Abstract
Infectious myositis may be caused by a broad range of bacterial, fungal, parasitic, and viral agents. Infectious myositis is overall uncommon given the relative resistance of the musculature to infection. For example, inciting events, including trauma, surgery, or the presence of foreign bodies or devitalized tissue, are often present in cases of bacterial myositis. Bacterial causes are categorized by clinical presentation, anatomic location, and causative organisms into the categories of pyomyositis, psoas abscess, Staphylococcus aureus myositis, group A streptococcal necrotizing myositis, group B streptococcal myositis, clostridial gas gangrene, and nonclostridial myositis. Fungal myositis is rare and usually occurs among immunocompromised hosts. Parasitic myositis is most commonly a result of trichinosis or cystericercosis, but other protozoa or helminths may be involved. A parasitic cause of myositis is suggested by the travel history and presence of eosinophilia. Viruses may cause diffuse muscle involvement with clinical manifestations, such as benign acute myositis (most commonly due to influenza virus), pleurodynia (coxsackievirus B), acute rhabdomyolysis, or an immune-mediated polymyositis. The diagnosis of myositis is suggested by the clinical picture and radiologic imaging, and the etiologic agent is confirmed by microbiologic or serologic testing. Therapy is based on the clinical presentation and the underlying pathogen.
INTRODUCTION
Myositis is defined as inflammation of a muscle, especially a voluntary muscle, characterized by pain, tenderness, swelling, and/or weakness. The many different etiologies of myositis include infection, autoimmune conditions, genetic disorders, medication adverse events, electrolyte disturbances, and diseases of the endocrine system (Table 1) (260). Some idiopathic cases of polymyositis are suspected to be related to infectious agents, especially viruses such as the paramyxoviruses or enteroviruses (120, 170, 264). However, conclusive evidence to date is lacking. Microorganisms may cause myositis via immune mechanisms without directly infecting the muscle (43). In addition, they may cause infectious myositis, which is defined as infection of skeletal muscle.
TABLE 1.
Causes of noninfectious myositis or myopathic symptoms
| Myopathy-related disease or manifestation |
|---|
| Idiopathic inflammatory myopathies |
| Polymyolitis |
| Dermatomyositis |
| Inclusion body myositis |
| Myositis associated with collagen vascular diseases |
| Polyarteritis nodosum |
| Wegener's granulomatosis |
| Systemic lupus erythematosus |
| Rheumatoid arthritis |
| Scleroderma |
| Sjogren's syndrome |
| Leukocytoclastic vasculitis |
| Hypersensitivity vasculitis |
| Polymyalgia rheumatica |
| Mixed connective tissue disease |
| Adult Still's disease |
| Myositis associated with malignancies |
| Other forms of myopathies |
| Inflammatory myopathies |
| Myositis associated with eosinophilia |
| Myositis ossificans |
| Giant cell myositis |
| Myopathies due to drugs and toxins |
| Diseases causing myopathic signs and symptoms |
| Metabolic myopathies |
| Inborn errors of metabolism |
| Disorders in glycogen metabolism |
| Lipid storage disorders |
| Mitochondrial myopathies |
| Endocrine disorders |
| Hypo- and hyperthyroidism |
| Acromegaly |
| Addison's disease |
| Cushing syndrome |
| Hyperaldosteronism |
| Hyperparathyroidism |
| Electrolyte abnormality |
| Nutritional deficiency |
| Neuropathic disorders |
| Muscular dystrophies |
| Others, including diseases of the neuromuscular junction |
| Medication-related adverse events |
| Other causes |
| Sarcoidosis |
| Amyloidosis |
| Behcet's disease |
| Atherosclerotic emboli |
Infectious myositis may be due to a wide variety of pathogens, including bacteria, fungi, parasites, and viruses (Table 2) (28, 42, 49, 121, 171, 174). Their clinical course may be acute, subacute, or chronic in nature. Although the diagnosis of the causative microbe requires cultures and/or other diagnostic testing, some clinical findings suggest the general category of the agent. For example, bacterial myositis usually presents as a focal muscle infection, whereas viruses and parasites are often more diffuse in nature, leading to generalized myalgias or multifocal myositis. Pyomyositis is defined as an acute intramuscular infection that is secondary to hematogenous spread of the microorganism into the body of a skeletal muscle; by definition, it is not secondary to a contiguous infection of the soft tissue or bone, nor due to penetrating trauma. Given the musculature's resistance to infection, bacterial myositis often occurs in the setting of muscular injury, surgery, ischemia, or the presence of a foreign body. Certain hosts, such as those with immunocompromising conditions, have a heightened risk of bacterial and fungal myositis (49, 207, 257).
TABLE 2.
Infectious causes of myositis
| Organism group | Organism(s)a |
|---|---|
| Gram-positive bacteria | Staphylococcus aureus* |
| Group A Streptococcus** | |
| Streptococcus (groups A, B, C, and G, S. pneumoniae, S. anginosus)*** | |
| Gram-negative bacteria | Aeromonas hydrophila*** |
| Burkholderia mallei, B. pseudomallei*** | |
| Citrobacter freundii** | |
| Enterobacter spp.** | |
| Escherichia coli** | |
| Haemophilus influenzae*** | |
| Klebsiella oxytoca, K. pneumoniae*** | |
| Morganella morganni*** | |
| Neisseria gonorrhoeae*** | |
| Pasteurella spp.*** | |
| Proteus spp.** | |
| Pseudomonas spp.** | |
| Salmonella spp.** | |
| Serratia marcescens*** | |
| Vibrio vulnificus*** | |
| Yersinia enterocolitica*** | |
| Anaerobic bacteria | Bacteroides spp.** |
| Clostridium spp.** | |
| Fusobacterium necrophorum and F. nucleatum*** | |
| Streptococcus spp. (anaerobic, e.g., Peptostreptococcus)** | |
| Veillonella spp.*** | |
| Mycobacterium spp. | Mycobacterium avium complex*** |
| Mycobacterium bovis*** | |
| Mycobacterium haemophilum*** | |
| Mycobacterium leprae*** | |
| Mycobacterium tuberculosis** | |
| Atypical bacteria | Actinomyces spp.*** |
| Bacillus spp.*** | |
| Bartonella spp.*** | |
| Borrelia burdorferi*** | |
| Brucella spp.** | |
| Coxiella burnetii*** | |
| Francisella tularensis*** | |
| Legionella pneumophila*** | |
| Leptospira spp.*** | |
| Mycoplasma pneumoniae** | |
| Nocardia spp.*** | |
| Rickettsia rickettsii and R. conorii*** | |
| Treponema pallidum*** | |
| Fungi | Aspergillus spp.*** |
| Blastomyces dermatitidis*** | |
| Candida spp.* | |
| Coccidioides spp.*** | |
| Cryptococcus neoformans*** | |
| Fusarium spp.*** | |
| Histoplasma capsulatum*** | |
| Pneumocystis jiroveci*** | |
| Parasites | Entamoeba histolytica*** |
| Echinococcus spp.*** | |
| Microspordia spp. (Brachiola, Trachipleistophora, Pleistophora)*** | |
| Onchocerca volvulus*** | |
| Plasmodium spp.** | |
| Sarcocystis spp.** | |
| Schistosoma spp.*** | |
| Spirometra mansonoides*** | |
| Taenia solium** | |
| Toxocara canis*** | |
| Toxoplasma gondii*** | |
| Trichinella spp.* | |
| Trypanosoma cruzi*** | |
| Viruses | Adenovirus*** |
| Cytomegalovirus*** | |
| Dengue virus*** | |
| Enteroviruses (coxsackie B virus and ECHO virus)** | |
| Epstein-Barr virus*** | |
| Hepatitis B and C viruses*** | |
| Herpes simplex virus 2*** | |
| HIV** | |
| HTLV-1** | |
| Influenza A and B viruses* | |
| Mumps virus*** | |
| Parainfluenza virus*** | |
| Parvovirus B19*** | |
| Varicella-zoster virus*** | |
| West Nile virus*** |
*, the most common cause of myositis in the causative organism category. Of note, S. aureus accounts for 90% of the pyomyositis cases in the tropics and 70% of cases in the developed world. The most common fungal agent is Candida spp., and influenza virus is the most frequent viral cause of myositis identified. The occurrence of parasitic myositis depends on geographic location; cases are rare in the United States. **, occasionally causes myositis. ***, rarely causes myositis; the literature mainly involves case reports of the organism causing myositis.
BACTERIAL INFECTIONS OF THE MUSCULATURE
Overview
Bacterial infections involving muscle are relatively uncommon. Myositis may result from contiguous sites of infection, penetrating trauma, vascular insufficiency, or by hematogenous dissemination. The infecting organism is related to the mechanism of the infection. For instance, an acute bacterial infection of skeletal muscle that is the result of hematogenous spread is most commonly due to Staphylococcus aureus. Infection in the setting of penetrating wounds or vascular insufficiency is often polymicrobial. Establishing the bacterial or other cause of myositis is crucial in the management of myositis cases; this should be accomplished by Gram and other microbiologic stains, followed by aerobic and anaerobic culture and sensitivity data. A wide variety of bacteria may cause myositis, as shown in Table 2. Bacterial infections of the musculature are often categorized according to the inciting event, clinical presentation, including anatomic location, and the causative organisms into the categories of pyomyositis, psoas abscess, S. aureus myositis, group A streptococcal necrotizing myositis, group B streptococcal myositis, clostridial gas gangrene, and nonclostridial myositis (28, 171) (Table 3).
TABLE 3.
Categories of bacterial pyogenic infections of the musculature
| Infection type | Causative agent(s) |
|---|---|
| Pyomyositis | Usually Staphylococcus aureusa |
| Psoas abscess | S. aureus, mixed gram-positive organisms, gram-negative aerobic and anaerobic organisms, Mycobacterium spp.a |
| Staphylococcus aureus myositis | S. aureus |
| Group A streptococcal necrotizing myositis | Streptococcus pyogenes |
| Group B streptococcal myositis | Streptococcus agalactiae |
| Clostridium species myositis, gas gangrene | Usually C. perfringens, but in spontaneous cases C. septicum |
| Nonclostridial myositis | |
| Anaerobic streptococcal myonecrosis | Peptostreptococcus; also may involve group A streptococci and S. aureus |
| Synergistic nonclostridial myonecrosis | Streptococci and mix of other organisms |
| Aeromonas myonecrosis | Aeromonas hydrophila |
| Vascular gangrene | Proteus, Bacillus, Bacteroides, and others |
See Table 1 for other etiologic agents.
Pyomyositis
Pyomyositis is defined as an acute intramuscular bacterial infection which is neither secondary to a contiguous infection of the soft tissue or bone nor due to penetrating trauma. Infections are a result of hematogenous spread and are usually due to S. aureus (8, 40, 42, 49). Of note, other metastatic infections or endocarditis generally are not present despite the occurrence of bacteremia.
Epidemiology.
Pyomyositis has been referred to as “tropical pyomyositis,” as most early cases were described in the tropics (8). In fact, in Africa, up to 4% of admissions may be due to pyomyositis (100). Pyomyositis in the tropics usually occurs among previously healthy persons and often among children. In the United States, cases were previously considered rare, with the first case described in 1971 (135). Some initial cases in the United States were among recent immigrants, while others were among long-time residents (42). During the past 2 decades, an increasing number of cases in the United States have been reported in the literature; a review from 1981 to 2002 noted 246 cases (49). The mean age at diagnosis was 34 years, but pyomyositis has developed over a wide range of ages (from 2 weeks to 92 years) (49). A male predominance of infection both within tropical and temperate regions has also been noted. Cases in the United States often occur among immunocompromised persons; a study found that 48% of pyomyositis cases among human immunodeficiency virus (HIV)-uninfected persons had at least one underlying medical condition (49). The rising incidence rates of pyomyositis cases within the United States are likely the result of increasing numbers of immunocompromised patients, including cancer patients receiving chemotherapy and those with rheumatologic conditions taking immunomodulatory agents, in addition to those with HIV infection, diabetes mellitus, and liver disease (49, 72, 246).
Patients with HIV appear to have an increased risk of developing these infections, which may be a result of several factors, including higher colonization rates with S. aureus, neutropenia, defective neutrophil and cell-mediated immune responses, use of intravenous lines, and predisposing muscle conditions due to the virus itself (i.e., HIV myopathy) or antiretroviral medications (e.g., mitochondrial myopathy associated with zidovudine) (28, 207, 257). Most cases have occurred among HIV patients with end-stage disease (CD4 cell count of <100 cells/mm3) (49). The true incidence or risk of pyomyositis among this group is unknown since it is not a reportable disease and large prospective studies have not been performed. The literature suggests that the number of pyomyositis cases has increased since the HIV epidemic began (42) and a comprehensive review of cases among HIV-infected persons noted several (n = 84) published cases among this group since 1987 (49).
Pathogenesis.
The pathogenesis of pyomyositis involves transient bacteremia in the setting of muscle injury. Bacteremia without concurrent muscle damage is unlikely to cause myositis, since healthy muscle is quite resistant to infection (151). For instance, in a study of staphylococcal bacteremia, <0.5% developed myositis (217). Muscle trauma or injury has been proposed as a prerequisite to the development of pyomyositis; however, many studies have reported a history of trauma or muscle injury in only 30 to 50% of cases (216). Trauma may also lead to hematoma formation that may be seeded during transient bacteremia. In addition to blunt trauma, overuse muscular injuries may be a risk factor for the development of pyomyositis. For instance, young athletes may be at a slightly higher risk of pyomyositis due to overuse injuries concurrent with asymptomatic episodes of bacteremia (45, 127). The mechanism may be a combination of overuse with muscle injury and the development of skin abrasions during athletic participation whereby bacteria enter the body (127). Given the infrequency of case reports of overuse injuries leading to pyomyositis (45, 127) and the large number of young athletes, the overall risk for pyomyositis in this group appears to be low. Several other conditions have been suggested in the pathogenesis of pyomyositis, including nutritional deficiencies, arboviral infections, and malaria, although supporting evidence is lacking (121). Finally, injection drug use, atopic dermatitis, and varicella may also be risk factors for pyomyositis due to the potential for bacteria to enter through areas of compromised skin integrity (82, 205, 216). In most cases, the exact inciting event which led to the development of pyomyositis remain obscure.
Microbiology.
As previously noted, S. aureus is the causative agent in the majority of cases, accounting for 90% of infections in the tropics and 60 to 70% of cases in the United States (40, 42, 49). Of note, recent reports show that community-acquired methicillin-resistant S. aureus (MRSA) is an important cause of pyomyositis (82, 144, 169, 193). A series of 26 cases of S. aureus myositis among children found that the majority (n = 16) of isolates were due to community-acquired MRSA (169). This case series also showed that patients with MRSA isolates which carried the Panton Valentine leukocidin genes had larger abscesses and required more drainage procedures (169). Two of the patients with MRSA pyomyositis developed life-threatening infections, including septic shock (169).
The second most common type of bacteria implicated in causing pyomyositis is group A streptococci. Other species of streptococci have been described, but these are overall uncommon; they may include groups B, C, and G streptococci as well as S. pneumoniae (12, 29, 46, 171, 232, 246, 255, 259, 265). Group B streptococci (S. agalactiae) is an opportunistic organism that is occasionally seen among diabetic patients who develop pyomyositis or necrotizing fasciitis (12, 29, 246). Less common bacterial causes of pyomyositis include gram-negative bacilli, anaerobes (e.g., Clostridium spp.), Bartonella spp. (103, 256), and Mycobacterium spp. (e.g., M. tuberculosis, M. avium complex) (131, 173, 211, 251). Another reported cause of pyomyositis is Fusobacterium necrophorum, which may lead to Lemierre's syndrome with septic thrombosis of the jugular vein, followed by septic emboli to the musculature causing pyomyositis (50); Fusobacterium nucleatum has also been noted to cause pyomyositis (20). Other atypical organisms include Actinomyces myositis described in the setting of alcoholism and poor dentition (104). The more unusual pathogens, such as gram-negative organisms (e.g., Salmonella, Bartonella) and fungi (e.g., Candida spp., Cryptococcus neoformans), are typically seen among immunocompromised patients (49). Table 2 shows the described bacterial etiologic agents of pyomyositis.
Clinical manifestations.
The evolution of pyomyositis may involve three stages (40). The first stage is typically subacute, occurring over 1 to 3 weeks, with local swelling and a “woody” texture, mild pain, and variable fevers. During this “invasive phase,” bacteria have begun to infect the muscle but a definitive purulent collection has not yet developed. The diagnosis is often confused with thrombosis, hematoma, contusion, muscle strain/rupture, or osteomyelitis. During the second or “suppurative” stage (which occurs at 10 to 21 days), tenderness and fevers become more pronounced. This is the stage at which the diagnosis of pyomyositis is usually established. Of note, local erythema and regional adenitis, which are common findings in soft tissue infections, are usually absent in pyomyositis. Imaging demonstrates a definitive abscess, and aspiration will yield pus at this stage. If the infection remains undiagnosed and untreated, intense local pain and fluctuance, as well as systemic findings, including sepsis, will develop as part of the third stage of pyomyositis.
Most pyomyositis cases involve only a single muscle group, but more diffuse involvement is described in 10 to 20% of cases. The large muscles of the lower extremities are most commonly affected (such as the quadriceps and gluteus muscle group), perhaps as a result of strenuous exercise or trauma leading to muscle injury (25, 45, 127). Any skeletal muscle group may be involved.
Differential diagnosis of pyomyositis may include a diverse number of clinical conditions involving bone (osteomyelitis), joints (septic arthritis), vessels (thrombosis), or muscle (hematoma or strain). Other conditions may also be confused with pyomyositis. When the iliopsoas muscle is involved, appendicitis may be suspected (262). Likewise, iliacus or obturator internus pyomyositis mimics septic arthritis of the hip, and pyriformis myositis may suggest an epidural abscess or sciatica (38, 41, 45).
Diagnosis.
Laboratory evaluation reveals leukocytosis with a left shift and an elevated erythrocyte sedimentation rate. The exception is that among HIV patients the white blood count is only elevated in 19% of cases due to their immunosuppressed condition (49). In the tropics, eosinophilia is also described, likely related to a concurrent parasitic infection. Muscle enzymes such as creatine kinase and aldolase are paradoxically normal despite muscle inflammation.
