Abstract
Pertussis, an acute respiratory infection caused by Bordetella pertussis, classically manifests as a protracted cough illness. The incidence of pertussis in the United States has been increasing in recent years. Immunity wanes after childhood vaccination, leaving adolescents and adults susceptible to infection. The transmission of pertussis in health care settings has important medical and economic consequences. Acellular pertussis booster vaccines are now available for use and have been recommended for all adolescents and adults. These vaccines are safe, immunogenic, and effective. Health care workers are a priority group for vaccination because of their increased risk of acquiring infection and the potential to transmit pertussis to high-risk patients. Health care worker vaccination programs are likely to be cost-effective, but further research is needed to determine the acceptability of pertussis vaccines among health care workers, the duration of immunity after booster doses, and the impact of vaccination on the management of pertussis exposures in health care settings.
INTRODUCTION
Pertussis is an acute respiratory infection that is characterized by prolonged cough illness, with significant associated morbidity and mortality. The causative agent, Bordetella pertussis, was first isolated in culture by Jules Bordet and Octave Gengou in 1906. Although pertussis vaccines have been administered routinely to children since the 1940s, immunity wanes over time and adults remain susceptible to disease. Health care workers are at particular risk of acquiring pertussis and may transmit the infection to high-risk susceptible patients and colleagues. A safe and effective pertussis booster vaccine for adolescents and adults is now available and has been recommended for routine use. Because of the importance of the transmission of pertussis in health care settings, vaccination of health care workers is a priority.
MICROBIOLOGY
Pertussis (whooping cough) is an acute respiratory infection caused primarily by Bordetella pertussis, although Bordetella parapertussis accounts for a small proportion of cases in the United States (31). B. pertussis is a gram-negative aerobic coccobacillus that has a tropism for respiratory epithelial cells. Several adhesion proteins, including filamentous hemagglutinin and pertactin, facilitate the binding of B. pertussis to ciliated epithelial cells (54). The organism produces several toxins that contribute to pathogenesis. Pertussis toxin, which is believed to be an important virulence factor for human disease, is a classic A:B-structure toxin that acts on G-protein-coupled adenylate cyclase pathways; the resultant increase in cyclic AMP impairs phagocyte function and increases respiratory secretions (54). The organism also produces several other toxins, including tracheal cytotoxin, which damages cells of the respiratory epithelium (54). B. pertussis has fastidious growth requirements and can be cultured using selective media, including Bordet-Gengou or Regan-Lowe agar.
EPIDEMIOLOGY
Pertussis is an endemic disease that occurs year-round, with epidemic cycles every 3 or 4 years. The incidence of reported pertussis in the United States has been steadily increasing over the past 2 decades (10, 13d, 21, 25, 95). This trend is occurring despite the fact that childhood vaccination rates are higher than they have ever been and that vaccine efficacy remains high (9, 13a). Some of this increase in disease may be due to improved diagnostic techniques and increased awareness of this infection (46, 53, 56, 74, 93, 95). However, several studies have suggested that immunity after vaccination wanes over time and protection may last only 10 to 15 years, leading to a growing population of susceptible adolescents and adults (35, 38, 77). Pertussis cases in adolescents and adults now account for the bulk of the recent increase in the United States, with more than half of reported cases now occurring in these age groups (13d).
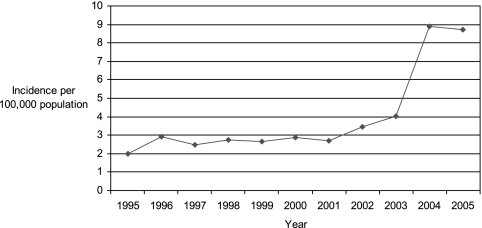
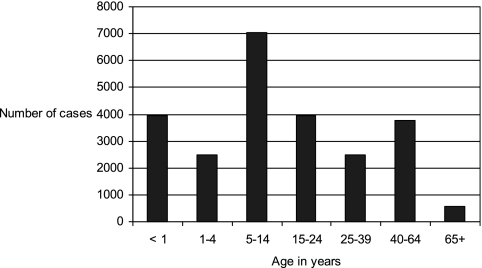
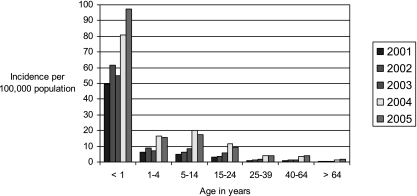
State health departments report probable and confirmed cases of pertussis to the Centers for Disease Control and Prevention (CDC). During 1997 to 2000, a total of 29,134 pertussis cases were reported to CDC, yielding an average annual incidence rate of 2.7 cases per 100,000 population. Although incidence rates remained highest among infants of <1 year of age (55.5 cases per 100,000), it was notable that 29% of the reported cases occurred in people 10 to 19 years of age and another 20% occurred in those 20 years of age and older (13d). Compared with surveillance data from 1994 to 1996, the incidence rate increased 62% among adolescents and 60% among adults (13d, 25). Figure 1 displays the annual incidence of pertussis in the United States during the years 1995 to 2005. In 2005, 60% of reported pertussis cases occurred among adolescents 10 to 18 years of age and adults aged ≥20 years (Fig. 2) (51). The change in incidence among specific age groups from 2001 to 2005 is displayed in Fig. 3.
FIG. 1.
U.S. pertussis incidence by year, 1995 to 2005. (Based on data from the CDC National Notifiable Diseases Surveillance System [http://www.cdc.gov/mmwr/summary.html].)
FIG. 2.
Numbers of reported pertussis cases by age, 2005. Of 25,616 reported cases in 2005, 15,354 occurred in persons of ≥10 years of age. (Based on data from the CDC National Notifiable Diseases Surveillance System [http://www.cdc.gov/mmwr/summary.html].)
FIG. 3.
U.S. pertussis incidence by age, 2001 to 2005. (Based on data from the CDC National Notifiable Diseases Surveillance System [http://www.cdc.gov/mmwr/summary.html].)
The increase in incidence rates of pertussis among adults has direct relevance to the epidemiology of pertussis in health care workers. In 1992, Wright et al. performed pertussis serology on a convenience sample of 73 emergency department employees and found that despite nearly universal childhood immunization, most employees had low antibody titers, placing them at risk of acquiring infection and subsequently transmitting pertussis to susceptible patients (94). Among health care workers, annual incidence rates of pertussis were 1.3% (95% confidence interval [CI], 0 to 3.5%) among 106 resident physicians and 3.6% (95% CI, 0 to 9.6%) among 39 emergency department nurses and physicians (92). Another study of 51 health care workers found that 90% of the subjects had an increase in antibodies to one or more pertussis antigens during a 5-year period (19).
TRANSMISSION
Pertussis is transmitted by large droplets that are produced during coughing, sneezing, or talking. These droplets generally travel less than 3 feet and can be deposited on mucosal surfaces of susceptible individuals. The attack rate for pertussis among close contacts of individuals with disease varies depending on the number and ages of contacts but is as high as 80 to 100% in some household contact studies (8, 36, 45, 73, 90). In a study by Bisgard et al., family members or relatives were the source of pertussis for 75% of infants 0 to 3 months of age and for 73% of infants 4 to 11 months of age; other sources for infant cases included child care contacts, neighbors, friends, or others (8). Mothers were a particularly common source of infection in this study, a finding that has been demonstrated in other studies as well (6, 16, 34). Adolescents are often infected by their peers; in one study of 664 adolescents and adults, schoolmates and friends were the source for 57% of the adolescent cases (18). In the same study, sources of infection for adult cases of pertussis included work colleagues (32%), relatives (14%), and friends (6%) (18). Children can also be a source of infection for susceptible adults (91). Importantly, health care workers may be a source of infection for infants. Several studies of pertussis outbreaks have documented that infants can acquire pertussis after exposure to infected health care personnel (23, 37, 44, 57).
