri.2007.11.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Patarroyo M, Blazar B, Pearson G, Klein E, Klein G. Induction of the EBV cycle in B-lymphocyte-derived lines is accompanied by increased natural killer (NK) sensitivity and the expression of EBV-related antigen(s) detected by the ADCC reaction. Int J Cancer. 1980;26:365–371. doi: 10.1002/ijc.2910260317. [DOI] [PubMed] [Google Scholar]
- Biggar R J, Chaturvedi A K, Goedert J J, Engels E A. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A K, Pfeiffer R M, Chang L, Goedert J J, Biggar R J, Engels E A. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- Goedert J J, Cote T R, Virgo P, Scoppa S M, Kingma D W, Gail M H, Jaffe E S, Biggar R J. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- Rabkin C S, Goedert J J, Biggar R J, Yellin F, Blattner W A. Kaposi’s sarcoma in three HIV-1-infected cohorts. J Acquir Immune Defic Syndr. 1990;3:S38–S43. [PubMed] [Google Scholar]
- Yang Y, Dong B, Mittelstadt P R, Xiao H, Ashwell J D. HIV Tat binds Egr proteins and enhances Egr-dependent transactivation of the Fas ligand promoter. J Biol Chem. 2002;277:19482–19487. doi: 10.1074/jbc.M201687200. [DOI] [PubMed] [Google Scholar]
- Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor γ/δ+ lymphocyte subsets during HIV infection. Clin Exp Immunol. 1989;75:206–210. [PMC free article] [PubMed] [Google Scholar]
- Malkovsky M, Fisch P, Mackenzie D, Bartz S R, Radtke B E, Wallace M, Manning J, Colizzi V, Pauza C D. Specificity and function of γ δ T lymphocytes. Folia Biol (Praha) 1992;38:293–306. [PubMed] [Google Scholar]
- Hayday A C. [γ][δ] Cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Huang J, Kerstann K W, Ahmadzadeh M, Li Y F, El-Gamil M, Rosenberg S A, Robbins P F. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenberg N, Perelson A S, Yerly S, Schockmel G A, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia B, Jennings C, Cauda R, Ortona L, Landay A L. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry. 1995;22:10–15. doi: 10.1002/cyto.990220103. [DOI] [PubMed] [Google Scholar]
- Sandberg J K, Fast N M, Palacios E H, Fennelly G, Dobroszycki J, Palumbo P, Wiznia A, Grant R M, Bhardwaj N, Rosenberg M G, Nixon D F. Selective loss of innate CD4(+) V α 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–7534. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.