Determining the causative organism is imperative to optimizing therapy. Cultures of the purulent material after radiologically guided drainage or open surgical procedure should be obtained. A Gram stain should be performed; other microbiologic stains are important when fungal, parasitic, or atypical bacterial agents are suspected (e.g., use of the acid-fast bacilli stain for Mycobacterium). Aerobic and anaerobic wound cultures should be performed as well as antimicrobial sensitivity testing. Cultures for Mycobacterium spp. and fungi should be considered, especially in an immunocompromised host. Two sets of aerobic and anaerobic blood cultures should also be obtained. Of note, bacteremia is often transient, as blood cultures are only positive in 5 to 35% of cases (49, 100, 171); the higher percentage of this range is from developed countries, which generally have improved blood culture capabilities.
The diagnosis of pyomyositis relies on imaging. Plain films may show soft tissue swelling, but their utility is limited. Ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) scans are more sensitive at detecting pyomyositis. CT scans may demonstrate focal abscess formation within the muscle by revealing a low-density area with a central fluid collection and surrounding rim of enhancement. MRI shows a hypointense central area with a gadolinium-enhanced rim and has the highest specificity especially for axial lesions. While MRI is the test of choice due to its high precision, most often cases are diagnosed using CT scans, as this method is usually more accessible. Radionuclide scans are usually not useful, since they are unable to precisely determine the site of infection (e.g., myositis versus fasciitis).
Treatment.
During the first stage, a distinct collection of pus is not present, and this stage often can be treated with antibiotics alone. Management of stage 2 or 3 pyomyositis involves surgical drainage either via CT- or ultrasound-guided drainage or via an open procedure. These procedures should be performed in an urgent manner and should ensure complete drainage of the purulent collection. Antibiotics are initially administered intravenously and should cover S. aureus. A β-lactamase-resistant penicillin, such as oxacillin or nafcillin, was commonly utilized in the past. Given the high rates of MRSA in both the community and hospital settings, initial therapy with vancomycin should be considered. Other antibiotic choices for MRSA pyomyositis include daptomycin, linezolid, tigecycline, and quinupristin-dalfopristin. Clindamycin may be useful, but due to increasing reports of resistance, this antibiotic should be reserved for an infection in which the organism shows sensitivity by D testing (136, 213). Since linezolid is bacteriostatic and tigecycline has low peak serum levels, these agents are not recommended in patients with concurrent bacteremia.
For immunocompromised patients, broad-spectrum antibiotics should be utilized, which will also include gram-negative and anaerobic coverage; in such cases, vancomycin and a carbapenem or β-lactam/β-lactamase inhibitor (e.g., piperacillin-tazobactam) is recommended. For anaerobic infections, clindamycin is an excellent agent. In addition, cases with both necrotizing myositis and fasciitis may benefit from the use of clindamycin, since it inhibits protein and toxin synthesis and is effective in a setting of large quantities of bacteria in various stages of replication due to the Eagle effect (65, 224). Antibiotics should be modified based on culture and sensitivity results. For instance, group A streptococcal infections are best treated with a combination of penicillin G and clindamycin (224).
Therapy is usually with intravenous antibiotics for the first 1 to 2 weeks and then oral antibiotics for a total of 4 to 6 weeks (216). A longer duration may be warranted in multifocal or severe disease or for certain organisms, such as Mycobacterium spp. Follow-up imaging to ensure resolution of the abscess is recommended. Prognosis is good, particularly when the infection is diagnosed during the first or second stage. The mortality rate has been cited as <1% to 4%. Complete recovery is typical; recurrence is uncommon but may occur among immunosuppressed patients or among those with atypical infections, such as Mycobacterium or Salmonella (49).
Psoas Abscess
A psoas abscess is defined as a purulent infectious collection within the psoas muscle. These infections have traditionally been classified separately from pyomyositis. Psoas abscesses are defined as primary if there is no obvious source of infection; these are typically due to occult hematogenous spread. Secondary psoas abscesses are due to contiguous spread from a nearby infection. Most commonly, infection involves only one of the psoas muscles; however, bilateral psoas abscesses have been described, especially in cases with concurrent Pott's disease (143).
Epidemiology.
Psoas abscess was first described in 1881 by Mynter (157). They are overall uncommon infections in the developed world. Approximately 400 cases have been described in the literature to date (91, 187), but this is an underestimate of the true number of cases. Cases of psoas abscesses in the United States at the beginning of the 20th century were largely due to Mycobacterium tuberculosis infections which involved bacterial spread between the vertebrae and psoas musculature. After the decline of tuberculosis in the United States, cases were mainly secondary psoas abscesses due to nearby gastrointestinal or genitourinary infections (248). Recently, the trend has shifted toward primary psoas abscesses occurring among immunocompromised patients, such as those undergoing cancer therapy or patients with HIV infection, as well as intravenous drug users (196, 236, 248). The number of psoas abscesses appears to have recently risen, likely due to the use of better imaging techniques (14, 187, 196).
All age groups may develop psoas abscesses, including neonates (159). The average age in most series is 30 to 50 years of age (91, 196, 248). Some studies have reported that primary psoas abscesses occur more frequently in younger age groups, presumably due to the higher rates of intravenous drug users and HIV infection within this age group (86, 187, 196). Secondary psoas abscesses often occur in older age groups, likely due to concurrent conditions predisposing to their development. There is no consistent gender predisposition for psoas abscesses (91).
Pathogenesis.
The psoas muscle is supplied by venous blood from the lumbar spine and has lymphatics overlying the muscle from nearby intraabdominal organs. The muscle extends from the 12th thoracic vertebrae to the 5th lumbar vertebrae down to the lesser trochanter of the femur. The psoas is also in close proximity to several intraabdominal structures, including the colon, appendix, terminal ileum, jejunum, ureters, renal pelvis, and pancreas, hence, it is quite susceptible to contiguous infection.
Secondary psoas abscesses develop as a result of spread of an infection from contiguous structures, such as concurrent vertebral infections (155) in the setting of S. aureus osteomyelitis or Pott's disease due to M. tuberculosis. Other routes may be from an intraabdominal source, most commonly gastrointestinal, which includes Crohn's disease, cancer, appendicitis, or diverticulitis. Less commonly, psoas abscesses may develop in relationship to genitourinary infections, such as a perinephric abscess, vaginal deliveries, cesarian surgery, abortion, or an infected retroperitoneal hematoma (28, 171, 199, 201, 209, 219, 236). The latter may occur in relation with a history of trauma.
Primary psoas abscesses arise from occult bacteremia and are usually due to S. aureus. These cases are often found among intravenous drug users as well as immunocompromised persons (196). Other patient populations which appear at higher risk for psoas abscesses include diabetics, the elderly, immunocompromised, and alcoholics (5, 14, 248). Of note, cases among patients with no risk factors have also been described. Regarding S. aureus, seeding of both the vertebrae and the psoas muscle may occur concurrently or may involve one area with contiguous spread into the other (182).
Microbiology.
Primary psoas abscesses due to hematogenous seeding involve S. aureus in approximately 80 to 90% of cases (91); recent cases have shown that community-acquired MRSA is a new cause of primary psoas abscesses (219, 254). Other pathogens have been described, including streptococci such as S. pneumoniae, S. milleri, or group A streptococci (14, 124). Figure 1 shows a multiloculated psoas abscess which developed insidiously in an HIV-positive patient who presented with fevers and back pain; the culture grew group A streptococci. Rarely, primary psoas abscesses are due to gram-negative organisms such as Escherichia coli, Pseudomonas, Haemophilus, Proteus, or Pasteurella species (66, 86, 91, 187, 222).
FIG. 1.
CT scan showing multiloculated primary iliopsoas abscess (arrow) due to group A streptococci in an HIV-infected patient.
Secondary psoas abscesses originating from intraabdominal structures are often polymicrobial and represent the normal flora of these sites. These include enteric gram-negative bacteria such as E. coli, Enterobacter, Salmonella, and Klebsiella species, as well as streptococci, Enterococcus spp., and anaerobes such as Clostridium spp. and Bacteroides spp. (185, 196). Vertebral osteomyelitis may also lead to infection of the psoas muscle and usually involves S. aureus (as mentioned above) or M. tuberculosis (94). More uncommon pathogens which may cause both osteomyelitis and psoas abscesses include Salmonella, Brucella, Nocardia, Coxiella burnetii, or fungal pathogens, such as Blastomyces dermatitidis or Coccidioides immitis (27, 92, 93, 185, 233, 261). Rare cases of Mycobacterium bovis have been described in association with bladder carcinoma therapy with Bacille Calmette-Guérin (122).
Clinical manifestations.
A high index of suspicion is needed to recognize a psoas abscess. The classic triad of fever, pain, and limp are often absent (133); instead only one or two findings are usually present, most commonly fever. Other symptoms may include back pain (especially with concurrent vertebral osteomyelitis), flank pain, limp, groin fullness or mass, malaise, anorexia, and weight loss (187, 248). Immunocompromised patients may be afebrile and present with minimal signs or symptoms. On examination, the following may be detected: fever; mass in the iliac fossa; abdominal, spinal, or iliac fossa tenderness; flexion and external rotation of the ipsilateral hip; and/or limping gait.
Diagnosis.
Laboratory testing may reveal leukocytosis, elevated erythrocyte sedimentation rate, and anemia. Blood cultures are positive in up to 70% of cases and are useful for establishing the causative organism (196). In cases of secondary psoas abscesses, pyuria and positive urine cultures may be found when the genitourinary tract is the originating site of infection. Wound cultures of the psoas drainage should be ordered for both aerobic and anaerobic bacteria. If M. tuberculosis is suspected, acid-fast bacilli stains and cultures should be obtained.
The diagnosis is established by radiological imaging. A psoas abscess may be detected on ultrasound evaluation, but this test is not as sensitive as CT or MRI scans; it may be used as an initial radiographic study, particularly in areas where more advanced imaging is not readily available. A negative ultrasound, however, does not rule out a psoas abscess. The diagnosis of a psoas abscess is often established by a CT scan, which is the most commonly used imaging technique in suspected cases. Radionuclide testing usually does not add additional information, unless there is concurrent bony involvement. If blood or wound cultures identify an enteric organism, further investigation to the source of the psoas abscess is warranted. This may include further abdominal imaging and a barium enema or colonoscopy.
Treatment.
Surgical drainage and intravenous antibiotics are recommended, similar to pyomyositis. Drainage may involve either CT-guided percutaneous drainage or an open surgical procedure; the choice often depends on the number and volume of the abscesses (15). For example, some experts suggest that cases of multiloculated psoas abscesses are best managed with an open surgical procedure via an extraperitoneal approach (14, 15, 155). Open surgical drainage may be preferentially utilized in cases of secondary psoas abscesses where there is concurrent gastrointestinal or genitourinary disease requiring surgical management. For instance, open drainage of the psoas abscess as well as resection and diversion of the involved bowel could be performed simultaneously. Debridement of nearby infections and the drainage of the psoas abscess should be complete to prevent recurrence. If a catheter is used to drain the abscess, it should remain until the cavity is obliterated. Cases of recurrence have been described, especially in cases where residual collections remained after the initial drainage procedure (86). In cases of recurrent psoas abscesses after a CT-guided procedure(s), open surgical drainage should be considered (248). Among patients with concurrent spinal infection, spinal surgery should be immediately performed in cases of neurologic deficits, spinal instability, or spinal cord compression.
Antibiotic therapy for a primary psoas abscess is generally with an antistaphylococcal antibiotic. Given descriptions of both nosocomial and community-acquired MRSA psoas abscesses (219, 254), vancomycin or one of the other effective antibiotics listed above should be administered. A broad-spectrum antibiotic should be utilized in cases originating intraabdominally to also cover enteric gram-negative organisms and anaerobes. This may include the addition of a carbapenem, β-lactam/β-lactamase inhibitor (e.g., piperacillin-tazobactam), or a combination of penicillin, gentamicin, and clindamycin. In cases where it is unclear if the psoas abscess is primary or secondary, broad-spectrum antibiotics along with vancomycin should be administered until further culture data are available. Antibiotics should be subsequently tailored to the culture and sensitivity results. The duration of antibiotics depends on the clinical response and the involvement of other tissues. Typically, antibiotics are continued for at least 3 weeks after drainage and fever resolution (236), but often they are given for 4 to 6 weeks. Follow-up imaging to assure complete drainage of the abscess is recommended. Patients with psoas abscesses due to M. tuberculosis should be treated with four-drug antituberculosis therapy until sensitivity data are available. The treatment course is generally 6 months in duration but should be extended if the clinical response is slow (26).
The prognosis for a localized psoas abscess is overall favorable especially if the diagnosis is established and treatment initiated early in the disease process. Primary psoas abscesses appear to have a better prognosis, with a mortality rate as low as 2%; secondary psoas abscesses have a mortality of approximately 20% (91). Factors predicting mortality in one study included age, APACHE score, bilateral abscesses, and postoperative or bony cause (15).
Bacterial Myositis
Acute bacterial myositis, defined as a diffuse muscle infection without an intramuscular abscess, occurs less commonly than pyomyositis and psoas abscess formation. Myositis compared to pyomyositis is more typically seen in adults rather than children. In addition, although staphylococcal myositis has been reported (4, 169), cases of myositis are usually due to a streptococcal infection (3). As with pyomyositis, a broad range of types of bacteria may lead to myositis, but gram-positive organisms predominate.
S. aureus myositis.
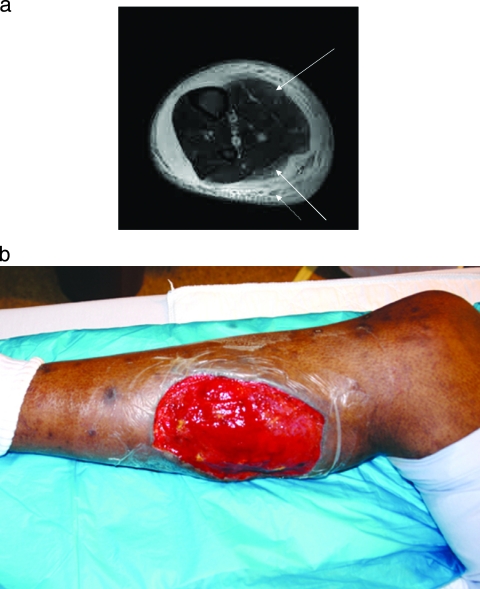
S. aureus may occasionally cause a diffuse myositis with rhabdomyolysis. One such case in the literature involved a 15-year-old boy who presented with all lower extremity muscle groups inflamed and tender. Unlike pyomyositis cases, the creatine kinase levels were markedly elevated and a muscle biopsy revealed muscle fiber degeneration with numerous gram-positive cocci at the muscle edges (4). Treatment is with emergent and thorough surgical debridement as well as antibiotic therapy directed against S. aureus. Myositis secondary to MRSA has also been described (169); therefore, empirical therapy should include vancomycin or another agent which covers MRSA until sensitivity data are available. Figure 2 depicts a case of community-acquired MRSA myositis of the lower extremity in an HIV-positive patient presenting with severe leg pain. MRI imaging demonstrated myositis of the gastrocnemius muscle along with subcutaneous abscesses (Fig. 2a). Urgent surgical debridement was performed finding purulent collections as well as inflamed and necrotic musculature; the wound culture grew MRSA. The patient required multiple surgical debridements along with a wound vacuum-assisted closure (VAC) to the 12- by 15-cm debrided area (Fig. 2b). Daptomycin was administered due to a vancomycin allergy.
FIG. 2.
(a) MRI T2-weighted image showing myositis of the gastrocnemius (both medial and lateral heads) (solid arrows) and multiple abscesses (dotted arrow) of the posterior compartment. Wound culture grew community-acquired methicillin-resistant Staphylococcus aureus. (b) Lower extremity after surgical debridement of the necrotic muscle.
Group A streptococcal necrotizing myositis.
Group A streptococci (GAS) may cause a variety of muscle infections, including pyomyositis, subacute myositis, acute myositis, and malignant myositis, in increasing order of severity (121). The most severe form is known as GAS necrotizing myositis, also called streptococcal myonecrosis or spontaneous streptococcal gangrenous myositis. Compared to pyomyositis or psoas abscesses, GAS necrotizing myositis is a less common entity but a significantly more aggressive and fatal infection (3, 200, 227). Cases are typically among men (2:1) and young adults; the disease usually occurs spontaneously without a history of penetrating trauma or an immunocompromising condition (3, 226, 227). The portal of entry is often unknown; some cases begin with a sore throat, suggesting that pharyngitis may have led to bacteremia and seeding of the musculature; however, most cases are without an antecedent illness (108). Nonsteroidal antiinflammatory medications are felt to be a risk factor for these infections (98).
The clinical presentation includes an initial prodromal stage with flu-like symptoms, which may include rash and myalgias. This evolves to intense local muscle pain that is disproportionate to clinical findings as well as local tense swelling and fevers. Although pyomyositis usually involves only one muscle group, GAS necrotizing myositis may have multiple sites of involvement. In addition, compared to pyomyositis, which evolves over several weeks, GAS necrotizing myositis is more rapidly progressive over a course of 1 to 4 days. Progression to bacteremia, toxic shock syndrome, and multiorgan failure can occur swiftly. Furthermore, due to the fulminant nature of this infection, intramuscular pressures may increase, leading to a compartment syndrome.
Laboratory findings show leukocytosis and elevated muscle enzymes; the latter is typically not seen in bacterial pyomyositis. Pathological examination of the involved muscle typically reveals muscle necrosis with gram-positive cocci in chains seen between the muscle bundles. In one case series and review, all patients had GAS cultured from the musculature (3). Bacteremia is common; one report demonstrated that 88% of cases had positive blood cultures when obtained (3). The differential diagnosis of these cases includes necrotizing fasciitis; differentiating features may include the presence of overlying violaceous skin appearance with the formation of bullae, which more commonly occurs with fasciitis rather than myositis. Of course, infrequently, these skin changes may be noted in myositis, and occasionally patients present with both fasciitis and myositis (189). MRI is useful for differentiating the necrotizing myositis and fasciitis but should not delay surgical management.