In the health care setting, droplet precautions should be implemented for patients with confirmed or suspected pertussis (76). These patients should be placed in a single room whenever feasible; if single rooms are not available, a spatial separation of ≥3 feet between beds should be maintained and the curtain should be drawn. Health care providers should wear a surgical mask (respirators are not required) when having close contact with the patient (within 3 feet). Droplet precautions should be maintained until the patient has received 5 days of effective antibiotic therapy (76).
CLINICAL MANIFESTATIONS
Pertussis infection classically manifests in three clinical phases. After an incubation period of 5 to 21 days, the catarrhal phase occurs first and includes mild nonspecific upper respiratory tract symptoms that are similar to a common cold. The second stage is characterized by cough, which is typically paroxysmal and may be accompanied by an inspiratory whoop or posttussive emesis. This stage may persist for weeks to months. Eventually patients enter the convalescent phase and the symptoms gradually resolve. Pertussis is referred to in some countries as the “100-day cough” because of the extended duration of the cough illness. Pertussis infection in infants may be associated with seizures, encephalopathy, apnea, and even death.
Protracted cough illness is common in adults with pertussis; in one study, 61% of adults were still coughing at an average of 94 days after cough onset (40). In another study, the mean duration of cough in adults was 12 weeks, and 55% coughed for more than 9 weeks (18). Among 664 adolescents and adults in that study, paroxysms of cough were present in 99% of cases, and other classic symptoms reported included apnea (87%), whoop (69%), and posttussive emesis (65%). Another study found that among 936 adults with pertussis, paroxysms (86%), vomiting (47%), apnea (44%), and whoop (41%) were common (40). Pertussis also may be associated with several important complications. In one study, sinusitis was the most common complication and occurred in 13% of patients with pertussis; other complications included otitis media (4%), urinary incontinence (4%), secondary bacterial pneumonia (4%), weight loss (3%), rib fracture (2%), and syncope (2%) (18). Similar complications were described by adults in another study, including difficulty sleeping (84%), weight loss (33%), urinary incontinence (28%), loss of consciousness (6%), and rib fracture (4%) (40). The morbidity associated with pertussis in adults can be severe and its economic impact quite substantial, with significant time missed from work for these individuals (18, 40, 67, 81, 95).
DIAGNOSIS AND TREATMENT
The Council of State and Territorial Epidemiologists has established a case definition for pertussis. A clinical case is defined as a cough illness lasting for ≥2 weeks with at least one of the following: paroxysms of coughing, inspiratory whoop, or posttussive vomiting without other apparent cause. A case is classified as confirmed if it includes an acute cough illness of any duration that is confirmed by culture or if it meets the clinical case definition and is either laboratory confirmed by PCR or epidemiologically linked to a laboratory-confirmed case. A case is classified as probable if it meets the clinical case definition but without laboratory confirmation or epidemiologic link to a laboratory-confirmed case.
The diagnosis of pertussis can be made in the laboratory by several methods. The gold standard for diagnosis is isolation of B. pertussis in culture of a nasopharyngeal aspirate or swab. Several factors may decrease the sensitivity of culture, including delays in specimen collection after onset of illness and receipt of antibiotic therapy (26, 78). PCR tests are rapid and sensitive, and the percentage of reported cases confirmed by PCR increased from 12% in 1997 to 44% in 2005 (13b). There is no standardization of PCR protocols, and wide variation exists among different laboratories (13b, 20). While the specificity of PCR testing is high, false-positive results can occur for several reasons, including DNA contamination or reliance on primers that are shared by other Bordetella species (13b, 20, 43, 69). For patients who present more than 2 weeks after cough onset, single-serum serology is another method to diagnose pertussis. Pertussis toxin is specific to B. pertussis, and in a Massachusetts study, a single antipertussis toxin antibody level above 20 μg/ml was found to be 63% sensitive (95% CI, 51 to 75%) in detecting bacteriologically confirmed cases of pertussis in persons of ≥11 years of age (46). The authors considered this serologic test to be a useful adjunct for diagnosis, particularly in cases where patients present late in the course of illness. The Massachusetts Department of Public Health State Laboratory Institute performs an antipertussis toxin immunoglobulin G test for diagnosis for patients of ≥11 years of age, but no other state health departments currently offer this test. Pertussis serology using commercially available reagents is not validated, and the results may be difficult to interpret (13b). No serologic assay is licensed for routine diagnostic testing in the United States, and CDC guidelines for laboratory confirmation of pertussis do not include the use of serology (36).
Treatment of pertussis early in the course of illness can reduce the duration and severity of symptoms as well as reduce the period of communicability of the disease (11, 82). Macrolide antibiotics (erythromycin, azithromycin, and clarithromycin) are recommended as the agents of choice for treating pertussis. Erythromycin use is associated with infantile hypertrophic pyloric stenosis in infants (33), and it is not known whether clarithromycin is safe for infants. Therefore, in infants of less than 1 month of age, azithromycin is the preferred choice. For those over 1 month of age, azithromycin and clarithromycin have fewer side effects and are generally better tolerated than erythromycin (particularly with regards to gastrointestinal upset). Multiple studies suggest that these agents are as effective as erythromycin for the treatment of pertussis (3, 4, 39, 66). The dose of azithromycin for infants of <1 month of age is 10 mg/kg of body weight daily for all 5 days; for persons of 1 month of age and older, the dose is 10 mg/kg (up to 500 mg) on day 1 followed by 5 mg/kg (up to 250 mg) daily on days 2 to 5. Individuals over 2 months of age who cannot receive a macrolide may be treated with a 14-day course of trimethoprim-sulfamethoxazole (82).
IMPACT OF PERTUSSIS IN HEALTH CARE SETTINGS
Because pertussis is often considered an illness of childhood, symptoms in adults may not be recognized as pertussis for days or weeks, resulting in transmission to other individuals both in the community and in health care settings. A study of university students found that the median duration of cough prior to diagnosis was 21 days among those with pertussis (53). Another study found that the average duration of illness before diagnosis among 14 adult cases was 12 days, and it was not uncommon for patients to have several physician visits before the diagnosis was made (62). In a study of health care workers, unrecognized pertussis infections (diagnosed by serology) were common, with an average annual rate of infection of 33% (19). The combination of waning immunity in adults who are health care workers and frequent exposure occurring in pediatric health care can lead to outbreaks that result in significant morbidity and cost.
Several large outbreaks of pertussis occurring in health care settings have been described (15, 37, 75). The index case may be a patient (30, 49), a health care worker (7, 22, 24, 44, 50, 80), or a visitor (79, 85). Subsequent exposures may result in transmission to a large number of health care workers and patients (22, 48). In one of the largest reported outbreaks during a community-wide epidemic of pertussis in Cincinnati in 1993, 206 hospital employees with respiratory disease were evaluated and 87 of them met the clinical or laboratory criteria for pertussis (15). Seventy-nine of these employees required 5-day furloughs while receiving antibiotic treatment, and a total of 622 employees received antibiotic prophylaxis or therapy based on exposures or disease. In one smaller reported outbreak, a single infected health care worker transmitted pertussis to several patients (one of whom later transmitted the infection to a different staff member), a ward clerk, and his wife (37). These outbreaks have demonstrated the relative ease with which pertussis cases can be amplified within the health care setting.
When pertussis exposures occur in health care institutions, proper management is crucial to preventing the transmission of infection. Patients or health care workers are considered to have been exposed if they have face-to-face exposure within 3 feet of a symptomatic case of pertussis (unless they were wearing a surgical mask at the time of the exposure); close contacts can also include those who have direct contact with the secretions of an infected source or those who share confined space with a symptomatic case for at least 1 h (82). The role of contaminated environmental surfaces in transmission is not clear. When it is determined that an exposure has occurred, the exposed individual(s) should receive antimicrobial prophylaxis in order to reduce the likelihood of symptomatic infection. Macrolides (such as azithromycin) are recommended as the first-line agents for postexposure prophylaxis, although trimethoprim-sulfamethoxazole is an acceptable alternative in cases of macrolide allergy (82). For adults, the recommended course of azithromycin is 5 days at a dose of 500 mg on day 1 and 250 mg per day on days 2 to 5. For health care workers who have been exposed, if the individual is within the incubation period of 5 to 21 days and has symptoms consistent with pertussis at the time the exposure is recognized, he or she should be treated as a case and furloughed from work until the completion of 5 days of azithromycin therapy.
ECONOMIC CONSEQUENCES OF PERTUSSIS IN HEALTH CARE SETTINGS
Pertussis cases and exposures can result in substantial medical costs (physician visits, emergency department visits, hospitalizations, diagnostic tests, prescription medications) and nonmedical costs (time lost from work, transportation, over-the-counter medications) for individuals. The societal cost of pertussis in adults is estimated to be $773 per case (40). Other studies have estimated societal costs of pertussis as high as $2,115 to $3,561 per case (42, 62), although these studies included children, who may suffer more-severe complications of pertussis.
Outbreaks of pertussis in health care institutions can be even more costly. In two hospital outbreaks in the state of Washington, the cost per case of pertussis from the hospital perspective ranged from $30,282 to $43,893 (5). The types of expenses incurred when pertussis outbreaks occur in health care settings include diagnostic testing, provision of antibiotic treatment or prophylaxis, costs associated with furlough of employees, and time spent by occupational health and infection control staff to track and identify exposed individuals, as well as costs associated with dissemination of information (such as mailing letters to exposed patients or families) (5, 13, 17, 88, 96).
The introduction of a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine for adults has the potential to directly impact the management of pertussis exposures in hospitals. In addition to decreasing the overall disease burden from pertussis, vaccination of health care workers may reduce the number of secondary cases and the labor, costs, and time associated with exposure management.
PERTUSSIS VACCINES
Pertussis-containing vaccines have been included in the routine childhood vaccination schedule in the United States since the 1940s. The current vaccine for young children is a combination of antigens of diphtheria, tetanus, and acellular pertussis (DTaP). Universal immunization with this vaccine is recommended for children under 7 years of age and is typically delivered as a five-dose series (2, 4, and 6 months of age, with boosters at 15 to 18 months and 4 to 6 years) (13c). The pertussis component of this vaccine always includes inactivated pertussis toxin (toxoid) as well as at least one other immunogen (such as filamentous hemagglutinin, pertactin, or fimbria types 2 and 3) derived from B. pertussis (1). The efficacies of different licensed DTaP vaccines are similar despite variation in specific components. The most common adverse events associated with DTaP vaccines include fever and local reactions such as erythema or swelling at the injection site (13f). Rarely, other more serious reactions may occur, including allergic reactions, seizures, hypotonic-hyporesponsive episodes, or prolonged crying. The frequency of adverse events has decreased since the introduction of acellular pertussis vaccines, which were licensed in the United States in 1991 and are less reactogenic than the whole-cell DTP vaccine used previously (1, 63).
Acellular pertussis boosters specifically formulated for adolescents and adults are now available for use in the United States. These combination Tdap vaccines include tetanus, diphtheria, and acellular pertussis, with reduced quantities of diphtheria and pertussis components compared with DTaP vaccines (Table 1). Currently, two Tdap products are licensed for use in the United States: Boostrix (GlaxoSmithKline Biologicals, Rixensart, Belgium), which is licensed only for adolescents aged 10 to 18 years, and Adacel (sanofi pasteur, Toronto, Ontario, Canada), which is licensed for adolescents and adults aged from 11 to 64 years. The Advisory Committee on Immunization Practices (ACIP), the national decision-making body for vaccine use in the United States, recommended in June 2005 that all adolescents routinely receive a single dose of Tdap to replace the next booster dose of Td (tetanus and diphtheria without a pertussis component) in order to prevent pertussis disease (12). In October 2005, the same recommendation was made for all adults (36). In addition, in February 2006, ACIP recommended Tdap for health care personnel as a priority group. They also suggested that hospital and ambulatory care facilities use approaches to optimize the uptake of vaccine such as education, convenient access, and provision of Tdap at no charge (36).
TABLE 1.
Composition per dose of selected vaccines containing pertussis componentsa
| Vaccine | Trade name | Manufacturer | Amt (μg) of indicated pertussis antigenc
|
Lf value ford:
|
||||
|---|---|---|---|---|---|---|---|---|
| PT | FHA | PRN | FIM | Diphtheria toxoid | Tetanus toxoid | |||
| DTaP | Infanrix | GSKb | 25 | 25 | 8 | 25 | 10 | |
| DTaP | Daptacel | sanofi pasteur | 10 | 5 | 3 | 5 | 15 | 5 |
| Tdap | Boostrix | GSK | 8 | 8 | 2.5 | 2.5 | 5 | |
| Tdap | Adacel | sanofi pasteur | 2.5 | 5 | 3 | 5 | 2 | 5 |
Adapted from reference 12.
GSK, GlaxoSmithKline Biologicals.
PT, pertussis toxin; FHA, filamentous hemagglutinin; PRN, pertactin; FIM, fimbriae.
Lf, limit of flocculation unit.
Several clinical trials have shown that Tdap vaccines are safe, immunogenic, and efficacious against cough illness in adolescents and adults (14, 27, 28, 64, 65, 82a, 83, 86, 89). In a randomized controlled trial with 4,480 participants of 11 to 64 years of age, Tdap vaccine produced high pertussis antibody concentrations which exceeded the titers measured after the vaccination of infants with the analogous DTaP vaccine (64). In another randomized controlled trial to assess the clinical efficacy of Tdap vaccine, 2,781 participants of 15 to 65 years of age were enrolled and followed for up to 2.5 years after receiving vaccine or placebo. Ten cases of pertussis that met the case definition occurred during the study; nine of these were in the control group and one occurred in a vaccine recipient, yielding an overall vaccine efficacy of 92% (95% CI, 32 to 99%) (89).
Serious adverse events after Tdap vaccine were uncommon in prelicensure studies (64, 89). The most common events were local reactions, including pain at the injection site (in up to 77% of recipients) and erythema or swelling (in approximately 20%). The most common systemic adverse event was headache, occurring in 20 to 30% of recipients, but other severe events were rare and occurred at a frequency similar to that seen for Td recipients. One particular adverse event of interest, entire-limb swelling, has occurred rarely after booster doses of DTaP (71, 72), and concerns have been raised that booster doses of Tdap at short intervals after Td might increase the frequency of this event. No cases of entire-limb swelling were reported for 3,017 Tdap recipients in a prelicensure reactogenicity study (64). Table 2 displays a summary of adverse events data from prelicensure Tdap studies.