Treatment requires immediate surgical exploration and debridement. Due to elevated compartment pressures, fasciotomies are often necessary. Repeated surgical evaluations are typically performed to ensure all infected or necrotic tissues are excised; occasionally, amputation is required. Wound, and often blood, cultures grow GAS. Other types of streptococci (group G) have been described, albeit less commonly (244).
Antibiotic therapy includes high-dose intravenous penicillin G (4 million units intravenously, every 4 h) in combination with clindamycin (600 to 900 mg intravenously, every 8 h). Clindamycin is included due to its greater efficacy in streptococcal infections involving a large burden of organisms in the stationary phase of growth (the “Eagle effect”), its long postantibiotic effect, and its inhibition of the production of toxin and other virulence factors via blocking protein synthesis (65, 224). Patients with concurrent streptococcal toxic shock syndrome may benefit from intravenous immunoglobulin, which may neutralize streptococcal exotoxins and has shown a possible benefit in small studies (54). The overall mortality of GAS myositis remains substantial at up to 85% (3, 108, 189).
Group B streptococcal myositis.
S. agalactiae may cause either pyomyositis or myositis, although cases in the literature are sparse. Group B streptococcal musculature infections may occur in association with underlying diabetes mellitus, peripheral vascular disease, malignancy, alcoholism, or other immunocompromising conditions (12, 29, 246). One reported case involved the presence of a foot ulcer and the subsequent development of bacteremia which may have resulted in seeding of the musculature (12). A rare case of myositis developing in the setting of endocarditis due to S. agalactiae has also been reported (168). Treatment is with penicillin and surgical debridement of the infected musculature.
Clostridium species myositis: gas gangrene.
Clostridial myonecrosis, better known as gas gangrene, is usually caused by C. perfringens. This infection occurs in a variety of settings, including traumatic wounds with soil contamination, surgery involving the bowel or biliary system or associated with septic abortions, vascular disease with arterial insufficiency often involving the extremities, and unhygienic injection of medications (e.g., epinephrine) or illicit drugs (e.g., heroin) (16). Common characteristics of inciting events include contamination of the site with Clostridium spp. (which exist in the soil and as part of the gastrointestinal flora of humans) and the presence of devitalized tissue; in addition, the presence of a foreign body has been implicated as a risk factor. Areas of low oxygen concentrations are ideal for these infections; anaerobic conditions impel Clostridium spp. to convert from the spore to the vegetative form, which produces toxins responsible for tissue damage and systemic manifestations.
Less commonly, clostridial myonecrosis may occur spontaneously without a prior wound. These cases are often due to C. septicum bacteremia and usually involve an underlying gastrointestinal process, such as occult colon cancer, bowel infarction, or neutropenic enterocolitis (68). Other Clostridium species (e.g., C. novyi, C. histolyticum, and C. sordellii) have been reported to cause gas gangrene, but less commonly.
Presenting symptoms include intense pain, edema, and a sweet-odorous discharge which occur several hours to a few days (6 h to 3 days) after injury. The wound is initially pale but may evolve to a bronze color with hemorrhagic bullae formation. Classic findings include the presence of gas in the tissues detected by either gas bubbles emitted from the wound or noted on radiographic films. Although gas is often thought to be a sine qua non for gas gangrene, the absence of crepitus or gas on examination should not deter consideration for this diagnosis. The failure of the muscle to contract on stimulation and the lack of bleeding of the wound in the operating room are also characteristic findings.
Laboratory data show leukocytosis and a hemolytic anemia due to the clostridial alpha-toxin. Bacteremia is noted in 15% of cases. Differential diagnosis includes other forms of fulminant myositis, including GAS necrotizing myositis and “nonclostridial myositis,” which is discussed below. Crepitant cellulitis, which is typically more superficial, may be considered in the differential diagnosis. Gram stain evaluation of the wound exudate usually shows a lack of neutrophils and an abundance of gram-positive bacilli with blunt ends.
Treatment requires emergent surgical exploration, debridement, and often excision of infected muscles and fasciotomies. Repeat surgical evaluations are often necessary to ensure that all infected and necrotic tissues have been adequately debrided. Antibiotic coverage is the same as for GAS necrotizing myositis, with both high-dose penicillin G and clindamycin (225). Since gas gangrene oftentimes arises from dirty wounds and involves other organisms in addition to Clostridium spp. in up to 50% of cases (95), broad coverage including antibiotics against gram-negative organisms (e.g., with the addition of a fluoroquinolone) is often initially employed. Coverage should be based on Gram stain and culture results.
The potential benefit of hyperbaric oxygen remains unclear. Theoretically it may be beneficial, since high concentrations of oxygen inhibit clostridial growth and halt alpha-toxin synthesis (36); however, studies are difficult to perform due to the sporadic nature of cases, hence, results to date have been inconclusive (59, 223, 249). The use of this therapy should not delay and/or be used in place of emergent surgical debridement along with antimicrobial therapy. Some have suggested the use of hyperbaric oxygen in cases in which surgical excision was incomplete due to location (e.g., paraspinal or abdominal areas). One issue that often limits the use of hyperbaric oxygen is the transfer of critically ill patients to centers with this therapy. The mortality rate remains 20 to 25%, and those who survive may have disfiguring wounds (95).
Nonclostridial myositis.
Four clinical entities are included in the category of nonclostridial myositis. These include anaerobic streptococcal myositis, synergistic nonclostridial myonecrosis, Aeromonas myonecrosis, and vascular gangrene (28, 171).
Anaerobic streptococcal myositis.
Anaerobic streptococcal myositis resembles clostridial myonecrosis with similar characteristics, including a foul copious exudate, gas in infected tissues, and extensive necrosis of the involved musculature. Differentiating characteristics may include a slower evolution of infection (subacute presentation evolving over 3 to 4 days versus acute presentation over 1 to 2 days), early overlying erythema (versus pallor), muscle contraction on stimulation, and numerous neutrophils in the exudate in cases of anaerobic streptococcal myositis. Causative organisms in this infection include anaerobic streptococci (e.g., Peptostreptococcus) but also may include GAS and S. aureus. Treatment is with early surgical exploration and debridement, along with gram-positive antibiotic coverage. If streptococci alone are the infecting organism(s), penicillin is the drug of choice.
Synergistic nonclostridial myonecrosis.
Synergistic nonclostridial myonecrosis is an infection largely of the subcutaneous tissues and fascia that may also extend into the muscle and overlying skin. The infection is often polymicrobial, consisting of aerobic and anaerobic organisms, such as streptococci (including Peptostreptococcus), Bacteroides, E. coli, Enterobacter, and Klebsiella. A rare case involving group B streptococci with peptostreptococci has been reported (167). Infections most commonly occur in immunosuppressed patients, such as those with neutropenia secondary to cancer therapy, or among diabetics. Management includes emergent and complete debridement of all infected tissues and broad-spectrum antibiotic therapy. A high mortality rate exists for this condition.
Aeromonas myonecrosis.
The third clinical condition is Aeromonas hydrophila myonecrosis, which usually occurs after penetrating trauma in a freshwater environment or tissue injury in association with contact with aquatic animals (96). Progression may be rapid and gas may form within the tissues, similar to gas gangrene. Diagnosis is established by collecting a wound specimen; Gram stain shows gram-negative straight rods with rounded ends, and the culture reveals Aeromonas. Therapy is with immediate surgical debridement and antibiotic therapy against Aeromonas, such as fluoroquinolones, a broad-spectrum or extended-spectrum cephalosporin (e.g., cefepime or ceftazidime), or trimethoprim-sulfamethoxazole. Myositis from Vibrio vulnificus has also been described and is often linked to salt water exposure (123); infections with Vibrio spp. have a predilection to occur among patients with liver disease, but infection may occur in immunocompetent hosts. Doxycycline, ceftazidime, and fluoroquinolones are the antibiotics of choice in these infections.
Vascular gangrene.
Vascular gangrene occurs in the setting of arterial insufficiency, which may arise after trauma or among diabetic patients. A variety of organisms can be implicated, including those listed for synergistic nonclostridial myonecrosis as well as Bacillus spp. and Proteus spp. (117). Therapy is also similar, with antibiotics modified based on intraoperative culture results.
Other Forms of Bacterial Myositis
As noted in the Introduction, a wide variety of bacterial agents may cause myositis (Table 2). Some agents are linked to a classified clinical entity as discussed above. Other bacteria may sporadically cause myalgias, myositis, myopathy, or acute rhabdomyolysis. For instance, endocarditis may cause diffuse myalgias; diagnosis is typically established by positive blood cultures and echocardiography. Syphilis may also be associated with myalgias, and clinical muscle disease has been reported in a few cases, with positive treponemal tests such as the microhemagglutination-Treponema pallidum test (64). Other causes of myalgias and/or myositis include Rocky Mountain spotted fever due to Rickettsia rickettsii, as well as other rickettsial (R. conorii) infections (21, 195); a positive serology is typically used to establish the diagnosis. A case of tularemia myositis has been reported with the diagnosis established by culture showing Francisella tularensis (67). Lyme disease, caused by Borrelia burgdorferi, can cause localized myositis of the orbits and other sites, often near areas of skin or joint involvement (99, 186). A case of rhabdomyolysis associated with Lyme disease has been reported (113). Diagnosis is with a positive Lyme serology confirmed by Western blot analysis.
Mycobacterium spp. may cause myositis by either direct extension from a contiguous source (e.g., an infected joint, bone, or abscess) or less commonly via bacteremia (250, 251). The most common site is a tuberculous psoas abscess which forms by extension from vertebral osteomyelitis (i.e., Pott's disease). Other examples include involvement of the intercostal muscles from extension from the pulmonary tract as well as leg striated muscles due to extension from the knee (251). Overall, muscle involvement in tuberculosis is uncommon, occurring in 1% or less of cases (251). Progression is typically slow and insidious in nature. Examination usually reveals swelling or mass in the affected muscle; local adenopathy is usually absent (250, 251). The lesion can progress with the formation of a sinus tract or simply calcify. Diagnosis is by direct sampling for histopathological examination and culture. Histopathology shows granulomatous inflammation, caseous necrosis, and giant cells; Ziehl-Neelsen staining reveals acid-fast bacilli (250, 251). Cultures become positive in weeks to months. Therapy requires standard antituberculosis medications based on susceptibility results and surgical excision. In addition to M. tuberculosis, infections with Mycobacterium avium complex, M. haemophilum, M. bovis, and M. leprae may involve the musculature (122, 131, 173, 211).
Acute rhabdomyolysis during bacterial infections can occur, but this clinical entity is more often associated with viral pathogens. The bacterium most commonly implicated in the development of rhabdomyolysis is Legionella sp.; other bacteria include streptococci, staphylococci, and gram-negative bacteria, such as Salmonella, Mycoplasma pneumoniae, Neisseria meningitidis, and Leptospira spp. (22, 158, 171, 214, 220, 238).
FUNGAL MYOSITIS
Fungal involvement of the musculature is uncommon but has been described in case reports. Most cases have involved immunocompromised patients; occasionally fungal myositis has been reported in immunocompetent persons. Given the rising prevalence rates of immunocompromised hosts due to advanced therapies for cancer and rheumatologic conditions, as well as the aging of the population, the number of fungal myositis cases may increase.
The symptoms of fungal myositis often overlap with that of bacterial myositis. History of severe immunosuppression and evidence of other sites of fungal infection may point to a fungal etiology. Fungal myositis may be due to Candida spp. (60, 80, 111, 206, 231), Cryptococcus neoformans (17, 78, 87, 102, 116, 165, 210), Histoplasma capsulatum (89, 241, 243), Coccidioides spp. (51, 239), Aspergillus spp. (112, 184), Pneumocystis jiroveci (172), and Fusarium spp. (204) (Table 2). Biopsy with culture is usually required to confirm the diagnosis of the fungal etiology; often the diagnosis of a fungal pathogen is not initially considered and is discovered by histopathologic examination or culture of the muscle tissue. Treatment is with surgical debridement and systemic antifungal agents.
Candida spp.
The most commonly reported cause of fungal myositis is Candida spp. Myositis may develop in the setting of systemic candidiasis. The first case was described in the 1970s in a patient with acute lymphoblastic leukemia which involved diffuse myositis (60). Factors which increase the risk of Candida spp. infections include severe granulocytopenia and broad-spectrum antibiotics; in addition, some have suggested that chemotherapeutic agents (e.g., vinca alkaloids) and steroid therapy may induce a subclinical myopathy which may predispose patients to a subsequent infectious myositis (231).
The triad of fever, rash, and muscle tenderness among candidemic patients suggests myositis (111). Most often the muscle involvement consists of diffuse, multiple microabscesses; however, larger, more discrete fungal abscesses can occur (80, 231). The most common causative organism is C. tropicalis, but a variety of Candida spp., including C. krusei and C. albicans, have been described (60, 80, 111, 231). Imaging with MRI, CT, or ultrasound is useful for localization and for biopsy (206). Muscle biopsy confirms the diagnosis by revealing budding yeast and pseudohyphae; blood cultures may be positive, especially in the setting of systemic infection. Since patients are often quite ill at the time of the development of myositis, muscle involvement is frequently diagnosed postmortem.
Therapy is with antifungal agents such as amphotericin B, an azole, or an echinocandin. Focal infectious collections should be surgically drained. Mortality rates are high. Early diagnosis and treatment is recommended to improve the survival rates; immunosuppressed patients with severe muscle pain should undergo prompt imaging and biopsy to evaluate for possible fungal myositis.
In addition to myositis due to Candida infections, a triad of chronic mucocutaneous candidiasis, myositis, and thymoma has been described (152). Myopathy appears to be autoimmune in nature in these cases, rather than being due to the fungal pathogen itself.
Cryptococcus neoformans
Cryptococcus neoformans infection may rarely present with infectious myositis (78, 87, 210). Most infections with this organism are asymptomatic; however, after the inhalation of the fungus, pulmonary, skin, or central nervous system (CNS) disease may occur. Cases of myositis usually occur in the setting of disseminated cryptococcal disease, but focal infections within the muscle have been reported (210).
Most cases occur among immunosuppressed persons; a review of the literature showed that cases have occurred in transplant recipients and patients with leukemia/lymphoma, AIDS, and hepatitis C viral infection (17, 78, 87, 102, 116, 165, 210). Patients often present with lower extremity pain and swelling. Other sites of myositis have included the paraspinal muscles (210). Some cases have involved cellulitis and necrotizing fasciitis in addition to myositis (87). Muscle biopsy demonstrates the presence of the fungus; histopathology may reveal the absence of inflammation but the presence of intracytoplasmic cryptococcal organisms. The polysaccharide capsule can be identified with mucicarmine or alcian blue stains (78). Diagnosis may be supported by a positive cryptococcal serum antigen. In a review of the literature, all cryptococcal myositis patients had a positive serum antigen test; however, the titers were low (1:16 to 1:32) among cases only involving the muscles (210). An estimated 60% have positive blood cultures (210). Treatment is with amphotericin B, flucytosine, and/or fluconazole as well as surgical debridement of focal purulent collections.
Histoplasma capsulatum
Histoplasmosis is endemic to the Ohio and Mississippi River valleys as well as to South/Central America, Africa, Australia, and Asia. Infection is via inhalation of the dimorphic fungal microconidia into the lungs. Infection is most commonly asymptomatic, but pneumonitis or dissemination may occur. Case reports of myositis exist in the literature, including a case of nodular myositis in an AIDS patient (89). Other cases presented as fulminant necrotizing myofascial infections, which occurred in a transplant recipient and a patient with a rheumatologic condition on immunosuppressive agents (241, 243). Diagnosis has been made by biopsy, with histopathology showing ovoid yeast cells using the Gomori-methenamine or Grocot silver stain and growth of the fungus on culture. The utility of serology or urine histoplasmosis antigen testing in cases of myositis is unclear; in one case, Histoplasma antigen was positive in the serum but negative in the urine (241). The use of these tests in disseminated disease may be useful; however, they can be falsely negative, especially in the setting of underlying immunosuppression. Surgical debridement and amphotericin B are the recommended therapies.
Coccidioides spp.
Coccidioidomycosis is a dimorphic fungal infection endemic to the southwestern United States, as well as areas in Mexico and Central/South America. After inhalation of C. immitis or C. posadasii, most infections are asymptomatic. One-third of cases manifest as pneumonia; 1 to 2% develop disseminated disease to the bones, skin, or CNS. Muscle involvement is rare (239); a case involving a cardiac transplant recipient manifested as a rash, severe myositis, and arthropathy (239). Coccidioides spp. muscular infections may involve the muscle body or occur as abscesses between muscle groups (51). Diagnosis is by biopsy of the involved muscles, which shows pathognomonic spherules filled with endospores and grows Coccidioides spp. on culture. Serology and complement fixation testing are available and are usually positive in disseminated disease. Although the usefulness of these tests for detecting coccidioidal myositis is limited by the small number of reported cases, test results have been positive in cases of muscular involvement (51). Initial treatment is typically with surgical debridement and amphotericin B.
Aspergillus spp.
Aspergillus is a fungal infection that occurs most commonly among immunocompromised persons. Myositis has been described as part of disseminated disease or as a focal infection (112). One case involved an elderly man receiving systemic corticosteroids for myelodysplastic syndrome who developed Aspergillus fumigatus myositis of the calf (184). Aspergillus paraspinal myositis developed after local steroid injections in another patient (112); a similar case involving Mucor spp. has also been reported in conjunction with receipt of a steroid injection (109). Most cases have involved immunosuppressed patients receiving steroids and/or cytotoxic therapy, or AIDS patients with low CD4 cell counts (106, 112). Interestingly, most patients who developed Aspergillus myositis had a history of other opportunistic infections (112).
The route of infection is usually hematogenous spread, although direct inoculation during steroid injection due to lack of disinfection before the procedure was hypothesized in one case (112). Diagnosis is by biopsy with fungal culture. The utility of the galactomann antigenemia test has not been studied in this setting to date (112). Treatment of Aspergillus is with voriconazole (106, 112) or amphotericin B. Most cases have resulted in death, likely due to the immunocompromised host state and the presence of disseminated disease in some of the cases.
Aspergillus has also been implicated in autoimmune myositis; one case report described a patient who developed myositis and pneumonitis after exposure to A. niger that was managed with corticosteroids (137). Unlike the cases of fungal myositis due to a direct infection noted above, this case was due to an immune-mediated myositis.
Pneumocystis jiroveci
Pneumocystis carinii, now known as P. jiroveci, is most notable for causing pneumonia among HIV-infected and other immunosuppressed patients. A single case of myositis presenting as an intramuscular mass in an AIDS patient has been described (172). Diagnosis is by biopsy showing granulomatous inflammation and organisms on Gomori-methenamine silver stain. Treatment is with trimethoprim-sulfamethaxazole.
Fusarium spp.
Fusarium spp. are ubiquitous in the soil and may cause disseminated infections in cancer patients and transplant recipients after entry through the gastrointestinal tract or skin, especially in the presence of an intravenous catheter. Infection may involve the sinus, eye, skin, or other sites. Three reports suggest that patients may develop myositis with symptoms of muscle pain and weakness as part of fusariosis (97, 150, 204). Fungemia as well as culture-positive skin lesions are often present in these cases (97, 204). Diagnosis is by biopsy and culture. Therapy is with voriconazole, posaconazole, or amphotericin B.
In addition to direct muscle involvement by the fungus, cases of myopathy due to mycotoxins after the ingestion of mold-damaged grain have been noted. These cases often also develop bone marrow suppression and have been referred to as “alimentary toxic aleukia” (145).
PARASITIC MYOSITIS
A variety of parasitic infections may encyst in the musculature. The most commonly reported parasitic causes of myositis are Trichinella spp. (trichinosis) (7, 35), Taenia solium (cysticercosis) (1, 69, 146, 198), and Toxoplasma gondii (toxoplasmosis) (176, 179, 191). However, a wide range of other parasites may cause myalgias or myositis. These include Trypanosoma cruzi (Chagas' disease) (62), Sarcocystis spp. (10, 237), Microsporidia spp. (30, 31, 44, 48, 52, 76, 132), Toxocara canis (247), Schistosoma spp. (190), Echinococcus spp. (202), Entamoeba histolytica (164), Spirometra mansonoides (sparganosis) (128), Plasmodium falciparum (malaria) (56, 149, 215), and Onchocerca volvulus (161) (Table 2). In addition, myositis caused by a new muspiceoid nematode was recently reported from Australia (18, 58).
Most parasitic infections which cause myositis are uncommon in the United States and are typically acquired abroad. The presence of eosinophilia and a travel history to a location of endemicity may suggest a possible parasitic etiology of myositis. Also of note, in the tropics, parasitic conditions such as the migration of Dracunculus medinensis (guinea worm) and Toxocara canis (visceral larva migrans) have been proposed as risk factors for cases of bacterial pyomyositis (130, 171, 183); it has been suggested that both the structural and immunologic changes due to migrating larvae may increase the risk of intramuscular pyogenic infections (130).
Trichinella spp.
Trichinosis is a disease that occurs after the ingestion of undercooked muscles of domestic or wild animals containing Trichinella spp.-encysted larvae; humans are incidental hosts. There are at least five species of Trichinella that can cause clinical infections in humans; the most common is T. spiralis. Other species include T. nativa (associated with eating bear meat), T. britovi, T. pseudospiralis, and T. nelsoni. In the United States, there has been a decline in the number of cases of trichinosis and a changing of its epidemiology. During the years 1997 to 2001, only 72 cases were reported in the United States (192). Traditionally, trichinosis has been associated with eating undercooked pork; however, in the United States, there has been a decline in cases involving commercial pork and an increasing number of cases associated with eating undercooked wild animals (e.g., polar bear, wild boar, walrus, cougar, and fox) and noncommercial, home-raised pork (192).
After ingestion of raw or undercooked meats, the gastric enzymes digest the muscle and release the larvae, which are resistant to acid and pass into the small intestine. There they burrow underneath the epithelium and develop into adult worms which may mate and release newborn larvae. Dissemination occurs via the lymphatic system and bloodstream to striated muscles, where the larvae encyst.
Most cases are subclinical. The presence and severity of clinical symptoms are related to the number of larvae ingested as well as host characteristics, such as age, size, and underlying conditions. The incubation period is shorter with larger ingestions. Gastrointestinal symptoms may occur during the first week (days 2 to 7) after ingestion and include nausea, abdominal pains, anorexia, vomiting, and either diarrhea or constipation. Systemic manifestations occur the following week (days 9 to 28) as the larvae penetrate the abdominal wall and disseminate throughout the body, causing symptoms of fevers, myalgias, conjunctival and splinter hemorrhages, and periorbital edema.
Muscle invasion may cause myalgias, swelling, and weakness; the presence and severity of these symptoms are related with the number of larvae per gram of muscle; <10 larvae/gram of muscle are often asymptomatic, while levels of >50 larvae/gram are usually associated with muscular symptoms. For instance, muscle symptoms occurred in 88% of patients during one outbreak, suggesting a high inoculum and burden of disease (7). Myositis initially occurs in the extraocular muscles, followed by the masseters and muscles of the diaphragm, neck, and larynx as well as the limbs; any striated muscle can be involved (35). Rarely, cases have included severe proximal muscle weakness simulating polymyositis (197). Symptoms and signs of myositis peak at 5 to 6 weeks after infection and then begin to wane after the larvae become encapsulated and calcified within the muscles. The degree of myositis may correlate with the level of eosinophilia (74). Some patients experience muscle aches, headaches, and mental apathy for weeks to months after infection. In one outbreak investigation, most patients had resolution of symptoms by 6 months; however, some continued to complain of muscle aches beyond this time (74).
The long-term course and prognosis of most cases are favorable. However, life-threatening manifestations may occur in approximately 2% of cases and are due to larvae passing through organs such as the heart, which may lead to myocarditis, congestive heart failure, and dysrhythmias; the lung, causing pneumonitis; and the brain, leading to meningoencephalitis, seizures, and focal neurologic deficits.
Diagnosis is suggested by the clinical history and the presence of eosinophilia; peripheral eosinophil counts are elevated starting in the second week of illness and may reach very high levels, with some cases with 50% eosinophils (181). Laboratory tests may also reveal leukocytosis, elevated immunoglobulin E, and increased muscle enzymes. The most common method of making the diagnosis is using serologic testing such as an enzyme-linked immunofluorescence assay, fluorescent antibody test, or the bentonite flocculation assay. Antibodies are usually detectable by 2 to 4 weeks after infection; a rising titer is highly suggested of the disease. Diagnosis can also be confirmed by biopsy of a swollen, tender superficial skeletal muscle, often the deltoid or gastrocnemius, near its insertion site. The specimen is compressed between glass slides or digested and then examined for larvae; a PCR test has been described (235).
Therapy in early infection is preferable to reduce the number of larvae in the gastrointestinal tract that may invade the muscles. One study showed that antihelminthic medications have little effect on the larvae within muscles (181). However, some studies have shown that muscle symptoms, enzyme levels, and residual larval infestation resolve more rapidly when treatment is administered (81, 253). Of note, viable larvae may persist despite therapy. The drug of choice is albendazole or alternatively mebendazole; thiabendazole is also active but is less tolerable. Patients should be advised to rest, and myalgias may be treated with analgesics. Severe forms of trichinosis, such as severe myositis, myocarditis, and neurologic complications, are often treated with albendazole in combination with corticosteroids (e.g., prednisone) (180). One study showed a benefit from the use of steroids in terms of hastening symptom resolution, but this study was not a randomized trial (212).
Prevention is by cooking meat to at least 60°C for 4 or more minutes or freezing to −15°C for 20 days. Since T. nativa larvae are resistant to freezing, bear meat must be adequately cooked. Public education regarding the consumption of wild game meat and noncommercial pork is advocated for further reduction in the number of cases in the United States (192).
Taenia solium
The pork tapeworm, Taenia solium, can cause gastroenteritis after a person ingests undercooked pork. Cysticercosis, on the other hand, in which infection becomes systemic, is acquired through the ingestion of T. solium eggs excreted in the feces of humans who are carrying the tapeworm; hence, even vegetarians may acquire this disease. Patients with a Taenia gastrointestinal infection may occasionally develop cysticercosis by fecal-oral autoinfection.
The true number of cases of cysticercosis is unknown, as most infections remain asymptomatic. A review of the literature reported over 1,000 diagnosed cases over the past 25 years in the United States and showed that most cases were acquired outside the United States (245); it also suggested that the prevalence of this infection may be rising, but this may have been impacted by the increased use of CNS imaging. Many U.S. cases occur in states bordering Mexico (especially California). A recent study reported that there were 221 deaths due to cysticercosis in the United States from 1990 to 2002, with Latinos and men being the most affected (221).
After ingestion, the eggs are activated by gastric juices and develop into invasive larvae that penetrate the intestinal wall. From the gastrointestinal tract, organisms disseminate via the bloodstream throughout the body; the larvae have a tropism for specific tissues, especially the CNS, eye, subcutaneous tissues, and muscles. Minimal immune response and inflammation are noted around the larvae, contributing to the lack of initial symptoms. The disease is often silent and usually is not recognized unless the cysticerci begin to degenerate within the CNS, evoking inflammation which leads to seizures or other neurologic signs; cysticercosis is the most common cause of adult-onset seizures worldwide. Other manifestations include retinitis and subcutaneous nodules under the skin.
Involvement of the musculature is usually asymptomatic and may be incidentally detected on radiographs showing calcified cysts referred to as having a “puffed rice” or “spindle-shaped” appearance. Of note, the process of death and calcification appears to occur earlier after infection within striated muscles than in the brain. Occasionally cases of myopathy as well as solitary intramuscular lesions initially mistaken as tumors have been reported (1, 69, 146). A case of disseminated muscular cysticercosis with myositis manifesting as diffuse myalgias and lower extremity weakness due to the inflammatory reaction around dying cysts has been reported (229).
A rare form of muscle involvement that entails large numbers of cysts within the muscle has been described in some developing countries and termed “pseudohypertrophic myopathy” (198, 242). In this condition, there is often symmetric enlargement and weakness of skeletal muscles, especially involving the calf muscles. The etiology of the disorder is unclear but likely results from host responses to the dying larvae (121).
Imaging of the soft tissue or muscles may have classic radiographic appearances of calcified lesions as noted above. In addition, MRI, CT, and ultrasound evaluations may show a clear cyst with a scolex (110). MRI is superior at visualizing the cysts, but calcifications are best seen on CT imaging. Stool specimens may show tapeworm eggs in cases of autoinfection. Confirmation is made by serologic testing such as with enzyme-linked immunosorbent assays using blood or cerebrospinal fluid specimens. Biopsies can also be useful; for example, subcutaneous nodules can be excised and histologically examined.
Treatment of the gastrointestinal form of the infection is with praziquantel or niclosamide. CNS disease is often managed with albendazole and steroids, especially with the presence of viable or degenerating cysts on imaging (57); subarachnoid or intraventricular cysts often require shunt placement and neuroendoscopic surgery, respectively. Muscle involvement with viable cysts is usually treated with albendazole or praziquantel; cases of myositis without evidence of viable cysts and which are asymptomatic may not require therapy (70). Large intramuscular lesions have been excised surgically and treated with albendazole in case reports (69). Patients with myositis should undergo imaging of the CNS to exclude brain involvement. Prevention strategies include improving sanitary conditions.
Toxoplasma gondii
Toxoplasma gondii is an intracellular parasite acquired via ingestion of undercooked meat (usually pork or lamb) containing T. gondii cysts, ingestion of food items contaminated with sporocysts from cat feces, or congenitally. The prevalence of infection varies geographically and increases steadily with age. In the United States, prevalence rates are generally 5 to 20%.
Acute infection among immunocompetent persons is usually asymptomatic. Some patients develop a clinical syndrome 1 to 3 weeks after infection consisting of a “mononucleosis-like” illness of fever, cervical adenopathy, myalgias, and malaise. Typically the illness self-resolves over several weeks. Immunocompromised patients, especially those with AIDS, may develop acute or reactivated disease also involving the CNS, eyes, lungs, or muscles (88).
Rare cases of polymyositis have been reported, mainly among immunocompromised hosts (88, 176, 179, 191). One case was reported in a patient with idiopathic CD4 lymphocytopenia (176), while other cases have been described among HIV-infected patients (88); such cases suggest that immunological disturbances may contribute to the development of inflammatory myositis due to T. gondii. Additional cases of myositis have been noted, including a child with who developed tetraplegia (33). Symptoms include weakness, wasting, and myalgias; many immunocompromised patients also have systemic signs, including fever, encephalitis, and multiorgan dysfunction (88).
Serological testing with a positive IgM confirms the acute diagnosis; reactivated disease is suggested by high IgG titers (88). Isolation of Toxoplasma in tissue culture or identification histologically can be performed. For instance, isolation of T. gondii from the muscle tissue in cases of myositis has been described (32). Treatment is generally not warranted, as infection is usually self-limited. In severe cases, including progressive myositis, sulfadiazine and pyrimethamine are indicated. Prevention is by avoiding undercooked meats and maintaining good personal hygiene.
Trypanosoma cruzi
Chagas' disease is found in the Americas with the highest rates in Bolivia and Brazil. Acquisition most commonly occurs via the bite of the reduviid bug; blood transfusions or congenital transmission may also occur. Most cases in the United States are among immigrants; the risk of disease acquisition during short-term travel is rare, as are infections acquired in the southwestern United States.
Most cases are asymptomatic; however, after an incubation period of 1 to 2 weeks, 10 to 20% of infected persons may develop a febrile illness. The hallmarks of the acute infection include a “chagoma” at the inoculation site, unilateral orbital edema (Romaña's sign), adenopathy, and hepatomegaly. Chronic disease is characterized by the onset of cardiomyopathy or megasyndromes of gastrointestinal tract.
Myositis of skeletal muscles may occur during the acute or chronic stages of Chagas' disease, but few data exist among human cases (47); most data are based on animal models showing the occurrence of myositis after experimental T. cruzi infection (140). This may be due to the fact that human infections are often subclinical or that muscular complaints may be overshadowed by other symptoms (47). Myositis of the lower and upper extremities has been described; in addition, cases of ocular myositis and polymyositis have been reported (47, 62). Reactivation of Chagas' disease occurs at a higher rate among AIDS patients, and these cases may also present with myositis (62, 75).
Diagnosis of acute infection is by blood smears showing motile trypomastigotes; during the indeterminate and chronic phases, parasitemia is less readily detectable. During these later stages, serology is typically utilized; xenodiagnosis may be available in areas of endemicity. Cases of myositis may be detected by muscle biopsy showing amastigotes in the muscle cells. Treatment of the acute, and perhaps indeterminate phases is with nifurtimox or benzidazole. Antiparasitic therapy in chronic disease is not recommended; rather, disease is managed using medications and procedures to improve symptoms. For example, heart disease is managed by cardiovascular medications and consideration for heart transplantation. Gastrointestinal megasyndromes may require surgical techniques. Prevention of Chagas' disease is by elimination of the insect vectors and screening of the blood supply.
Sarcocystis spp.
Sarcocystosis is a worldwide tissue protozoan infection most commonly seen in southeastern Asia; cases from India, Africa, South America, and Europe have also been reported. An outbreak among U.S. servicemen training in Malaysia was recently reported (10). Humans are incidentally infected through ingestion of Sarcocystis organisms and may develop one of two clinical syndromes. Humans may be the definitive host for some Sarcocystis species (e.g., S. hominis and S. suihominis) after ingestion of undercooked meat (beef or pork) giving rise to enteritis. Humans may also be an intermediate host after ingestion of food items contaminated with sporocysts from the feces of a carnivore, which leads to disseminated disease including the formation of muscle cysts; myositis develops in these cases as the cysts disintegrate and is referred to as sarcosporidiosis.
Only a few clinical cases have been described, including 41 cases reported involving the skeletal muscles and 11 involving the myocardium (10, 19). However, subclinical infections are common, especially among residents in areas of endemicity (i.e., southeastern Asia); an autopsy study showed a prevalence of 20% of skeletal muscle sarcocystosis (258).
Symptoms of skeletal myositis include painful muscle swellings, which occur episodically, lasting from days to weeks. Symptoms may be due to both direct parasitic activity and from immune-mediated responses (10). Myalgias, weakness, tenderness, and fasiculations are commonly described. Other symptoms may include fevers, rash, bronchospasm, and subcutaneous nodules. Symptoms of myositis may be chronic and relapsing in nature (237).
Diagnosis is suggested by peripheral eosinophilia and travel in an endemic area. Elevated creatine kinase, aldolase, and liver function tests can be seen (10). A serologic test using Western blot analysis has been described (10, 90), but the diagnosis is usually confirmed by muscle biopsy showing sarcocysts. Treatment is largely symptomatic; corticosteroids are used to reduce symptoms. Some reports have suggested symptomatic benefit from albendazole or cotrimoxazole therapy (10, 237), but controlled trials are lacking. Recurrent or persistent symptoms of myositis may occur, suggesting a chronic Sarcocystis infection (114). Prevention is by avoidance of food items that may be contaminated by feces of wild animals.
Microsporidia spp.
Microsporidia are protozoan parasites found widespread in nature, especially in fish and insects. The route of human infection is unclear but may involve eating undercooked meats or via insect bites. Microsporidiosis has become more recognized as a human pathogen since the HIV epidemic, as most infections occur among immunosuppressed persons (44, 132). AIDS patients with CD4 cell counts of <100 cells/mm3 are at highest risk. The use of tumor necrosis factor alpha antagonists among patients with rheumatologic conditions has also been cited as a risk factor (48). There are reports of disseminated disease in immunocompetent persons, but these are uncommon, and infections in this group are typically asymptomatic. The disease spectrum of microsporidiosis includes diarrhea, keratoconjunctivitis, hepatitis, cholangitis, genitourinary infections, myositis, and other sites of disseminated disease.
There are currently 15 species known to infect humans; of these, Trachipleistophora hominis and Trachipleistophora anthropophthera, Pleistophora ronneofiei, and also Brachiola algerae, Brachiola vesicularum, and Brachiola connori have been reported to cause myositis (52). Microsporidia myositis has recently been reviewed, with only nine human cases reported in the literature (52). Clinical symptoms include muscle pain, weakness, wasting, and fever. Creatine kinase levels are often elevated. Diagnosis is by muscle biopsy with light and electron microscopic examination. Treatment is with albendazole; therapy with both albendazole and itraconazole in combination has been reported with mixed results (31, 48). Prevention measures are unclear, since the route of infection is unknown; however, sanitary measures and avoidance of insect bites may be useful.