TABLE 2.
Adverse events after vaccination with Adacela
| Adverse event | Intensity | Frequency after Adacel (%) |
|---|---|---|
| Pain at injection site | Any | 65.7 |
| Moderate | 15.1 | |
| Severe | 1.1 | |
| Erythema at injection site | Any | 24.7 |
| Moderate | 8.0 | |
| Severe | 6.2 | |
| Swelling at injection site | Any | 21.0 |
| Moderate | 7.6 | |
| Severe | 5.8 | |
| Axillary node swelling | Any | 6.5 |
| Moderate | 1.2 | |
| Severe | 0.1 | |
| Fever of ≥38°C | Any | 1.4 |
| Moderate | 0.4 | |
| Severe | 0 | |
| Chills | Any | 8.1 |
| Moderate | 1.3 | |
| Severe | 0.7 | |
| Nausea | Any | 9.2 |
| Moderate | 2.5 | |
| Severe | 0.8 | |
| Vomiting | Any | 3.0 |
| Moderate | 1.0 | |
| Severe | 0.5 | |
| Diarrhea | Any | 10.3 |
| Moderate | 2.2 | |
| Severe | 0.5 | |
| Headache | Any | 33.9 |
| Moderate | 11.4 | |
| Severe | 2.8 | |
| Generalized body ache/myalgias | Any | 21.9 |
| Moderate | 6.1 | |
| Severe | 1.2 | |
| Fatigue | Any | 24.3 |
| Moderate | 6.9 | |
| Severe | 1.3 | |
| Arthralgia/swollen joints | Any | 9.1 |
| Moderate | 2.5 | |
| Severe | 0.5 | |
| Rash | Any | 2.0 |
Adapted from reference 36.
Pregnancy is not a contraindication for use of Tdap. Pregnant women were excluded from prelicensure vaccine trials, and safety data are not available. The American Academy of Pediatrics (AAP) recommends that pregnant adolescents be given the same consideration for immunization as adolescents who are not pregnant (2). ACIP has recommended that pregnant women who previously have not received a dose of Tdap should be vaccinated in the immediate postpartum period (13e). However, in situations where pregnant women are at high risk for acquiring pertussis or transmitting pertussis to vulnerable individuals, providers may choose to administer Tdap during pregnancy. Both AAP and ACIP recommend that when Tdap is administered during pregnancy, the second or third trimester is preferred when feasible (2, 13e). Both Tdap manufacturers (GlaxoSmithKline and sanofi pasteur) have established registries for women who receive Tdap during pregnancy, and providers are encouraged to report to these registries when such instances occur. Tdap may also be given to women who are breastfeeding or plan to breastfeed.
ACIP has recommended that adolescents 11 to 18 years of age and adults 19 to 64 years of age receive a single dose of Tdap (0.5 ml administered intramuscularly) to replace a single dose of Td if it has been ≥10 years since their last tetanus booster (or ≥5 years if Tdap is being administered for wound prophylaxis). Shorter intervals (down to 2 years after Td) are likely to be safe based on data from a Canadian study (29). Because health care personnel are at higher risk of acquiring pertussis infection and may transmit infection to high-risk patients, ACIP has recommended that Tdap be administered for this group at an interval as short as 2 years after Td. This recommendation applies to health care personnel who have direct patient contact, which includes (but is not limited to) physicians and other primary care providers, nurses, aides, respiratory therapists, radiology technicians, students (e.g., medical, nursing, and other), dentists, social workers, chaplains, volunteers, and dietary and clerical workers (36).
HEALTH CARE WORKER VACCINATION PROGRAMS
Providing Tdap vaccine for health care workers is one method to reduce the morbidity associated with the transmission of pertussis in health care settings. Such vaccination programs are expected to be costly from the hospital perspective. The cost of the vaccine at our institution in 2007 was $34 per dose (Children's Hospital Boston pharmacy, written communication, November 2007). In addition to direct vaccine costs, occupational health services would require staffing resources to deliver vaccines to employees. Any vaccine-related adverse events that result in staff missing work would also have an associated cost for the institution.
Given the significant costs associated with vaccine purchase and administration, cost-effectiveness analyses are useful to guide decisions about vaccination programs for health care workers. Routine vaccination of adults with Tdap was found to be cost-effective in a previously published model (41). Furthermore, a model that focused exclusively on vaccinating health care workers demonstrated that the hospital would save $2.38 in net return for every dollar invested in a Tdap vaccination program (13). These studies suggest that pertussis vaccination campaigns for health care workers are likely to be cost-effective from the health care system and societal perspectives.
Although cost is an important factor in the development of vaccination programs for health care workers, other variables merit consideration as well. The acceptability of pertussis booster vaccines among health care workers has not been well studied. Influenza vaccine programs have demonstrated that medical personnel often have misconceptions regarding vaccines (32, 47, 70), and coverage rates remain low despite education and encouragement (87). It is not clear that health care workers will be any more willing to undergo pertussis vaccination, and the perceived obstacles to a successful pertussis vaccination program need to be identified. Issues of vaccine safety are also likely to be an important factor in the ultimate acceptability of pertussis vaccination campaigns for health care workers, and further data regarding the safety of Tdap in adults are needed, particularly in regards to short intervals of administration after Td.
Our institution employed several strategies to vaccinate health care workers. After institution-wide education and dissemination of the new ACIP recommendations for vaccination, we began by targeting “high-risk” providers who were likely to have contact with pertussis cases (such as staff from the emergency department, the infectious disease and pulmonary services, and radiology). Occupational health services staff members traveled to each of these locations and administered Tdap vaccines on site during designated time periods. We then held a publicized Tdap vaccine clinic in a centralized location in the main hospital on 5 consecutive days, during which all employees could receive the vaccine. Subsequently, vaccine continues to be available to all hospital staff free of charge in our occupational health services clinic, and Tdap is actively encouraged for unvaccinated employees who are seen there during unrelated visits as well.
UNANSWERED QUESTIONS
Further research is needed to answer a number of challenging questions regarding Tdap vaccination of health care workers. First, since the vaccine is approved only for adults up to age 64, should older health care workers be vaccinated? As the cohort of baby boomers ages, it is likely that the number of health care workers of 65 years of age and older will increase. Prelicensure trials did not address the safety or efficacy of vaccine in this cohort of the population. Although it is likely that the benefits of vaccination will still outweigh the risks among those over 65, the absence of data makes it difficult for health care institutions to address concerns for these employees.
Second, the duration of immunity to pertussis after a single dose of Tdap and the potential need for additional booster doses is currently unknown. Further research to address this issue will be crucial, especially given the increased and ongoing risk of exposure to pertussis in the health care setting as well as the potential safety concerns associated with multiple doses of vaccine components over the course of a lifetime.
Another dilemma created by introduction of Tdap vaccine relates to diagnostic testing for pertussis. Culture and PCR tests are most sensitive when performed within 14 days after cough onset (although nucleic acid from the organism may be detectable by PCR for an additional 1 to 2 weeks). For patients who present late in the course of their clinical symptoms (i.e., more than 2 weeks after cough onset), serologic testing is often the best way to make the diagnosis. Detection of serum immunoglobulin G to pertussis toxin is the diagnostic test of choice in this setting for adults (although, as previously described, Massachusetts is the only state that performs single-serum serology outside of commercial labs, and only in patients ≥11 years of age). According to the Massachusetts Department of Public Health, serology for pertussis is uninterpretable for persons who have received Tdap vaccine within the prior 3 years (48a), leaving no reliable method for making the diagnosis in vaccinated individuals who present late in the course of their illness. A diagnostic method that could reliably diagnose pertussis in recent vaccine recipients would benefit infection control or public health staff who need to decide whether to initiate contact tracing in order to identify other cases or make decisions about postexposure prophylaxis for exposed individuals.