Toxocara canis
Toxocara canis, the dog tapeworm, is acquired by humans via the accidental ingestion of Toxocara eggs. Children are most commonly affected due to their exposure while playing in contaminated areas. The two predominant syndromes include visceral larva migrans (manifesting as fever, hepatomegaly, and wheezing) and ocular larva migrans (visual loss). Cases involving transient myositis have been reported (247), as have cases of myocarditis (2). Toxocara migration has also been cited as a potential risk factor for the development of bacterial pyomyositis (130, 183). Eosinophilia is present, and the diagnosis is usually made by serologic testing. Therapy is with albendazole for cases of visceral larva migrans, whereas its use in ocular disease is not beneficial; laser photocoagulation may be useful for eradicating migrating ocular larvae. Patients with severe symptoms as well as ocular involvement should receive corticosteroids. In the case reports regarding skeletal myositis, symptoms were short term and self-resolved without specific treatment (247). Prevention is by precluding children from playing in areas potentially contaminated with Toxocara eggs.
Other Parasites
Schistosoma species may cause myalgias during the migration of schistosomula and egg deposition. The syndrome of Katayama fever usually occurs six weeks after exposure to fresh water from which cercariae can penetrate through the skin (190). Most cases of Katayama fever are due to Schistosoma japonicum (found in Asia) or Schistosoma mansoni (found in Africa, South America, and the Arabian Peninsula). In addition to myalgias, individuals with Katayama fever typically present with fever, chills, cough, headache, abdominal tenderness, and urticaria resembling a serum sickness-like disease (190).
Another form of muscle involvement after S. mansoni infection has been described in reports involving localized and diffuse muscle weakness and atrophy (141, 142); confirmation regarding the association between Schistosoma infection and the development of myopathy is needed. Diagnosis of a schistosomal infection is suggested by travel history, eosinophilia, and perhaps a history of swimmers itch. Stool examination using the Kato thick smear technique to identify schistosome eggs or serologic tests can establish the diagnosis. Treatment is with praziquantel. Therapy in acute disease is advised to prevent the possible long-term complications of schistosomiasis including hepatosplenic or genitourinary disease. Prevention is by avoidance of swimming in contaminated water.
Echinococcus spp. are found in the Middle East, Central/South America, Russia, and parts of Europe and usually cause cystic disease of the lungs or intraabdominal organs. Occasionally, echinococci cause skeletal muscular infections in <1 to 4% of cases. Patients may present with symptoms of a slowly growing mass or acute pain due to cyst rupture. Alternatively, muscular involvement may be incidentally detected on radiographic studies showing a calcified Echinococcus cyst (34, 202). The thigh is a common location for cysts, but paraspinal, pelvic, deltoid, and psoas locations have also been described (73, 202, 230). Some cases were initially misdiagnosed as tumors (11, 107). One report suggested that preexisting muscle disease, such as myotonia congenital, may increase the risk for echinococcal involvement of skeletal muscles (202); however, most cases have occurred among patients without preexisting muscle disease. Disease is acquired through the ingestion of contaminated food or water with echinococcal eggs. Diagnosis is confirmed with a biopsy showing the multilaminar cyst membrane characteristic of this infection; serologic tests may be obtained but may be negative in skeletal infections (230, 240). A PCR has been described to detect Echinococcus spp. DNA within the cyst fluid (240). Treatment of muscular lesions is primary, with surgical removal as well as albendazole therapy (9, 34). A modified percutaneous approach for drainage was recently described for muscular lesions (166). Chemotherapy with albendazole should be given before surgery if there is a risk of spillage of cyst that may lead to an anaphylactic reaction. Some cases have used combination therapy with both albendazole and praziquantel in the setting of spillage of cyst contents during surgery (230) and in inoperable hydatid disease (129).
Entamoeba infections most often result in gastrointestinal infection and liver abscesses. Case reports of abscess formation in the skeletal musculature have been rarely reported, including one case with a psoas abscess due to E. histolytica (164). Experimental animal models have demonstrated that Entamoeba may lead to muscular abscesses (39), but clinically cases have been overall uncommon. Treatment is with surgical drainage and metronidazole.
Spirometra spp. cause sparganosis, a cestode infection acquired by drinking contaminated water or, in Asia, by ingesting raw snakes or frogs. Symptomatic infections are characterized by the appearance of subcutaneous nodules. Occasionally, Spirometra can involve the brain and other critical locations. Scant cases of muscular involvement have been reported, including orbital myositis and pyomyositis of the thigh (125, 128). Diagnosis is by biopsy of the lesion. Treatment is with surgical debridement along with praziquantel.
Malaria is acquired through the bite of a female Anopheles mosquito. Infection with Plasmodium falciparum commonly causes myalgias and/or myositis. In addition, biochemical changes and histopathologic evidence of skeletal muscle damage (by muscle enzyme elevations or muscle biopsy results, respectively) have been frequently noted (56, 149). Rhabdomyolysis and myoglobinuria with acute renal failure are rare complications of severe P. falciparum infection (215). Rhabdomyolysis due to other Plasmodium spp. (e.g., P. vivax) has been very rarely noted; one case report occurred in a patient with a concurrent muscular enzyme deficiency (177). Diagnosis is established by identification of Plasmodium spp. on thin and thick blood smears. Therapy is with antimalarial agents, such as quinidine gluconate or quinine sulfate for severe cases.
Onchocerca volvulus causes river blindness in Africa and Central/South America after a person is bitten by a Simulium black fly. Most commonly, skin disease, nodules over bony prominences, and eye disease develop. Eosinophilic myositis has been reported in a single case (161). Diagnosis is by biopsy showing microfilariae; ivermectin is the drug of choice.
VIRAL MYOSITIS
A variety of viruses may cause myalgias, polymyositis, or virus-associated rhabdomyolysis. Often the symptoms of myositis are diffuse in nature, and patients have other symptoms and signs attributable to the causative viral pathogen. The most commonly reported causes of viral myositis in the United States are influenza A and B viruses (6, 85, 101, 138, 147, 154). Several other viral pathogens, including enteroviruses (23, 63, 79, 84, 115, 252), HIV (37, 53), human T-cell leukemia-lymphoma virus (HTLV) type 1 (153, 162), and hepatitis viruses (B and C) (61, 148, 163, 175) may cause myositis and are discussed below.
Influenza A and B Viruses
Influenza virus infections occur almost every winter in the Northern Hemisphere and typically manifest as fever, headache, cough, and rhinorrhea. Myalgias may also be a common initial complaint (occurring before or along with the systemic symptoms) and are usually diffuse in nature. Most influenza cases manifest as an upper respiratory infection or pneumonia. Nonpulmonary complications include myositis, myocarditis, and meningoencephalitis (228).
Myositis due to influenza was first clinically described in the 1950s as “myalgias cruris epidemica” and was proven to be due to the influenza virus in 1970 (138, 154). It is characterized by the sudden onset of calf pain often accompanied by difficulty with ambulation. Myositis is distinct from the initial symptoms of myalgias by its later onset, more focal location, and more severe intensity. Myositis is an overall common finding occurring in 6% and 34% of children with type A or B infection, respectively (101). Muscle involvement is more common in influenza B virus infections, perhaps due to the presence of a unique protein (NB protein) used for viral entry which may make B viruses more myotrophic than influenza A viruses (24, 101, 208); more studies are needed.
Most cases of myositis have been described in school-aged children (mean age, 8 years); children may be more susceptible to muscle involvement due to the influenza virus's tropism toward more immature muscle cells seen in animal studies (55, 208). Cases have been described among adults, including the elderly, albeit, less frequently (6, 263).
A review of 316 cases found that muscle symptoms usually begin in the early convalescent phase of the illness approximately 3 days (range 0 to 18 days) after the onset of fever and respiratory symptoms (6). Boys are most commonly affected, with a 2:1 predominance (6). Calf muscles are often involved; examination may reveal tenderness on palpation and the presence of gait difficulties (6). Among cases of influenza virus-associated myositis, in two-thirds of cases, the calf muscles were affected alone, whereas one-third of cases occurred in multiple muscular groups in addition to the calf muscles.
Laboratory tests usually reveal an elevated creatine kinase level; lactic dehydrogenase and aspartate aminotransferase levels may also be increased (6, 101). If an electromyogram is performed, there are myopathic changes. Diagnosis is usually made by the clinical history, presence of influenza in the community, and by detecting influenza virus in nasopharyngeal specimens. Muscle biopsy is not routinely recommended; however, the virus has been isolated from muscle tissue (85). Interestingly, histopathologic examination of muscle reveals degeneration and necrosis, with overall little inflammatory infiltrates; in mild cases, the findings may be patchy (6, 55).
Treatment is symptomatic, as myositis is self-limited, usually resolving within a mean of 3 days (range, 1 to 30 days) (6). Antiviral medications (e.g., neuraminidase inhibitors) are useful for decreasing the duration of respiratory and systemic symptoms if administered within 36 h of symptom onset. However, myositis occurs after the onset of systemic symptoms, and hence this window of time is usually surpassed. The efficacy of antiviral treatment for acute benign myositis is unknown and hence is not currently recommended.
Influenza virus-associated rhabdomyolysis has also been described. In fact, a recent study of the most common viruses implicated in rhabdomyolysis included influenza virus, HIV, and enteroviral infections in descending order of frequency, with 42% of cases being due to influenza virus (214). In a review of over 300 cases of influenza myositis, 3% were accompanied by rhabdomyolysis (6). Influenza A is more often associated with rhabdomyolysis than type B, and its occurrence is more frequents in girls (6); both findings are opposite to that seen in acute benign myositis. Cases have been noted in both children and adults (8, 154). Influenza virus-associated rhabdomyolysis may be complicated by renal failure and the development of a compartment syndrome (6, 101).
Enteroviruses
Enteroviruses, including coxsackieviruses (group A and B) and enteric cytopathogenic human orphan (ECHO) viruses, may also cause myositis (115, 119). Most common among this group is coxsackievirus B infections manifesting as pleurodynia (also known as Bornholm disease) (252). Infections are typically noted among children who present in the summer or fall with paroxysms of severe, sharp chest pains. The costochondral muscles may be tender on palpation.
In addition to pleurodynia, cases of rhabdomyolysis due to coxsackieviruses and ECHO viruses have been reported (23, 63, 79, 119, 178). Cases due to coxsackieviruses occurred over a wide range of ages (9 to 57 years) with a male/female ratio of 0.6 (79). A variety of serotypes caused these infections, including A9, A16, and B2-B6. The pathogenesis of myositis is somewhat uncertain, but muscle biopsies have shown degenerative necrosis of the muscle fibers and picornavirus-like structures (84). Further evidence of coxsackieviruses' ability to directly cause myositis is that the virus has been shown to produce muscle damage in animal models. Diagnosis is usually made on the basis of the clinical picture alone. The virus can be detected in stool samples or by serologic testing; a fourfold rise in the titer suggests an acute infection. Muscle biopsies are not warranted. Therapy is symptomatic; the disease usually resolves in several days, but recurrences of pleurodynia have been described in up to one-fourth of cases (252).
HIV
HIV infection can cause a wide range of skeletal disorders, including myopathy, polymyositis, and rhabdomyolysis (37, 53, 71). HIV has been found in lymphoid cells surrounding the muscle tissue, but not within muscle fibers, suggesting that the pathogenesis of myositis is immune or cytokine mediated (264). A case of polymyositis in a patient coinfected with HIV and hepatitis C virus did not find either virus in muscle examination using PCR (188). Muscular disease may occur anytime during HIV infection, including during acute infection or during late-stage disease (118). Diagnosis is often by exclusion; other causes of myopathy that are common among HIV-infected patients must be excluded, such as medications (e.g., zidovudine and didanosine), opportunistic infections, and wasting syndromes with muscle weakness (37, 118). Management of cases involves supportive care, including anti-inflammatory medications; one review showed that immunosuppressive agents (e.g., corticosteroids, azathioprine, and methotrexate) may be useful (118).
HTLV-1
HTLV-1 is the cause of adult T-cell leukemia/lymphoma and tropical spastic paraparesis/HTLV-1-associated myelopathy. It is endemic to the Caribbean, Africa, Japan, and the southern United States. Polymyositis has been diagnosed in association with HTLV-1 infections in locations of endemicity (153, 162). Like HIV, the pathological mechanism is thought to be immunologically mediated (134, 264).
Hepatitis B and C Viruses
Hepatitis viral infections have been associated with a variety of musculoskeletal syndromes, including polyarthritis, cryoglobulinemia, and polymyalgia rheumatica. Both hepatitis B and C viral infections have also been associated with the development of polymyositis in case reports (61, 148, 163, 175). One case developed polymyositis in association with hepatitis B surface antigen-positive hepatitis; the severity of the myositis appeared to be associated with hepatitis flares in this case (163). Symptoms include myalgias and proximal muscle weakness. Polymyositis due to hepatitis B or C virus is likely an immune-mediated process; patients have been treated with corticosteroids.
Other Viruses
A variety of viruses have been described as causes of benign acute myositis and/or rhabdomyolysis. These include parainfluenza virus (156, 266), adenovirus (194), and respiratory syncytial virus (160). Often there is a history of an upper respiratory or gastrointestinal illness. The diagnosis in these cases is usually suggested by serologic studies or by culture results from nasopharyngeal or stool specimens. Rarely, muscle biopsies have been performed in an attempt to identify the virus by immunofluorescent studies, electron microscopy, or virus isolation. Whether myositis caused by these viruses is due to direct infection of the muscles or as immune-mediated phenomena (by an “innocent bystander” mechanism or by immunologic cross-reactivity due to homology between viral antigens and muscle proteins) is often unclear in these reports (160). Most biopsies have not revealed a direct viral effect, suggesting an immune-mediated pathogenesis.
Other viruses linked to myositis include herpetic viruses, such as cytomegalovirus (139, 160), Epstein-Barr virus (178, 203, 234), herpes simplex virus (203), and varicella-zoster virus (13). Dengue virus has also been associated with myalgias and myositis; a recent report described a severe case of myositis due to dengue virus (77). Finally, evidence that other viruses, such as paramyxovirus (mumps), parvovirus B19, and West Nile virus, cause myositis is limited to date and requires further study (83, 105, 120, 126, 218, 264).
CONCLUSION
Infectious agents which cause myositis include bacterial, fungal, parasitic, and viral pathogens. Manifestations are variable, including local muscle abscesses, diffuse infectious myositis, generalized myalgias, and acute rhabdomyolysis. Some pathogens are associated with a specific clinical presentations (Clostridium perfringens leading to gas gangrene), whereas other microbes (e.g., viruses) can lead to a variety of types of muscle involvement, including both direct infection and immune- or toxin-mediated injury. Bacterial and fungal myositis is typically localized to a specific muscle group and in temperate areas often occurs among immunosuppressed patients. Viral myositis is often diffuse in nature, while parasitic myositis often has a suggestive travel history and concurrent eosinophilia. Diagnosis is based on the clinical picture and the associated laboratory and radiological data. Treatment often requires a multispecialty approach, including infectious disease specialists, radiologists, and surgeons.