Perhaps the most important unresolved issue regarding the use of Tdap vaccine in health care settings is whether vaccinated health care workers should still receive postexposure prophylaxis when exposed to a case of pertussis. Currently, CDC recommends that health care institutions continue postexposure prophylaxis for vaccinated health care workers who have unprotected exposure to pertussis (36). The stated rationale for this recommendation is that more research is necessary to determine the effectiveness of Tdap in preventing pertussis infection in health care workers and its effectiveness in preventing transmission of pertussis from vaccinated health care workers to patients. However, one of the primary benefits of widespread health care worker vaccination would be to eliminate the need for postexposure prophylaxis or other control interventions, which are associated with significant costs and resource utilization. In recognition of this issue, CDC also advised that health care facilities could consider an alternative management strategy for exposed vaccinated health care workers. This strategy might consist of daily monitoring of exposed health care workers for early signs and symptoms of pertussis, with prompt treatment and furlough if such symptoms do develop. Because there is minimal risk of transmission before the development of symptoms, and because the incubation period extends only to 21 days, this strategy might be feasible in certain institutions with the appropriate infrastructure and resources and particularly in settings where the patient population is at lower risk of severe disease if transmission were to occur. However, if research confirms that the vaccine is highly effective in preventing the infection of health care workers and transmission to patients, then the potential to avoid postexposure prophylaxis and other control measures for vaccinated exposed individuals would be highly valued.
CONCLUSIONS
Pertussis remains an important cause of morbidity and mortality, and its incidence is rising among adolescents and adults. Tdap vaccine is generally safe and effective, and its availability as a booster against pertussis for health care workers may be the most effective way to reduce the risk of pertussis infection and transmission in health care settings. Studies suggest that health care worker vaccination campaigns are likely to be cost-effective, but further research is required to clarify questions regarding the duration of immunity and the impact of health care worker vaccination on the management of pertussis exposures. Despite these gaps in knowledge, it is expected that the widespread vaccination of health care workers with Tdap will provide important protection not only to vaccine recipients but also to the vulnerable patients for whom they care.
REFERENCES
- 1.American Academy of Pediatrics. 2007. Pertussis (whooping cough), p. 498-520. In L. K. Pickering (ed.), Red Book: 2006 report of the Committee on Infectious Diseases, 26th ed. American Academy of Pediatrics, Elk Grove Village, IL. http://aapredbook.aappublications.org/cgi/content/full/2006/1/3.96.
- 2.American Academy of Pediatrics. 2 January 2008, accession date. Prevention of pertussis among adolescents: recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. http://www.cispimmunize.org/ill/dtp/TdapAAPPolicy.pdf. [DOI] [PubMed]
- 3.Aoyama, T., K. Sunakawa, S. Iwata, Y. Takeuchi, and R. Fujii. 1996. Efficacy of short-term treatment of pertussis with clarithromycin and azithromycin. J. Pediatr. 129:761-764. [DOI] [PubMed] [Google Scholar]
- 4.Bace, A., T. Zrnic, J. Begovac, N. Kuzmanovic, and J. Culig. 1999. Short-term treatment of pertussis with azithromycin in infants and young children. Eur. J. Clin. Microbiol. Infect. Dis. 18:296-298. [DOI] [PubMed] [Google Scholar]
- 5.Baggett, H. C., J. S. Duchin, W. Shelton, D. M. Zerr, J. Heath, I. R. Ortega-Sanchez, and T. Tiwari. 2007. Two nosocomial pertussis outbreaks and their associated costs—King County, Washington, 2004. Infect. Control Hosp. Epidemiol. 28:537-543. [DOI] [PubMed] [Google Scholar]
- 6.Baron, S., E. Njamkepo, E. Grimprel, P. Begue, J. Desenclos, J. Drucker, and N. Guiso. 1998. Epidemiology of pertussis in French hospitals in 1993 and 1994: thirty years after a routine use of vaccination. Pediatr. Infect. Dis. J. 17:412-418. [DOI] [PubMed] [Google Scholar]
- 7.Bassinet, L., M. Matrat, E. Njamkepo, S. Aberrane, B. Housset, and N. Guiso. 2004. Nosocomial pertussis outbreak among adult patients and healthcare workers. Infect. Control Hosp. Epidemiol. 25:995-997. [DOI] [PubMed] [Google Scholar]
- 8.Bisgard, K. M., F. B. Pascual, K. R. Ehresmann, C. A. Miller, C. Cianfrini, C. E. Jennings, C. A. Rebmann, J. Gabel, S. L. Schauer, and S. M. Lett. 2004. Infant pertussis: who was the source? Pediatr. Infect. Dis. J. 23:985-989. [DOI] [PubMed] [Google Scholar]
- 9.Bisgard, K. M., P. Rhodes, B. L. Connelly, D. Bi, C. Hahn, S. Patrick, M. P. Glode, and K. R. Ehresmann. 2005. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998-2001. Pediatrics 116:e285-e294. [DOI] [PubMed] [Google Scholar]
- 10.Black, S. 1997. Epidemiology of pertussis. Pediatr. Infect. Dis. J. 16:S85-S89. [DOI] [PubMed] [Google Scholar]
- 11.Bortolussi, R., B. Miller, M. Ledwith, and S. Halperin. 1995. Clinical course of pertussis in immunized children. Pediatr. Infect. Dis. J. 14:870-874. [DOI] [PubMed] [Google Scholar]
- 12.Broder, K. R., M. M. Cortese, J. K. Iskander, K. Kretsinger, B. A. Slade, K. H. Brown, C. M. Mijalski, T. Tiwari, E. J. Weston, A. C. Cohn, P. U. Srivastava, J. S. Moran, B. Schwartz, and T. V. Murphy. 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 55:1-34. [PubMed] [Google Scholar]
- 13.Calugar, A., I. R. Ortega-Sanchez, T. Tiwari, L. Oakes, J. A. Jahre, and T. V. Murphy. 2006. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin. Infect. Dis. 42:981-988. [DOI] [PubMed] [Google Scholar]
- 13a.Centers for Disease Control and Prevention. 2003. National, state, and urban area vaccination levels among children aged 19-35 months—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 52:728-732. [PubMed] [Google Scholar]
- 13b.Centers for Disease Control and Prevention. 2007. Outbreaks of respiratory illness mistakenly attributed to pertussis—New Hampshire, Massachusetts, and Tennessee, 2004-2006. MMWR Morb. Mortal. Wkly. Rep. 56:837-842. [PubMed] [Google Scholar]
- 13c.Centers for Disease Control and Prevention. 1997. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 46(RR7):1-25. [PubMed] [Google Scholar]
- 13d.Centers for Disease Control and Prevention. 2002. Pertussis—United States, 1997-2000. MMWR Morb. Mortal. Wkly. Rep. 51:73-76. [PubMed] [Google Scholar]
- 13e.Centers for Disease Control and Prevention. 20 December 2007, accession date. Prevention of tetanus, diphtheria and pertussis among pregnant women: provisional ACIP recommendations for the use of Tdap vaccine, 2006. http://www.cdc.gov/vaccines/recs/provisional/downloads/tdap-preg.pdf.
- 13f.Centers for Disease Control and Prevention. 2000. Use of diphtheria toxoid-tetanus toxoid-acellular pertussis vaccine as a five-dose series. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 49:1-8. [PubMed] [Google Scholar]
- 14.Chapman, T. M., and K. L. Goa. 2003. Reduced-antigen combined diphtheria-tetanus-acellular pertussis vaccine (Boostrix). Drugs 63:1407-1416. [DOI] [PubMed] [Google Scholar]
- 15.Christie, C. D., A. M. Glover, M. J. Willke, M. L. Marx, S. F. Reising, and N. M. Hutchinson. 1995. Containment of pertussis in the regional pediatric hospital during the Greater Cincinnati epidemic of 1993. Infect. Control Hosp. Epidemiol. 16:556-563. [DOI] [PubMed] [Google Scholar]
- 16.Crowcroft, N. S., R. Booy, T. Harrison, L. Spicer, J. Britto, Q. Mok, P. Heath, I. Murdoch, M. Zambon, R. George, and E. Miller. 2003. Severe and unrecognised: pertussis in UK infants. Arch. Dis. Child. 88:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, J. P. 2005. Clinical and economic effects of pertussis outbreaks. Pediatr. Infect. Dis. J. 24:S109-S116. [DOI] [PubMed] [Google Scholar]
- 18.De Serres, G., R. Shadmani, B. Duval, N. Boulianne, P. Dery, M. D. Fradet, L. Rochette, and S. A. Halperin. 2000. Morbidity of pertussis in adolescents and adults. J. Infect. Dis. 182:174-179. [DOI] [PubMed] [Google Scholar]
- 19.Deville, J. G., J. D. Cherry, P. D. Christenson, E. Pineda, C. T. Leach, T. L. Kuhls, and S. Viker. 1995. Frequency of unrecognized Bordetella pertussis infections in adults. Clin. Infect. Dis. 21:639-642. [DOI] [PubMed] [Google Scholar]
- 20.Dragsted, D. M., B. Dohn, J. Madsen, and J. S. Jensen. 2004. Comparison of culture and PCR for detection of Bordetella pertussis and Bordetella parapertussis under routine laboratory conditions. J. Med. Microbiol. 53:749-754. [DOI] [PubMed] [Google Scholar]
- 21.Farizo, K. M., S. L. Cochi, E. R. Zell, E. W. Brink, S. G. Wassilak, and P. A. Patriarca. 1992. Epidemiological features of pertussis in the United States, 1980-1989. Clin. Infect. Dis. 14:708-719. [DOI] [PubMed] [Google Scholar]
- 22.Friedman, D. S., C. R. Curtis, S. L. Schauer, S. Salvi, H. Klapholz, T. Treadwell, J. Wortzman, K. M. Bisgard, and S. M. Lett. 2004. Surveillance for transmission and antibiotic adverse events among neonates and adults exposed to a healthcare worker with pertussis. Infect. Control Hosp. Epidemiol. 25:967-973. [DOI] [PubMed] [Google Scholar]
- 23.Gehanno, J. F., M. Pestel-Caron, C. Marguet, M. Nouvellon, and I. Gueit. 1998. Pertussis outbreak in an outpatient hospital staff. Arch. Pediatr. 5:92-93. [DOI] [PubMed] [Google Scholar]
- 24.Gehanno, J. F., M. Pestel-Caron, M. Nouvellon, and J. F. Caillard. 1999. Nosocomial pertussis in healthcare workers from a pediatric emergency unit in France. Infect. Control Hosp. Epidemiol. 20:549-552. [DOI] [PubMed] [Google Scholar]
- 25.Guris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 26.Hallander, H. O. 1999. Microbiological and serological diagnosis of pertussis. Clin. Infect. Dis. 28:S99-106. [DOI] [PubMed] [Google Scholar]
- 27.Halperin, S. A., B. Smith, M. Russell, D. Scheifele, E. Mills, P. Hasselback, C. Pim, W. Meekison, R. Parker, P. Lavigne, and L. Barreto. 2000. Adult formulation of a five component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine is safe and immunogenic in adolescents and adults. Pediatr. Infect. Dis. J. 19:276-283. [DOI] [PubMed] [Google Scholar]
- 28.Halperin, S. A., B. Smith, M. Russell, P. Hasselback, R. Guasparini, D. Skowronski, W. Meekison, R. Parker, P. Lavigne, and L. Barreto. 2000. An adult formulation of a five-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids is safe and immunogenic in adolescents and adults. Vaccine 18:1312-1319. [DOI] [PubMed] [Google Scholar]
- 29.Halperin, S. A., L. Sweet, D. Baxendale, A. Neatby, P. Rykers, B. Smith, M. Zelman, D. Maus, P. Lavigne, and M. D. Decker. 2006. How soon after a prior tetanus-diphtheria vaccination can one give adult formulation tetanus-diphtheria-acellular pertussis vaccine? Pediatr. Infect. Dis. J. 25:195-200. [DOI] [PubMed] [Google Scholar]
- 30.Halsey, N. A., M. A. Welling, and R. M. Lehman. 1980. Nosocomial pertussis: a failure of erythromycin treatment and prophylaxis. Am. J. Dis. Child. 134:521-522. [DOI] [PubMed] [Google Scholar]
- 31.He, Q., M. K. Viljanen, H. Arvilommi, B. Aittanen, and J. Mertsola. 1998. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA 280:635-637. [DOI] [PubMed] [Google Scholar]
- 32.Heimberger, T., H. G. Chang, M. Shaikh, L. Crotty, D. Morse, and G. Birkhead. 1995. Knowledge and attitudes of healthcare workers about influenza: why are they not getting vaccinated? Infect. Control Hosp. Epidemiol. 16:412-415. [DOI] [PubMed] [Google Scholar]
- 33.Honein, M. A., L. J. Paulozzi, I. M. Himelright, B. Lee, J. D. Cragan, L. Patterson, A. Correa, S. Hall, and J. D. Erickson. 1999. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet 354:2101-2105. [DOI] [PubMed] [Google Scholar]
- 34.Izurieta, H. S., T. A. Kenyon, P. M. Strebel, A. L. Baughman, S. T. Shulman, and M. Wharton. 1996. Risk factors for pertussis in young infants during an outbreak in Chicago in 1993. Clin. Infect. Dis. 22:503-507. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson, D. 1988. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br. Med. J. (Clin. Res. ed.) 296:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kretsinger, K., K. R. Broder, M. M. Cortese, M. P. Joyce, I. Ortega-Sanchez, G. M. Lee, T. Tiwari, A. C. Cohn, B. A. Slade, J. K. Iskander, C. M. Mijalski, K. H. Brown, and T. V. Murphy. 2006. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recommend. Rep. 55:1-37. [PubMed] [Google Scholar]
- 37.Kurt, T. L., A. S. Yeager, S. Guenette, and S. Dunlop. 1972. Spread of pertussis by hospital staff. JAMA 221:264-267. [PubMed] [Google Scholar]