REFERENCES
- 1.Abdelwahab, I. F., M. J. Klein, G. Hermann, and M. Abdul-Quader. 2003. Solitary cysticercosis of the biceps brachii in a vegetarian: a rare and unusual pseudotumor. Skeletal Radiol. 32:424-428. [DOI] [PubMed] [Google Scholar]
- 2.Abe, K., H. Shimokawa, T. Kubota, Y. Nawa, and A. Takeshita. 2002. Myocarditis associated with visceral larva migrans due to Toxocara canis. Intern. Med. 41:706-708. [DOI] [PubMed] [Google Scholar]
- 3.Adams, E. M., S. Gudmundsson, D. E. Yocum, R. C. Haselby, W. A. Craig, and W. R Sundstrom. 1985. Streptococcal myositis. Arch. Intern. Med. 145:1020-1023. [PubMed] [Google Scholar]
- 4.Adamski, G. B., E. H. Garin, W. E. Ballinger, and S. T. Shulman. 1980. Generalized nonsuppurative myositis with staphylococcal septicemia. J. Pediatr. 96:964-967. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal, S. N., A. J. Dwivedi, and M. Khan. 2002. Primary psoas abscess. Dig. Dis. Sci. 47:2103-2105. [DOI] [PubMed] [Google Scholar]
- 6.Agyeman, P., A. Duppenthaler, U. Heininger, and C. Aebi. 2004. Influenza-associated myositis in children. Infection 32:199-203. [DOI] [PubMed] [Google Scholar]
- 7.Akar, S., O. Gurler, E. Pozio, F. Onen, I. Sari, E. Gerceker, A. J. Gunes, B. Akinci, M. Birlik, and N. Akkoc. 2007. Frequency and severity of musculoskeletal symptoms in humans during an outbreak of trichinellosis caused by Trichinella britovi. J. Parasitol. 93:341-344. [DOI] [PubMed] [Google Scholar]
- 8.Ansaloni, L. 1996. Tropical pyomyositis. World J. Surg. 20:613-617. [DOI] [PubMed] [Google Scholar]
- 9.Arazi, M., M. Erikoglu, K. Odev, R. Memik, and M. Ozdemir. 2005. Primary echinococcus infestation of the bone and muscles. Clin. Orthop. Relat. Res. 432:234-241. [DOI] [PubMed] [Google Scholar]
- 10.Arness, M. K., J. D, Brown, J. P. Dubey, R. C. Neafie, and D. E. Granstrom. 1999. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am. J. Trop. Med. Hyg. 61:548-553. [DOI] [PubMed] [Google Scholar]
- 11.Ates, M., and M. Karakaplan. 2007. Hydatid cyst in the biceps and gluteus muscles: case report. Surg. Infect. 8:475-478. [DOI] [PubMed] [Google Scholar]
- 12.Back, S. A., T. O'Neill, G. Fishbein, and G. Gwinup. 1990. A case of group B streptococcal pyomyositis. Rev. Infect. Dis. 12:784-787. [DOI] [PubMed] [Google Scholar]
- 13.Badilla, J., and P. J. Dolman. 2007. Orbital myositis involving the oblique muscles associated with herpes zoster ophthalmicus. Ophthal. Plast. Reconstr. Surg. 23:411-413. [DOI] [PubMed] [Google Scholar]
- 14.Bagul, N. B., A. M. Abeysekara, and S. Jacob. 2008. Primary psoas abscess due to Streptococcus milleri. Ann. Clin. Microbiol. Antimicrob. 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baier, P. K., G. Arampatzis, A. Imdahl, and U. T. Hopt. 2006. The iliopsoas abscess: aetiology, therapy, and outcome. Langenbecks Arch. Surg. 391:411-417. [DOI] [PubMed] [Google Scholar]
- 16.Bangsberg, D. R., J. I. Rosen, T. Aragón, A. Campbell, L. Weir, and F. Perdreau-Remington. 2002. Clostridial myonecrosis cluster among injection drug users: a molecular epidemiology investigation. Arch. Intern. Med. 162:517-522. [DOI] [PubMed] [Google Scholar]
- 17.Barber, B. A., J. M. Crotty, R. G. Washburn, and P. S. Pegram. 1995. Cryptococcus neoformans myositis in a patient with AIDS. Clin. Infect. Dis. 21:1510-1511. [DOI] [PubMed] [Google Scholar]
- 18.Basuroy, R., R. Pennisi, T. Robertson, R. Norton, J. Stokes, J. Reimers, and J. Archer. 2008. Parasitic myositis in tropical Australia. Med. J. Aust. 188:254-256. [DOI] [PubMed] [Google Scholar]
- 19.Beaver, P. C., K. Gadgil, and P. Morera. 1979. Sarcocystis in man: a review and report of five cases. Am. J. Trop. Med. Hyg. 28:819-844. [PubMed] [Google Scholar]
- 20.Beavers, B. R. 1992. Fusobacterium nucleatum pyomyositis. Orthopedics 15:208-211. [DOI] [PubMed] [Google Scholar]
- 21.Behar, D. M., and H. Ben-Ami. 2001. Myositis accompanying Rickettsia conorii infection. Isr. Med. Assoc. J. 3:471-472. [PubMed] [Google Scholar]
- 22.Berger, R. P., and R. M. Wadowksy. 2000. Rhabdomyolysis associated with infection by Mycoplasma pneumoniae: a case report. Pediatrics 105:433-436. [DOI] [PubMed] [Google Scholar]
- 23.Berlin, B. S., N. M. Simon, and R. N. Bovner. 1974. Myoglobinuria precipitated by viral infection. JAMA 227:1414-1415. [PubMed] [Google Scholar]
- 24.Betakova, T., M. V. Nermut, and A. J. Hay. 1996. The NB protein is an integral component of the membrane of influenza B virus. J. Gen. Virol. 77:2689-2694. [DOI] [PubMed] [Google Scholar]
- 25.Bickels, J., L. Ben-Sira, A. Kessler, and S. Wientroub. 2002. Primary pyomyositis. J. Bone Joint Surg. Am. 84:2277-2286. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg, H. M., W. J. Burman, R. E. Chaison, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, A. A. Vernon, et al. 2003. Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 27.Breton, G., Y. Yahiaoui, L. Deforges, A. Lebrun, M. Michel, and B. Godeau. 2007. Psoas abscess: an unusual manifestation of Q fever. Eur. J. Intern. Med. 18:66-68. [DOI] [PubMed] [Google Scholar]
- 28.Brook, I. 2007. Microbiology and management of soft tissue and muscle infections. Int. J. Surg. 30:1-11. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell, D. S., G. W. Kernodle, and H. F. Seigler. 1986. Pectoralis pyomyositis: an unusual cause of chest wall pain in a patient with diabetes mellitus and rheumatoid arthritis. J. Rheumatol. 13:434-436. [PubMed] [Google Scholar]
- 30.Cali, A., P. M. Takvorian, E. Keohane, and L. M. Weiss. 1997. Opportunistic microsporidian infections associated with myositis. J. Eukaryot. Microbiol. 44:86S. [DOI] [PubMed] [Google Scholar]
- 31.Cali, A., P. M. Takvorian, S. Lewin, M. Rendel, C. S. Sian, M. Wittner, H. B. Tanowitz, E. Keohane, and L. M. Weiss. 1998. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J. Eukaryot. Microbiol. 45:240-251. [DOI] [PubMed] [Google Scholar]
- 32.Calicó, I., E. Caballero, O. Martinez, L. Arcalís Arce, L. Llopart, I. Ocaña, and C. Juste. 1991. Isolation of Toxoplasma gondii from immunocompromised patients using tissue culture. Infection 19:340-342. [DOI] [PubMed] [Google Scholar]
- 33.Calore, E. E., R. Minkovski, Z. Khoury, A. C. Seguro, N. M. Perez Calore, and M. J. Cavaliere. 2000. Skeletal muscle pathology in 2 siblings infected with Toxoplasma gondii. J. Rheumatol. 27:1556-1559. [PubMed] [Google Scholar]
- 34.Cankorkmaz, L., H. Ozturk, G. Koyluoglu, M. H. Atalar, and M. S. Arslan. 2007. Intermuscular hydatid cyst in a 4-year-old child: a case report. J. Pediatr. Surg. 42:1946-1948. [DOI] [PubMed] [Google Scholar]
- 35.Capo, V., and D. D. Despommier. 1996. Clinical aspects of infection with Trichinella spp. Clin. Microbiol. Rev. 9:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapnick, E. K., and E. I. Abter. 1996. Necrotizing soft-tissue infections. Infect. Dis. Clin. North Am. 10:835-855. [DOI] [PubMed] [Google Scholar]
- 37.Chariot, P., E. Ruet, F. J. Authier, Y. Lévy, and R. Gherardi. 1994. Acute rhabdomyolysis in patients infected by human immunodeficiency virus. Neurology 44:1692-1696. [DOI] [PubMed] [Google Scholar]
- 38.Chen, W. S., and Y. L. Wan. 1996. Iliacus pyomyositis mimicking septic arthritis of the hip joint. Arch. Orthop. Trauma Surg. 115:233-235. [DOI] [PubMed] [Google Scholar]
- 39.Chiari, L., J. Guerrero, and C. Negrão dos Santos. 1978. Experimental muscular amebiasis in hamsters as a biological model. Z. Parasitenkd. 56:107-114. [DOI] [PubMed] [Google Scholar]
- 40.Chiedozi, L. C. 1979. Pyomyositis. Review of 205 cases in 112 patients. Am. J. Surg. 137:255-259. [DOI] [PubMed] [Google Scholar]
- 41.Chong, K. W., and B. K. Tay. 2004. Piriformis pyomyositis: a rare cause of sciatica. Singapore Med. J. 45:229-231. [PubMed] [Google Scholar]
- 42.Christin, L., and G. A. Sarosi. 1992. Pyomyositis in North America: case reports and review. Clin. Infect. Dis. 15:668-677. [DOI] [PubMed] [Google Scholar]
- 43.Christopher-Stine, L., and P. H. Plotz. 2004. Myositis: an update on pathogenesis. Curr. Opin. Rheumatol. 16:700-706. [DOI] [PubMed] [Google Scholar]
- 44.Chupp, G. L., J. Alroy, L. S. Adelman, J. C. Breen, and P. R. Skolnik. 1993. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin. Infect. Dis. 16:15-21. [DOI] [PubMed] [Google Scholar]
- 45.Chusid, M. J., W. C. Hill, J. A. Bevan, and J. R. Sty. 1998. Proteus pyomyositis of the piriformis muscle in a swimmer. Clin. Infect. Dis. 26:194-195. [DOI] [PubMed] [Google Scholar]
- 46.Collazos, J., A. Fernández, E. Martínez, J. Mayo, and J. M. de la Viuda. 1996. Pneumococcal pyomyositis. Case report, review of the literature, and comparison with classic pyomyositis caused by other bacteria. Arch. Intern. Med. 156:1470-1474. [DOI] [PubMed] [Google Scholar]
- 47.Cossermelli, W., H. Friedman, E. H. Pastor, M. R. Nobre, A. Manzione, M. E. Camargo, and M. Shiroma. 1978. Polymyositis in Chagas's disease. Ann. Rheum. Dis. 37:277-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coyle, C. M., L. M. Weiss, L. V. Rhodes III, A. Cali, P. M. Takvorian, D. F. Brown, G. S. Visvesvara, L. Xiao, J. Naktin, E. Young, M. Gareca, G. Colasante, and M. Wittner. 2004. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N. Engl. J. Med. 351:42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crum, N. F. 2004. Bacterial pyomyositis in the United States. Am. J. Med. 117:420-428. [DOI] [PubMed] [Google Scholar]
- 50.Crum-Cianflone, N. F., and R. Mayer. 2008. An unusual case of Lemierre's syndrome presenting as pyomyositis. Am. J. Med. Sci. 335:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crum-Cianflone, N. F., A. A. Truett, N. Teneza-Mora, R. C. Maves, H. M. Chun, M. F. Bavaro, and B. R. Hale. 2006. Unusual presentations of coccidioidomycosis: a case series and review of the literature. Medicine (Baltimore) 85:263-277. [DOI] [PubMed] [Google Scholar]
- 52.Curry, A., N. J. Beeching, J. D. Gilbert, G. Scott, P. L. Rowland, and B. J. Currie. 2005. Trachipleistophora hominis infection in the myocardium and skeletal muscle of a patient with AIDS. J. Infect. 51:e139-e144. [DOI] [PubMed] [Google Scholar]
- 53.Dalakas, M. C., G. H. Pezeshkpour, M. Gravell, and J. L. Sever. 1986. Polymyositis associated with AIDS retrovirus. JAMA 256:2381-2383. [PubMed] [Google Scholar]
- 54.Darenberg, J., N. Ihendyane, J. Sjölin, E. Aufwerber, S. Haidl, P. Follin, J. Andersson, A. Norrby-Teglund, et al. 2003. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 37:333-340. [DOI] [PubMed] [Google Scholar]
- 55.Davis, L. E., and M. Kornfeld. 2001. Experimental influenza B viral myositis. J. Neurol. Sci. 187:61-67. [DOI] [PubMed] [Google Scholar]
- 56.Davis, T. M., E. Pongponratan, W. Supanaranond, S. Pukrittayakamee, T. Helliwell, P. Holloway, and N. J. White. 1999. Skeletal muscle involvement in falciparum malaria: biochemical and ultrastructural study. Clin. Infect. Dis. 29:831-835. [DOI] [PubMed] [Google Scholar]
- 57.Del Brutto, O. H., K. L. Roos, C. S. Coffey, and H. H. García. 2006. Meta-analysis. Cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann. Intern. Med. 145:43-51. [DOI] [PubMed] [Google Scholar]
- 58.Dennett, X., S. J. Siejka, J. R. Andrews, I. Beveridge, and D. M. Spratt. 1998. Polymyositis caused by a new genus of nematode. Med. J. Aust. 168:226-227. [DOI] [PubMed] [Google Scholar]
- 59.Dias, M. D., B. Fontes, R. S. Poggetti, and D. Birolini. 2008. Hyperbaric oxygen therapy: types of injury and number of sessions: a review of 1506 cases. Undersea Hyperb. Med. 35:53-60. [PubMed] [Google Scholar]
- 60.Diggs, C. H., G. M. Eskenasy, J. C. Sutherland, and P. H. Wiernik. 1976. Fungal infection of muscle in acute leukemia. Cancer 38:1771-1772. [DOI] [PubMed] [Google Scholar]
- 61.Di Muzio, A., B. Bonetti, M. Capasso, L. Panzeri, E. Pizzigallo, N. Rizzuto, and A. Uncini. 2003. Hepatitis C virus infection and myositis: a virus localization study. Neuromuscul. Disord. 13:68-71. [DOI] [PubMed] [Google Scholar]
- 62.dos Santos Sde, S., G. M. Almeida, M. L. Monteiro, P. Gemignani, M. I. Duarte, C. M. Toscano, and A. A. Barone. 1999. Ocular myositis and diffuse meningoencephalitis from Trypanosoma cruzi in an AIDS patient. Trans. R. Soc. Trop. Med. Hyg. 93:535-536. [DOI] [PubMed] [Google Scholar]
- 63.Dunnet, J., J. Y. Paton, and C. E. Robertson. 1981. Acute renal failure and Coxsackie viral infection. Clin. Nephrol. 16:262-263. [PubMed] [Google Scholar]
- 64.Durston, J. H., and F. J. Jefferiss. 1975. Syphilitic myositis. Br. J. Vener. Dis. 51:141-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eagle, H. 1952. Experimental approach to the problem of treatment failure with penicillin. I. Group A streptococcal infection in mice. Am. J. Med. 13:389-399. [DOI] [PubMed] [Google Scholar]
- 66.el Hassani, S., E.-M. Echarrab, R. Bensabbah, A. Attaibi, H. Kabiri, K. Bourki, S. Balafrej, and N. Hajjaj-Hassouni. 1998. Primary psoas abscess. A review of 16 cases. Rev. Rhum. Engl. ed. 65:555-559. [PubMed] [Google Scholar]
- 67.Eliasson, H., and E. Back. 2003. Myositis and septicaemia caused by Francisella tularensis biovar holarctica. Scand. J. Infect. Dis. 35:510-511. [DOI] [PubMed] [Google Scholar]
- 68.El-Masry, S. 2005. Spontaneous gas gangrene associated with occult carcinoma of the colon: a case report and review of the literature. Int. Surg. 90:245-247. [PubMed] [Google Scholar]
- 69.Ergen, F. B., B. Turkbey, U. Kerimoglu, K. Karaman, K. Yorganc, and A. Saglam. 2005. Solitary cysticercosis in the intermuscular area of the thigh: a rare and unusual pseudotumor with characteristic imaging findings. J. Comput. Assist. Tomogr. 29:260-263. [DOI] [PubMed] [Google Scholar]
- 70.Evans, C. A. W., H. H. Garcia, and R. H. Gilman. 2000. Cysticercosis, p. 862-866. In G. W. Hunter, G. T. Strickland, A. J. Magill (ed.), Hunter's tropical medicine and emerging infectious diseases, 8th ed. W.B. Saunders Company, Philadelphia, PA.