- 38.Lambert, H. J. 1965. Epidemiology of a small pertussis outbreak in Kent County, Michigan. Public Health Rep. 80:365-367. [PMC free article] [PubMed] [Google Scholar]
- 39.Lebel, M. H., and S. Mehra. 2001. Efficacy and safety of clarithromycin versus erythromycin for the treatment of pertussis: a prospective, randomized, single blind trial. Pediatr. Infect. Dis. J. 20:1149-1154. [DOI] [PubMed] [Google Scholar]
- 40.Lee, G. M., S. Lett., S. Schauer, C. LeBaron, T. V. Murphy, D. Rusinak, and T. A. Lieu. 2004. Societal costs and morbidity of pertussis in adolescents and adults. Clin. Infect. Dis. 39:1572-1580. [DOI] [PubMed] [Google Scholar]
- 41.Lee, G. M., T. V. Murphy, S. Lett., M. Cortese, K. Kretsinger, S. Schauer, and T. A. Lieu. 2007. Cost effectiveness of pertussis vaccination in adults. Am. J. Prev. Med. 32:186-193. [DOI] [PubMed] [Google Scholar]
- 42.Lee, L. H., and M. E. Pichichero. 2000. Costs of illness due to Bordetella pertussis in families. Arch. Fam. Med. 9:989-996. [DOI] [PubMed] [Google Scholar]
- 43.Lievano, F. A., M. A. Reynolds, A. L. Waring, J. Ackelsberg, K. M. Bisgard, G. N. Sanden, D. Guris, A. Golaz, D. J. Bopp, R. J. Limberger, and P. F. Smith. 2002. Issues associated with and recommendations for using PCR to detect outbreaks of pertussis. J. Clin. Microbiol. 40:2801-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linnemann, C. C., Jr., N. Ramundo, P. H. Perlstein, S. D. Minton, and G. S. Englender. 1975. Use of pertussis vaccine in an epidemic involving hospital staff. Lancet ii:540-543. [DOI] [PubMed] [Google Scholar]
- 45.Long, S. S., C. J. Welkon, and J. L. Clark. 1990. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J. Infect. Dis. 161:480-486. [DOI] [PubMed] [Google Scholar]
- 46.Marchant, C. D., A. M. Loughlin, S. M. Lett., C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169:1297-1305. [DOI] [PubMed] [Google Scholar]
- 47.Martinello, R. A., L. Jones, and J. E. Topal. 2003. Correlation between healthcare workers' knowledge of influenza vaccine and vaccine receipt. Infect. Control Hosp. Epidemiol. 24:845-847. [DOI] [PubMed] [Google Scholar]
- 48.Martinez, S. M., C. A. Kemper, D. Haiduven, S. H. Cody, and S. C. Deresinski. 2001. Azithromycin prophylaxis during a hospitalwide outbreak of a pertussis-like illness. Infect. Control Hosp. Epidemiol. 22:781-783. [DOI] [PubMed] [Google Scholar]
- 48a.Massachusetts Department of Public Health, Bureau of Communicable Disease Control. 20 December 2007, accession date. Pertussis. Massachusetts Department of Public Health, Bureau of Communicable Disease Control. http://www.mass.gov/Eeohhs2/docs/dph/disease_reporting/guide/pertussis.pdf.
- 49.Matlow, A. G., S. Nelson, R. Wray, and P. Cox. 1997. Nosocomial acquisition of pertussis diagnosed by polymerase chain reaction. Infect. Control Hosp. Epidemiol. 18:715-716. [DOI] [PubMed] [Google Scholar]
- 50.McCall, B. J., M. Tilse, B. Burt, P. Watt, M. Barnett, and J. G. McCormack. 2002. Infection control and public health aspects of a case of pertussis infection in a maternity health care worker. Commun. Dis. Intell. 26:584-586. [DOI] [PubMed] [Google Scholar]
- 51.McNabb, S. J., R. A. Jajosky, P. A. Hall-Baker, D. A. Adams, P. Sharp, W. J. Anderson, J. J. Aponte, G. F. Jones, D. A. Nitschke, C. A. Worsham, and R. A. Richard, Jr. 2007. Summary of notifiable diseases—United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1-92. [PubMed] [Google Scholar]
- 52.Reference deleted.
- 53.Mink, C. M., J. D. Cherry, P. Christenson, K. Lewis, E. Pineda, D. Shlian, J. Dawson, and D. A. Blumberg. 1992. A search for Bordetella pertussis infection in university students. Clin. Infect. Dis. 14:464-471. [DOI] [PubMed] [Google Scholar]
- 54.Murray, P. R., K. S. Rosenthal, and M. A. Pfaller. 2005. Medical microbiology, 5th ed. Elsevier Mosby, Philadelphia, PA.
- 55.Reference deleted.
- 56.Nennig, M. E., H. R. Shinefield, K. M. Edwards, S. B. Black, and B. H. Fireman. 1996. Prevalence and incidence of adult pertussis in an urban population. JAMA 275:1672-1674. [PubMed] [Google Scholar]
- 57.Nouvellon, M., J. F. Gehanno, M. Pestel-Caron, C. Weber, J. F. Lemeland, and N. Guiso. 1999. Usefulness of pulsed-field gel electrophoresis in assessing nosocomial transmission of pertussis. Infect. Control Hosp. Epidemiol. 20:758-760. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Reference deleted.
- 60.Reference deleted.
- 61.Reference deleted.
- 62.Pichichero, M. E., and J. Treanor. 1997. Economic impact of pertussis. Arch. Pediatr. Adolesc. Med. 151:35-40. [DOI] [PubMed] [Google Scholar]
- 63.Pichichero, M. E., M. A. Deloria, M. B. Rennels, E. L. Anderson, K. M. Edwards, M. D. Decker, J. A. Englund, M. C. Steinhoff, A. Deforest, and B. D. Meade. 1997. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children. Pediatrics 100:772-788. [DOI] [PubMed] [Google Scholar]
- 64.Pichichero, M. E., M. B. Rennels, K. M. Edwards, M. M Blatter, G. S. Marshall, M. Bologa, E. Wang, and E. Mills. 2005. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults. JAMA 293:3003-3011. [DOI] [PubMed] [Google Scholar]
- 65.Pichichero, M. E., M. M. Blatter, W. A. Kennedy, J. Hedrick, D. Descamps, and L. R. Friedland. 2006. Acellular pertussis vaccine booster combined with diphtheria and tetanus toxoids for adolescents. Pediatrics 117:1084-1093. [DOI] [PubMed] [Google Scholar]
- 66.Pichichero, M. E., W. J. Hoeger, and J. R. Casey. 2003. Azithromycin for the treatment of pertussis. Pediatr. Infect. Dis. J. 22:847-849. [DOI] [PubMed] [Google Scholar]
- 67.Postels-Multani, S., H. J. Schmitt, C. H. Wirsing von Konig, H. L. Bock, and H. Bogaerts. 1995. Symptoms and complications of pertussis in adults. Infection 23:139-142. [DOI] [PubMed] [Google Scholar]
- 68.Reference deleted.