- 71.Fabricius, E. M., I. Hoegl, and W. Pfaeffl. 1991. Ocular myositis as first presenting symptom of human immunodeficiency virus (HIV-1) infection and its response to high-dose cortisone treatment. Br. J. Ophthalmol. 75:696-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falagas, M. E., P. I. Rafailidis, A. Kapaskelis, and G. Peppas. 2008. Pyomyositis associated with hematological malignancy: case report and review of the literature. Int. J. Infect. Dis. 12:120-125. [DOI] [PubMed] [Google Scholar]
- 73.Feki, W., S. Ghozzi, R. Khiari, J. Ghorbel, H. Elarbi, H. Khouni, and N. Ben Rais. 2008. Multiple unusual locations of hydatid cysts including bladder, psoas muscle and liver. Parasitol. Int. 57:83-86. [DOI] [PubMed] [Google Scholar]
- 74.Ferraccioli, G. F., M. Mercadanti, F. Salaffi, F. Bruschi, M. Melissari, and E. Pozio. 1988. Prospective rheumatological study of muscle and joint symptoms during Trichinella nelsoni infection. Q. J. Med. 69:973-984. [PubMed] [Google Scholar]
- 75.Ferreira, M. S., A. Nishioka Sde, M. T. Silvestre, A. S. Borges, F. R. Nunes-Araújo, and A. Rocha. 1997. Reactivation of Chagas' disease in patients with AIDS: report of three new cases and review of the literature. Clin. Infect. Dis. 25:1397-1400. [DOI] [PubMed] [Google Scholar]
- 76.Field, A. S., D. J. Marriott, S. T. Milliken, B. J. Brew, E. U. Canning, J. G. Kench, P. Darveniza, and J. L. Harkness. 1996. Myositis associated with a newly described microsporidian, Trachipleistophora hominis, in a patient with AIDS. J. Clin. Microbiol. 34:2803-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finsterer, J., and K. Kongchan. 2006. Severe, persisting, steroid-responsive Dengue myositis. J. Clin. Virol. 35:426-428. [DOI] [PubMed] [Google Scholar]
- 78.Flagg, S. D., Y. J. Chang, C. P. Masuell, S. Natarajan, G. Hermann, and M. H. Mendelson. 2001. Myositis resulting from disseminated cryptococcosis in a patient with hepatitis C cirrhosis. Clin. Infect. Dis. 32:1104-1107. [DOI] [PubMed] [Google Scholar]
- 79.Fodili, F., and E. F. van Bommel. 2003. Severe rhabdomyolysis and acute renal failure following recent Coxsackie B virus infection. Neth. J. Med. 61:177-179. [PubMed] [Google Scholar]
- 80.Fornadley, J. A., G. S. Parker, L. S. Rickman, and S. Paparello. 1990. Candida myositis manifesting as a discrete neck mass. Otolaryngol. Head Neck Surg. 102:74-76. [DOI] [PubMed] [Google Scholar]
- 81.Fourestié, V., M. E. Bougnoux, T. Ancelle, M. Liance, F. Roudot-Thoraval, H. Naga, M. Pairon-Pennachioni, J. L. Rauss, and A. Lejonc. 1988. Randomized trial of albendazole versus tiabendazole plus flubendazole during an outbreak of human trichinellosis. Parasitol. Res. 75:36-41. [DOI] [PubMed] [Google Scholar]
- 82.Fowler, A., and A. Mackay. 2006. Community-acquired methicillin-resistant Staphylococcus aureus pyomyositis in an intravenous drug user. J. Med. Microbiol. 55:123-125. [DOI] [PubMed] [Google Scholar]
- 83.Fox, S. A., B. K. Ward, P. D. Robbins, F. L. Mastaglia, and N. R. Swanson. 1996. Inclusion body myositis: investigation of the mumps virus hypothesis by polymerase chain reaction. Muscle Nerve 19:23-28. [DOI] [PubMed] [Google Scholar]
- 84.Fukuyama, Y., T. Ando, and J. Yokota. 1977. Acute fulminant myoglobinuric polymyositis with picornavirus-like crystals. J. Neurol. Neurosurg. Psychiatry 40:775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gamboa, E. T., A. B. Eastwood, A. P. Hays, J. Maxwell, and A. S. Penn. 1979. Isolation of influenza virus from muscle in myoglobinuric polymyositis. Neurology 29:1323-1335. [DOI] [PubMed] [Google Scholar]
- 86.Garner, J. P., P. D. Meiring, K. Ravi, and R. Gupta. 2007. Psoas abscess: not as rare as we think? Colorectal Dis. 9:269-274. [DOI] [PubMed] [Google Scholar]
- 87.Gave, A. A., R. Torres, and L. Kaplan. 2004. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg. Infect. 5:309-313. [DOI] [PubMed] [Google Scholar]
- 88.Gherardi, R., M. Baudrimont, F. Lionnet, J. M. Salord, C. Duvivier, C. Michon, M. Wolff, and C. Marche. 1992. Skeletal muscle toxoplasmosis in patients with acquired immunodeficiency syndrome: a clinical and pathological study. Ann. Neurol. 32:535-542. [DOI] [PubMed] [Google Scholar]
- 89.Goel, D., A. K. Prayaga, N. Rao, and P. Damodaram. 2007. Histoplasmosis as a cause of nodular myositis in an AIDS patient diagnosed on fine needle aspiration cytology. A case report. Acta Cytolog. 51:89-91. [DOI] [PubMed] [Google Scholar]
- 90.Granstrom, D. E., J. P. Dubey, S. W. Davis, R. Fayer, J. C. Fox, K. B. Poonacha, R. C. Giles, and P. F. Comer. 1993. Equine protozoal myeloencephalitis: antigen analysis of cultured Sarcocystis neurona merozoites. J. Vet. Diagn. Investig. 5:88-90. [DOI] [PubMed] [Google Scholar]
- 91.Gruenwald, I., J. Abrahamson, and O. Cohen. 1992. Psoas abscess: case report and review of the literature. J. Urol. 147:1624-1626. [DOI] [PubMed] [Google Scholar]
- 92.Hamdad, F., B. Vidal, Y. Douadi, G. Laurans, B. Canarelli, G. Choukroun, V. Rodriguez-Nava, P. Boiron, B. Beaman, and F. Eb. 2007. Nocardia nova as the causative agent in spondylodiscitis and psoas abscess. J. Clin. Microbiol. 45:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hardjasudarma, M., B. Willis, C. Black-Payne, and R. Edwards. 1995. Pediatric spinal blastomycosis: case report. Neurosurgery 37:534-536. [DOI] [PubMed] [Google Scholar]
- 94.Harrigan, R. A., F. H. Kauffman, and M. B. Love. 1995. Tuberculous psoas abscess. J. Emerg. Med. 13:493-498. [DOI] [PubMed] [Google Scholar]
- 95.Hart, G. B., R. C. Lamb, and M. B. Strauss. 1983. Gas gangrene. J. Trauma 23:991-1000. [DOI] [PubMed] [Google Scholar]
- 96.Heckerling, P. S., T. M. Stine, J. C. Pottage, Jr., S. Levin, and A. A. Harris. 1983. Aeromonas hydrophila myonecrosis and gas gangrene in a nonimmunocompromised host. Arch. Intern. Med. 143:2005-2007. [PubMed] [Google Scholar]
- 97.Helm, T. N., D. H. Longworth, G. S. Hall, B. J. Bolwell, B. Fernandez, and K. J. Tomecki. 1990. Case report and review of resolved fusariosis. J. Am. Acad. Dermatol. 23:393-398. [DOI] [PubMed] [Google Scholar]
- 98.Hird, B., and K. Byrne. 1994. Gangrenous streptococcal myositis: case report. J. Trauma-Injury Infect. Crit. Care 36:589-591. [PubMed] [Google Scholar]
- 99.Holmgren, A. R., and E. L. Matteson. 2006. Lyme myositis. Arthritis Rheum. 54:2697-2700. [DOI] [PubMed] [Google Scholar]
- 100.Horn, C. V., and S. Master. 1968. Pyomyositis tropicans in Uganda. East Afr. Med. J. 45:463-471. [PubMed] [Google Scholar]
- 101.Hu, J. J., C. L. Kao, P. I. Lee, C. M. Chen, C. Y. Lee, C. Y. Lu, and L. M. Huang. 2004. Clinical features of influenza A and B in children and association with myositis. J. Microbiol. Immunol. Infect. 37:95-98. [PubMed] [Google Scholar]
- 102.Hurd, D. D., D. B. Staub, R. I Roelofs, and L. P. Dehner. 1989. Profound muscle weakness as the presenting feature of disseminated cryptococcal infection. Rev. Infect. Dis. 11:970-974. [DOI] [PubMed] [Google Scholar]
- 103.Husain, S., and N. Singh. 2002. Pyomyositis associated with bacillary angiomatosis in a patient with HIV infection. Infection 30:50-53. [DOI] [PubMed] [Google Scholar]
- 104.Hussain, S., A. Sequeira, A. Malik, and J. Huang. 2006. Disseminated actinomycosis with multifocal muscular involvement. J. La. State Med. Soc. 158:189-191. [PubMed] [Google Scholar]
- 105.Ichinose, K., K. Migita, T. Sekita, H. Hamada, H. Ezaki, S. Mukoubara, and K. Eguchi. 2004. Parvovirus B19 infection and myofasciitis. Clin. Rheumatol. 23:184-185. [DOI] [PubMed] [Google Scholar]
- 106.Ichiyasu, H., A. Yamamura, M. Honda, S. Okamoto, K. Tsumori, T. Okamoto, K. Sato, M. Matsumoto, and H. Kohrogi. 2006. Successful treatment by voriconazole for pulmonary and adductor magnus muscle aspergillosis induced by immunosuppressive therapy for hypersensitivity pneumonia. Nihon Kokyuki Gakkai Zasshi 44:754-760. [PubMed] [Google Scholar]
- 107.Insabato, L., G. Marino, F. Fazioli, V. Iacono, M. Mascolo, and L. Palombini. 2007. Primary intramuscular infestation of Echinococcus granulosus misdiagnosed as a soft tissue tumor: a case report. Acta Cytol. 51:631-633. [DOI] [PubMed] [Google Scholar]
- 108.Jahnson, L., L. Berggren, E. Björsell-Ostling, J. Bonnerstig, and H. Holmberg. 1992. Streptococcal myositis. Scand. J. Infect. Dis. 24:661-665. [DOI] [PubMed] [Google Scholar]
- 109.Jain, J. K., A. Markowitz, P. V. Khilanani, and C. B. Lauter. 1978. Localized mucormycosis following intramuscular corticosteroid. Case report and review of the literature. Am. J. Med. Sci. 275:209-216. [DOI] [PubMed] [Google Scholar]
- 110.Jankharia, B. G., G. B. Chavhan, P. Krishnan, and B. Jankharia. 2005. MRI and ultrasound in solitary muscular and soft tissue cysticercosis. Skeletal Radiol. 34:722-726. [DOI] [PubMed] [Google Scholar]
- 111.Jarowski, C. I., M. A. Fialk, H. W. Murray, G. J. Gottlieb, M. Coleman, C. R. Steinberg, and R. T. Silver. 1978. Fever, rash, and muscle tenderness. A distinctive clinical presentation of disseminated candidiasis. Arch. Intern. Med. 138:544-546. [PubMed] [Google Scholar]
- 112.Javier, R. M., J. Sibilia, A. S. Lugger, S. Natarajan-Ame, J. J. Kuntz, and R. Herbrecht. 2001. Fatal Aspergillus fumigatus myositis in an immunocompetent patient. Eur. J. Clin. Microbiol. Infect. Dis. 20:810-813. [DOI] [PubMed] [Google Scholar]
- 113.Jeandel, C., C. Perret, H. Blain, P. Jouanny, F. Penin, and M. C. Laurain. 1994. Rhabdomyolysis with acute renal failure due to Borrelia burgdorferi. J. Intern. Med. 235:191-192. [DOI] [PubMed] [Google Scholar]
- 114.Jeffrey, H. C. 1974. Sarcosporidiosis in man. Trans. R. Soc. Trop. Med. Hyg. 68:17-29. [DOI] [PubMed] [Google Scholar]
- 115.Jehn, U. W., and M. K. Fink. 1980. Myositis, myoglobinemia, and myoglobinuria associated with enterovirus echo 9 infection. Arch. Neurol. 37:457-458. [DOI] [PubMed] [Google Scholar]
- 116.Jiménez-Lucho, V. E., R. Del Busto, and E. J. Fisher. 1985. Disseminated cryptococcosis with muscular involvement. J. Infect. Dis. 151:567. [DOI] [PubMed] [Google Scholar]
- 117.Johnson, D. A., P. L. Aulicino, and J. G. Newby. 1984. Bacillus cereus-induced myonecrosis. J. Trauma 24:267-270. [DOI] [PubMed] [Google Scholar]
- 118.Johnson, R. W., F. M. Williams, S. Kazi, M. M. Dimachkie, and J. D. Reveille. 2003. Human immunodeficiency virus-associated polymyositis: a longitudinal study of outcome. Arthritis Rheum. 49:172-178. [DOI] [PubMed] [Google Scholar]
- 119.Josselson, J., T. Pula, and J. H. Sadler. 1980. Acute rhabdomyolysis associated with an echovirus 9 infection. Arch. Intern. Med. 140:1671-1672. [PubMed] [Google Scholar]
- 120.Kallajoki, M., T. Hyypiä, P. Halonen, C. Orvell, B. K. Rima, and K. Kalimo. 1991. Inclusion body myositis and paramyxoviruses. Hum. Pathol. 22:29-32. [DOI] [PubMed] [Google Scholar]
- 121.Kallen, P. S., J. S. Louie, K. M. Nies, and A. S. Bayer. 1982. Infectious myositis and related syndromes. Semin. Arthritis Rheum. 11:421-439. [DOI] [PubMed] [Google Scholar]
- 122.Katz, D. S., H. Wogalter, R. F. D'Esposito, and B. A. Cunha. 1992. Mycobacterium bovis vertebral osteomyelitis and psoas abscess after intravesical BCG therapy for bladder carcinoma. Urology 40:63-66. [DOI] [PubMed] [Google Scholar]
- 123.Kelly, M. T., and W. F. McCormick. 1981. Acute bacterial myositis caused by Vibrio vulnificus. JAMA 246:72-73. [PubMed] [Google Scholar]
- 124.Kern, L., C. Rassbach, and M. Ottolini. 2006. Streptococcal pyomyositis of the psoas: case reports and review. Pediatr. Emerg. Care 22:250-253. [DOI] [PubMed] [Google Scholar]
- 125.Kim, J. R., and J. M. Lee. 2001. A case of intramuscular sparganosis in the sartorius muscle. J. Korean Med. Sci. 16:378-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kishore, J., and J. Singh. 2006. Detection of parvovirus B19 in a case of erythema infectiosum with myositis. Indian Pediatr. 43:814-817. [PubMed] [Google Scholar]
- 127.Koutures, C. G., M. Savoia, and R. A. Pedowitz. 2000. Staphylococcus aureus thigh pyomyositis in a collegiate swimmer. Clin. J. Sport Med. 10:297-299. [DOI] [PubMed] [Google Scholar]
- 128.Kubota, T., and M. Itoh. 2007. Sparganosis associated with orbital myositis. Jpn. J. Ophthalmol. 51:311-312. [DOI] [PubMed] [Google Scholar]
- 129.Lam, K. S., A. Faraj, R. C. Mulholland, and R. G. Finch. 1997. Medical decompression of vertebral hydatidosis. Spine 22:2050-2055. [DOI] [PubMed] [Google Scholar]
- 130.Lambertucci, J. R., A. Rayes, J. C. Serufo, D. M. Teixeira, R. Gerspacher-Lara, E. Nascimento, G. Brasileiro Filho, and A. C. Silva. 1998. Visceral larva migrans and tropical pyomyositis: a case report. Rev. Inst. Med. Trop. Sao Paulo 40:383-385. [DOI] [PubMed] [Google Scholar]
- 131.Lawn, S. D., T. A. Bicanic, and D. C. Macallan. 2004. Pyomyositis and cutaneous abscesses due to Mycobacterium avium: an immune reconstitution manifestation in a patient with AIDS. Clin. Infect. Dis. 38:461-463. [DOI] [PubMed] [Google Scholar]
- 132.Ledford, D. K., M. D. Overman, and A. Gonzalvo. 1985. Microsporidioisis myositis in a patient with the acquired immunodeficiency syndrome. Ann. Intern. Med. 102:628-630. [DOI] [PubMed] [Google Scholar]
- 133.Lee, Y. T., C. M. Lee, S. C. Su, C. P. Liu, and T. E. Wang. 1999. Psoas abscess: a 10 year review. J. Microbiol. Immunol. Infect. 32:40-46. [PubMed] [Google Scholar]
- 134.Leon-Monzon, M., I. Illa, and M. C. Dalakas. 1994. Polymyositis in patients infected with human T-cell leukemia virus type I: the role of the virus in the cause of the disease. Ann. Neurol. 36:643-649. [DOI] [PubMed] [Google Scholar]
- 135.Levin, M. J., and P. Gardner. 1971. “Tropical” pyomyositis: an unusual infection due to Staphylococcus aureus. N. Engl. J. Med. 284:196-198. [DOI] [PubMed] [Google Scholar]
- 136.Lewis, J. S., II, and J. H. Jorgensen. 2005. Inducible clindamycin resistance in staphylococci: should clinicians and microbiologists be concerned? Clin. Infect. Dis. 40:280-285. [DOI] [PubMed] [Google Scholar]
- 137.Lonneux, M., N. Nolard, I. Philippart, A. Henkinbrant, J. Hamels, J. Fastrez, and J. P. d'Odemont. 1995. A case of lymphocytic pneumonitis, myositis, and arthritis associated with exposure to Aspergillus niger. J. Allergy Clin. Immunol. 95:1047-1049. [DOI] [PubMed] [Google Scholar]
- 138.Lundberg, A. 1957. Myalgia cruris epidemica. Acta Paediatr. 46:18-31. [DOI] [PubMed] [Google Scholar]
- 139.Maeda, M., A. Maeda, H. Wakiguchi, N. Murakami, T. Sata, T. Okada, and T. Kurashige. 2000. Polymyositis associated with primary cytomegalovirus infection. Scand. J. Infect. Dis. 32:212-214. [DOI] [PubMed] [Google Scholar]
- 140.Maldonado, I. R., M. L. Ferreira, E. R. Camargos, E. Chiari, and C. R. Machado. 2004. Skeletal muscle regeneration and Trypanosoma cruzi-induced myositis in rats. Histol. Histopathol. 19:85-93. [DOI] [PubMed] [Google Scholar]
- 141.Mannsour, S. E., P. M. Bauman, H. H. Reese, and G. F. Otto. 1965. Myopathy in mice experimentally infected with Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 59:87-89. [DOI] [PubMed] [Google Scholar]
- 142.Mansours, E.-D., and H. Reese. 1964. A previously unreported myopathy in patients with schistosomiasis. Neurology 14:355-361. [DOI] [PubMed] [Google Scholar]
- 143.Maron, R., D. Levine, T. E. Dobbs, and W. M. Geisler. 2006. Two cases of pott disease associated with bilateral psoas abscesses: case report. Spine 31:E561-E564. [DOI] [PubMed] [Google Scholar]
- 144.Martínez-Aguilar, G., A. Avalos-Mishaan, K. Hulten, W. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2004. Community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus musculoskeletal infections in children. Pediatr. Infect. Dis. J. 23:701-706. [DOI] [PubMed] [Google Scholar]
- 145.Mayer, C. F. 1953. Endemic panmyelotoxicosis in the Russian grain belt. I. The clinical aspects of alimentary toxic aleukia (ATA); a comprehensive review. Mil. Surg. 113:173-189. [PubMed] [Google Scholar]
- 146.McGrill, R. J. 1948. Cysticercosis resembling myopathy. Lancet 6:728-730. [DOI] [PubMed] [Google Scholar]
- 147.Middleton, P. J., R. M. Alexander, and M. T. Szymanski. 1970. Severe myositis during recovery from influenza. Lancet ii:533-535. [DOI] [PubMed] [Google Scholar]
- 148.Mihas, A. A., J. D. Kirby, and S. P. Kent. 1978. Hepatitis B antigen and polymyositis. JAMA 239:221-222. [PubMed] [Google Scholar]
- 149.Miller, K. D., N. J. White, J. A. Lott, J. M. Roberts, and B. M. Greenwood. 1989. Biochemical evidence of muscle injury in African children with severe malaria. J. Infect. Dis. 159:139-142. [DOI] [PubMed] [Google Scholar]
- 150.Minor, Jr., R. L., M. A. Pfaller, R. D. Gingrich, and L. J. Burns. 1989. Disseminated Fusarium infections in patients following bone marrow transplantation. Bone Marrow Transplant. 4:653-658. [PubMed] [Google Scholar]
- 151.Miyake, H. 1904. Beitrage (Beitraege) zur Kenntnis der sogenannten Myositis Infectiosa. Mitt. Grenzgeb. Med. Chir. 13:155-198. [Google Scholar]
- 152.Montes, L. F., R. Ceballos, M. D. Cooper, M. N. Bradley, and D. E. Bockman. 1972. Chronic mucocutaneous candidiasis, myositis, and thymoma. A new triad. JAMA 222:1619-1623. [PubMed] [Google Scholar]
- 153.Morgan, O. S., P. Rodgers-Johnson, C. Mora, and G. Char. 1989. HTLV-1 and polymyositis in Jamaica. Lancet ii:1184-1187. [DOI] [PubMed] [Google Scholar]
- 154.Morgensen, J. L. 1974. Myoglobinuria and renal failure associated with influenza. Ann. Intern. Med. 80:362-363. [DOI] [PubMed] [Google Scholar]
- 155.Mückley, T., T. Schütz, M. Kirschner, M. Potulski, G. Hofmann, and V. Bühren. 2003. Psoas abscess: the spine as a primary source of infection. Spine 28:E106-E113. [DOI] [PubMed] [Google Scholar]
- 156.Muto, S., K. Tabei, Y. Asano, and S. Hosoda. 1987. A case of rhabdomyolysis in chronic renal failure. Jpn. J. Med. 26:76-80. [DOI] [PubMed] [Google Scholar]
- 157.Mynter, H. 1881. Acute psoitis. Buffalo Med. Surg. J. 21:202-210. [PMC free article] [PubMed] [Google Scholar]
- 158.Naschitz, J. E., D. Yeshurum, and I. Shagrawi. 1989. Rhabdomyolysis in pneumococcal sepsis. Am. J. Med. 87:479-480. [DOI] [PubMed] [Google Scholar]
- 159.Natsume, H., T. Nakanishi, Y. Nakagawa, and Y. Igarashi. 1997. A case of an iliopsoas abscess in a neonate. Acta Paediatr. Jpn. 39:459-461. [DOI] [PubMed] [Google Scholar]
- 160.Naylor, C. D., A. M. Jevnikar, and N. J. Witt. 1987. Sporadic viral myositis in two adults. CMAJ 137:819-821. [PMC free article] [PubMed] [Google Scholar]
- 161.Neumann, H., R. Herz, and C. Baum. 1985. Granulomatose und eosinophilic myositis due to Onchocerca volvulus. Pathologe 6:101-107. [PubMed] [Google Scholar]
- 162.Nishikai, M., and A. Sato. 1991. Human T lymphotropic virus type I and polymyositis and dermatomyositis in Japan. Arthritis Rheum. 34:791-792. [DOI] [PubMed] [Google Scholar]
- 163.Nojima, T., M. Hirakata, S. Sato, T. Fujii, A. Suwa, T. Mimori, and Y. Ikeda. 2000. A case of polymyositis associated with hepatitis B infection. Clin. Exp. Rheumatol. 18:86-88. [PubMed] [Google Scholar]
- 164.O'Leary, C., and R. Finch. 1992. Amoebic psoas and liver abscesses. Postgrad. Med. J. 68:972-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O'Neill, K. M., A. H. Ormsby, and R. A. Prayson. 1998. Cryptococcal myositis: a case report and review of the literature. Pathology 30:316-317. [DOI] [PubMed] [Google Scholar]
- 166.Ormeci, N., R. Idilman, S. Akyar, M. Palabiyikoglu, S. Coban, H. Erdem, and F. Ekiz. 2007. Hydatid cysts in muscle: a modified percutaneous treatment approach. Int. J. Infect. Dis. 11:204-288. [DOI] [PubMed] [Google Scholar]
- 167.Overkamp, D., M. Pfohl, R. Klier, B. Domres, and R. M. Schmülling. 1992. Spontaneous gas-forming bacterial myonecrosis caused by group B streptococci and peptostreptococci. Clin. Investig. 70:441-443. [DOI] [PubMed] [Google Scholar]
- 168.Owen, A. 1991. Endocarditis due to Streptococcus agalactiae presenting with myositis. Int. J. Cardiol. 33:333-334. [DOI] [PubMed] [Google Scholar]
- 169.Pannaraj, P. S., K. G. Hulten, B. E. Gonzalez, E. O. Mason, and S. L. Kaplan. 2006. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 43:953-960. [DOI] [PubMed] [Google Scholar]
- 170.Parasca, I., L. Damian, and A. Albu. 2006. Infectious muscle disease. Rom. J. Intern. Med. 44:131-141. [PubMed] [Google Scholar]
- 171.Pasternack, M. S., and M. N. Swartz. 2005. Myositis. p. 1194-1204. In G. L. Mandel, J. E. Bennett, and R. D. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 6th ed. Elsevier, Churchill Livingstone, United Kingdom.