- 69.Qin, X., E. Galanakis, E. T. Martin, and J. A. Englund. 2007. Multitarget PCR for diagnosis of pertussis and its clinical implications. J. Clin. Microbiol. 45:506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qureshi, A. M., N. J. Hughes, E. Murphy, and W. R. Primrose. 2004. Factors influencing uptake of influenza vaccination among hospital-based health care workers. Occup. Med. (London) 54:197-201. [DOI] [PubMed] [Google Scholar]
- 71.Rennels, M. B. 2003. Extensive swelling reactions occurring after booster doses of diphtheria-tetanus-acellular pertussis vaccines. Semin. Pediatr. Infect. Dis. 14:196-198. [DOI] [PubMed] [Google Scholar]
- 72.Rennels, M. B., M. A. Deloria, M. E. Pichichero, G. A. Losonsky, J. A. Englund, B. D. Meade, E. L. Anderson, M. C. Steinhoff, and K. M. Edwards. 2000. Extensive swelling after booster doses of acellular pertussis-tetanus-diphtheria vaccines. Pediatrics 105:e12. [DOI] [PubMed] [Google Scholar]
- 73.Schellekens, J., C. H. von Konig, and P. Gardner. 2005. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr. Infect. Dis. J. 24:S19-S24. [DOI] [PubMed] [Google Scholar]
- 74.Senzilet, L. D., S. A. Halperin, J. S. Spika, M. Alagaratnam, A. Morris, and B. Smith. 2001. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin. Infect. Dis. 32:1691-1697. [DOI] [PubMed] [Google Scholar]
- 75.Shefer, A., L. Dales, M. Nelson, B. Werner, R. Baron, and R. Jackson. 1995. Use and safety of acellular pertussis vaccine among adult hospital staff during an outbreak of pertussis. J. Infect. Dis. 171:1053-1056. [DOI] [PubMed] [Google Scholar]
- 76.Siegel, J. D., E. Rhinehart, M. Jackson, L. Chiarello, and the Healthcare Infection Control Practices Advisory Committee. 2007. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings, June 2007. http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf. [DOI] [PMC free article] [PubMed]
- 77.Skowronski, D. M., G. De Serres, D. MacDonald, W. Wu, C. Shaw, J. Macnabb, S. Champagne, D. M. Patrick, and S. A. Halperin. 2002. The changing age and seasonal profile of pertussis in Canada. J. Infect. Dis. 185:1448-1453. [DOI] [PubMed] [Google Scholar]
- 78.Sotir, M. J., D. L. Cappozzo, D. M. Warshauer, C. E. Schmidt, T. A. Monson, J. L. Berg, J. A. Zastrow, G. W. Gabor, and J. P. Davis. 2007. Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county-wide outbreak focused among adolescents and adults. Clin. Infect. Dis. 44:1216-1219. [DOI] [PubMed] [Google Scholar]
- 79.Spearing, N. M., R. L. Horvath, and J. G. McCormack. 2002. Pertussis: adults as a source in healthcare settings. Med. J. Aust. 177:568-569. [DOI] [PubMed] [Google Scholar]
- 80.Steketee, R. W., D. G. Burstyn, S. G. Wassilak, W. N. Adkins, Jr., M. B. Polyak, J. P. Davis, and C. R. Manclark. 1988. A comparison of laboratory and clinical methods for diagnosing pertussis in an outbreak in a facility for the developmentally disabled. J. Infect. Dis. 157:441-449. [DOI] [PubMed] [Google Scholar]
- 81.Thomas, P. F., P. B. McIntyre, and B. B. Jalaludin. 2000. Survey of pertussis morbidity in adults in western Sydney. Med. J. Aust. 173:74-76. [DOI] [PubMed] [Google Scholar]
- 82.Tiwari, T., T. V. Murphy, and J. Moran. 2005. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recommend. Rep. 54:1-16. [PubMed] [Google Scholar]
- 82a.Tran Minh, N. N., Q. He, A. Ramalho, A. Kaufhold, M. K. Viljanen, H. Arvilommi, and J. Mertsola. 1999. Acellular vaccines containing reduced quantities of pertussis antigens as a booster in adolescents. Pediatrics 104:e70. [DOI] [PubMed] [Google Scholar]
- 83.Turnbull, F. M., T. C. Heath, B. B. Jalaludin, M. A. Burgess, and A. C. Ramalho. 2000. A randomized trial of two acellular pertussis vaccines (dTpa and pa) and a licensed diphtheria-tetanus vaccine (Td) in adults. Vaccine 19:628-636. [DOI] [PubMed] [Google Scholar]
- 84.Reference deleted.
- 85.Valenti, W. M., P. H. Pincus, and M. K. Messner. 1980. Nosocomial pertussis: possible spread by a hospital visitor. Am. J. Dis. Child. 134:520-521. [DOI] [PubMed] [Google Scholar]
- 86.Van der Wielen, M., P. Van Damme, E. Joossens, G. Francois, F. Meurice, and A. Ramalho. 2000. A randomised controlled trial with a diphtheria-tetanus-acellular pertussis (dTpa) vaccine in adults. Vaccine 18:2075-2082. [DOI] [PubMed] [Google Scholar]
- 87.Walker, F. J., J. A. Singleton, P. Lu, K. G. Wooten, and R. A. Strikas. 2006. Influenza vaccination of healthcare workers in the United States, 1989-2002. Infect. Control Hosp. Epidemiol. 27:257-265. [DOI] [PubMed] [Google Scholar]
- 88.Ward, A., J. Caro, L. Bassinet, B. Housset, J. A. O'Brien, and N. Guiso. 2005. Health and economic consequences of an outbreak of pertussis among healthcare workers in a hospital in France. Infect. Control Hosp. Epidemiol. 26:288-292. [DOI] [PubMed] [Google Scholar]
- 89.Ward, J. I., J. D. Cherry, S. J. Chang, S. Partridge, H. Lee, J. Treanor, D. P. Greenberg, W. Keitel, S. Barenkamp, D. I. Bernstein, R. Edelman, and K. Edwards. 2005. Efficacy of an acellular pertussis vaccine among adolescents and adults. N. Engl. J. Med. 353:1555-1563. [DOI] [PubMed] [Google Scholar]
- 90.Wirsing von Konig, C. H., S. Postels-Multani, H. Bogaerts, H. L. Bock, S. Laukamp, S. Kiederle, and H. J. Schmitt. 1998. Factors influencing the spread of pertussis in households. Eur. J. Pediatr. 157:391-394. [DOI] [PubMed] [Google Scholar]
- 91.Wirsing von Konig, C. H., S. Postels-Multani, H. L. Bock, and H. J. Schmitt. 1995. Pertussis in adults: frequency of transmission after household exposure. Lancet 346:1326-1329. [DOI] [PubMed] [Google Scholar]
- 92.Wright, S. W., M. D. Decker, and K. M. Edwards. 1999. Incidence of pertussis infection in healthcare workers. Infect. Control Hosp. Epidemiol. 20:120-123. [DOI] [PubMed] [Google Scholar]
- 93.Wright, S. W., K. M. Edwards, M. D. Decker, and M. H. Zeldin. 1995. Pertussis infection in adults with persistent cough. JAMA 273:1044-1046. [PubMed] [Google Scholar]
- 94.Wright, S. W., K. M. Edwards, M. D. Decker, and M. M. Lamberth. 1994. Pertussis seroprevalence in emergency department staff. Ann. Emerg. Med. 24:413-417. [DOI] [PubMed] [Google Scholar]
- 95.Yih, W. K., S. M. Lett., F. N. des Vignes, K. M. Garrison, P. L. Sipe, and C. D. Marchant. 2000. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989-1998. J. Infect. Dis. 182:1409-1416. [DOI] [PubMed] [Google Scholar]
- 96.Zivna, I., D. Bergin, J. Casavant, S. Fontecchio, S. Nelson, A. Kelley, S. Mathis, Z. Melvin, R. Erlichman, and R. T. Ellison. 2007. Impact of Bordetella pertussis exposures on a Massachusetts tertiary care medical system. Infect. Control Hosp. Epidemiol. 28:708-712. [DOI] [PubMed] [Google Scholar]