- 172.Pearl, G. S., and B. Sieger. 1996. Granulomatous Pneumocystis carinii myositis presenting as an intramuscular mass. Clin. Infect. Dis. 22:577-578. [DOI] [PubMed] [Google Scholar]
- 173.Pearson, J. M., R. J. Rees, and A. G. Weddell. 1970. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr. Rev. 41:155-166. [DOI] [PubMed] [Google Scholar]
- 174.Peng, S. L. 2002. Rheumatic manifestations of parasitic diseases. Semin. Arthritis Rheum. 31:228-247. [DOI] [PubMed] [Google Scholar]
- 175.Pittsley, R. A., M. A. Shearn, and L. Kaufman. 1978. Acute hepatitis B simulating dermatomyositis. JAMA 239:959. [PubMed] [Google Scholar]
- 176.Plonquet, A., G. Bassez, F. J. Authier, J. M. Dray, J. P. Farcet, and R. K. Gherardi. 2003. Toxoplasmic myositis as a presenting manifestation of idiopathic CD4 lymphocytopenia. Muscle Nerve 27:761-765. [DOI] [PubMed] [Google Scholar]
- 177.Poels, P. J., W. M. Dolmans, and F. J. Gabreëls. 1993. Rhabdomyolysis associated with malaria tertiana in a patient with myoadenylate deaminase deficiency. Trop. Geogr. Med. 45:83-86. [PubMed] [Google Scholar]
- 178.Poels, P. J., J. A. Ewals, E. M. Joosten, and A. M. Van Loon. 1989. Rhabdomyolysis associated with simultaneous Epstein-Barr virus infection and isolation of echovirus 6 from muscle: a dual infection. J. Neurol. Neurosurg. Psychiatry 52:412-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pollock, J. L. 1979. Toxoplasmosis appearing to be dermatomyositis. Arch. Dermatol. 115:736-737. [DOI] [PubMed] [Google Scholar]
- 180.Pozio, E., M. A. Gomez Morales, and J. Dupouy-Camet. 2003. Clinical aspects, diagnosis and treatment of trichinellosis. Expert Rev. Anti. Infect. Ther. 1:471-482. [DOI] [PubMed] [Google Scholar]
- 181.Pozio, E., D. Sacchini, L. Sacchi, A. Tamburrini, and F. Alberici. 2001. Failure of mebendazole in the treatment of humans with Trichinella spiralis infection at the stage of encapsulating larvae. Clin. Infect. Dis. 32:638-642. [DOI] [PubMed] [Google Scholar]
- 182.Priest, D. H., and J. E. Peacock, Jr. 2005. Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South. Med. J. 98:854-862. [DOI] [PubMed] [Google Scholar]
- 183.Rayes, A. A., V. Nobre, D. M. Teixeira, J. C. Serufo, G. B. Filho, C. M. Antunes, and J. R. Lambertucci. 2000. Tropical pyomyositis and human toxocariasis: a clinical and experimental study. Am. J. Med. 109:422-425. [DOI] [PubMed] [Google Scholar]
- 184.Reboli, A. C., R. F. Reilly, and R. J. Jacobson. 1987. Aspergillus myositis in a patient with a myelodysplastic syndrome. Mycopathology 97:117-119. [DOI] [PubMed] [Google Scholar]
- 185.Reddix, R. N. Jr., J. P. Montoya, and D. L. Hurley. 2007. Late recurrent Salmonella sacroiliac osteomyelitis with psoas abscess in a non-sickle cell adult: case report. Am. J. Orthop. 36:E5-E6. [PubMed] [Google Scholar]
- 186.Reimers, C. D., J. de Koning, U. Neubert, V. Preac-Mursic, J. G. Koster, W. Müller-Felber, D. E. Pongratz, and P. H. Duray. 1993. Borrelia burgdorferi myositis: report of eight patients. J. Neurol. 240:278-283. [DOI] [PubMed] [Google Scholar]
- 187.Ricci, M. A., F. B. Rose, and K. K. Meyer. 1986. Pyogenic psoas abscess: worldwide variations in etiology. World J. Surg. 10:834-843. [DOI] [PubMed] [Google Scholar]
- 188.Richardson, S. J., F. Lopez, S. Rojas, S. Cho, M. Holodniy, B. Herndier, and J. Katz. 2001. Multinodular polymyositis in a patient with human immunodeficiency and hepatitis C virus coinfection. Muscle Nerve 24:433-437. [DOI] [PubMed] [Google Scholar]
- 189.Roggiani, M., and P. M. Schlievert. 1994. Streptococcal toxic shock syndrome, including necrotizing fasciitis and myositis. Curr. Opin. Infect. Dis. 7:423-426. [Google Scholar]
- 190.Ross, A. G., D. Vickers, G. R. Olds, S. M. Shah, and D. P. McManus. 2007. Katayama syndrome. Lancet Infect. Dis. 7:218-224. [DOI] [PubMed] [Google Scholar]
- 191.Rowland, L. P., and M. Greer. 1961. Toxoplasmic polymyositis. Neurology 11:367-370. [DOI] [PubMed] [Google Scholar]
- 192.Roy, S. L., A. S. Lopez, and P. M. Schantz. 2003. Trichinellosis surveillance—United States, 1997-2001. MMWR Surveill. Summ. 52:1-8. [PubMed] [Google Scholar]
- 193.Ruiz, M. E., S. Yohannes, and C. G. Wladyka. 2005. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 352:1488-1489. [DOI] [PubMed] [Google Scholar]
- 194.Sakata, H., G. Taketazu, K. Nagaya, M. Shirai, R. Sugai, K. Ikegami, and S. Maruyama. 1998. Outbreak of severe infection due to adenovirus type 7 in a paediatric ward in Japan. J. Hosp. Infect. 39:207-211. [DOI] [PubMed] [Google Scholar]
- 195.San Jose, A., J. A. Bosch, A. Arderiu, J. Montalban, and M. Vilardell. 1988. Myositis due to Rickettsia conorii infection. Trans. R. Soc. Trop. Med. Hyg. 82:346. [DOI] [PubMed] [Google Scholar]
- 196.Santaella, R. O., E. K. Fishman, and P. A. Lipsett. 1995. Primary vs secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch. Surg. 130:1309-1313. [DOI] [PubMed] [Google Scholar]
- 197.Santos Durán-Ortiz, J., I. García-de la Torre, G. Orozco-Barocio, G. Martínez-Bonilla, A. Rodríguez-Toledo, and L. Herrera-Zárate. 1992. Trichinosis with severe myopathic involvement mimicking polymyositis. Report of a family outbreak. J. Rheumatol. 19:310-312. [PubMed] [Google Scholar]
- 198.Sawhney, B. B., J. S. Chopra, A. K. Banerji, and P. L. Wahi. 1976. Pseudohypertrophic myopathy in cysticercosis. Neurology 26:270-272. [DOI] [PubMed] [Google Scholar]
- 199.Saylam, K., V. Anaf, and C. Kirkpatrick. 2002. Successful medical management of multifocal psoas abscess following cesarean section: report of a case and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 102:211-214. [DOI] [PubMed] [Google Scholar]
- 200.Schattner, A., E. Hay, B. Lifschitz-Mercer, S. Gorbacz, and Z. Bentwich. 1989. Fulminant streptococcal myositis. Ann. Emerg. Med. 18:320-322. [DOI] [PubMed] [Google Scholar]
- 201.Scheepers, N. J., P. F. van Bommel, and O. P. Bleker. 1993. Psoas abscess related to spontaneous abortion, intra-uterine contraceptive device and curettage. Acta Obstet. Gynecol. Scand. 72:223-224. [DOI] [PubMed] [Google Scholar]
- 202.Schimrigk, K., and W. Emser. 1978. Parasitic myositis by Echinococcus alveolaris. Report of a family with myotonia congenita. Eur. Neurol. 17:1-7. [DOI] [PubMed] [Google Scholar]
- 203.Schlesinger, J. J., D. Gandara, and K. G. Bensch. 1978. Myoglobinuria associated with herpes-group viral infections. Arch. Intern. Med. 138:422-424. [PubMed] [Google Scholar]
- 204.Schneller, F. R., S. C. Gulati, I. B. Cunningham, R. J. OReilly, H. J. Schmitt, and B. D. Clarkson. 1990. Fusarium infections in patients with hematologic malignancies. Leuk. Res. 14:961-966. [DOI] [PubMed] [Google Scholar]
- 205.Schreck, P., P. Schreck, J. Bradley, and H. Chambers. 1996. Musculoskeletal complications of varicella. J. Bone Joint Surg. Am. 78:1713-1719. [DOI] [PubMed] [Google Scholar]
- 206.Schwartz, D. M., and E. R. Morgan. 2008. Multimodality imaging of Candida tropicalis myositis. Pediatr. Radiol. 38:473-476. [DOI] [PubMed] [Google Scholar]
- 207.Schwartzman, W. A., M. W. Lambertus, C. A. Kennedy, and M. B. Goetz. 1991. Staphylococcal pyomyositis in patients infected by the human immunodeficiency virus. Am. J. Med. 90:595-600. [PubMed] [Google Scholar]
- 208.Servidei, S., A. F. Miranda, and E. T. Gamboa. 1987. Infectivity of influenza B virus in cultured human muscle. Acta Neuropathol. 73:67-76. [DOI] [PubMed] [Google Scholar]
- 209.Shahabi, S., J. P. Klein, and P. F. Rinaudo. 2002. Primary psoas abscess complicating a normal vaginal delivery. Obstet. Gynecol. 99:906-909. [DOI] [PubMed] [Google Scholar]
- 210.Sharma, M., R. Khatib, B. A. Jones, and M. G. Fakih. 2002. Cryptococcus neoformans myositis without dissemination. Scand. J. Infect. Dis. 34:858-859. [DOI] [PubMed] [Google Scholar]
- 211.Shih, J. Y., P. R. Hsueh, Y. L. Chang, S. F. Lin, L. J. Teng, and K. T. Luh. 1998. Pyomyositis due to Mycobacterium haemophilum in a patient with polymyositis and long-term steroid use. Clin. Infect. Dis. 26:505-507. [DOI] [PubMed] [Google Scholar]
- 212.Shimoni, Z., Z. Klein, P. Weiner, M. V. Assous, and P. Froom. 2007. The use of prednisone in the treatment of trichinellosis. Isr. Med. Assoc. J. 9:537-539. [PubMed] [Google Scholar]
- 213.Siberry, G. K., T. Tekle, K. Carroll, and J. Dick. 2003. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin. Infect. Dis. 37:1257-1260. [DOI] [PubMed] [Google Scholar]
- 214.Singh, U., and W. M. Scheld. 1996. Infectious etiologies of rhabdomyolysis: three case reports and review. Clin. Infect. Dis. 22:642-649. [DOI] [PubMed] [Google Scholar]
- 215.Sinniah, R., and W. Lye. 2000. Acute renal failure from myoglobinuria secondary to myositis from severe falciparum malaria. Am. J. Nephrol. 20:339-343. [DOI] [PubMed] [Google Scholar]
- 216.Small, L. N., and J. J. Ross. 2005. Tropical and temperate pyomyositis. Infect. Dis. Clin. North Am. 19:981-989. [DOI] [PubMed] [Google Scholar]
- 217.Smith, I. M., and A. B. Vickers. 1960. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet i:1318-1322. [PubMed] [Google Scholar]
- 218.Smith, R. D., S. Konoplev, G. DeCourten-Myers, and T. Brown. 2004. West Nile virus encephalitis with myositis and orchitis. Hum. Pathol. 35:254-258. [DOI] [PubMed] [Google Scholar]
- 219.Sokolov, K. M., E. Kreye, L. G. Miller, C. Choi, and A. W. Tang. 2007. Postpartum iliopsoas pyomyositis due to community-acquired methicillin-resistant Staphylococcus aureus. Obstet. Gynecol. 110:535-538. [DOI] [PubMed] [Google Scholar]
- 220.Solbrig, M. V., J. H. Sher, and R. W. Kula. 1987. Rhabdomyolysis in leptospirosis (Weil's disease). J. Infect. Dis. 156:692-693. [DOI] [PubMed] [Google Scholar]
- 221.Sorvillo, F. J., C. DeGiorgio, and S. H. Waterman. 2007. Deaths from cysticercosis, United States. Emerg. Infect. Dis. 13:230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Steiner, F. T., A. S. Brem, and G. Peter. 1987. Psoas muscle abscess due to Pasteurella multocida. J. Urol. 137:487-488. [DOI] [PubMed] [Google Scholar]
- 223.Stevens, D. L., A. E. Bryant, K. Adams, and J. T. Mader. 1993. Evaluation of therapy with hyperbaric oxygen for experimental infection with Clostridium perfringens. Clin. Infect. Dis. 17:231-237. [DOI] [PubMed] [Google Scholar]
- 224.Stevens, D. L., A. E. Gibbons, R. Bergstrom, and V. Winn. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23-28. [DOI] [PubMed] [Google Scholar]
- 225.Stevens, D. L., B. M. Laine, and J. E. Mitten. 1987. Comparison of single and combination antimicrobial agents for prevention of experimental gas gangrene caused by Clostridium perfringens. Antimicrob. Agents Chemother. 31:312-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Ries, P. M. Schlievert, and E. Kaplan. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 227.Subramanian, K. N., and K. S. Lam. 2003. Malignant necrotising streptococcal myositis: a rare and fatal condition. J. Bone Joint Surg. Br. 85:277-278. [DOI] [PubMed] [Google Scholar]
- 228.Tabbutt, S., M. Leonard, R. I. Godinez, M. Sebert, J. Cullen, T. L. Spray, and D. Friedman. 2004. Severe influenza B myocarditis and myositis. Pediatr. Crit. Care Med. 5:403-406. [DOI] [PubMed] [Google Scholar]
- 229.Takayanagui, O. M., and L. Chimelli. 1998. Disseminated muscular cysticercosis with myositis induced by praziquantel therapy. Am. J. Trop. Med. Hyg. 59:1002-1003. [DOI] [PubMed] [Google Scholar]
- 230.Thursky, K., and J. Torresi. 2001. Primary muscle hydatidosis of the thigh: management of a complicated case with combination adjunctive albendazole and praziquantel chemotherapy. Clin. Infect. Dis. 32:E65-E68. [DOI] [PubMed] [Google Scholar]
- 231.Tsai, S. H., Y. J. Peng, and N. C. Wang. 2006. Pyomyositis with hepatic and perinephric abscesses caused by Candida albicans in a diabetic nephropathy patient. Am. J. Med. Sci. 331:292-294. [DOI] [PubMed] [Google Scholar]
- 232.Tumeh, S. S., G. J. Butler, J. H. Maguire, and J. S. Nagel. 1988. Pyogenic myositis: CT evaluation. J. Comput. Assist. Tomogr. 12:1002-1005. [DOI] [PubMed] [Google Scholar]
- 233.Turunc, T., Y. Z. Demiroglu, H. Uncu, S. Colakoglu, and H. Arslan. 2007. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J. Infect. 55:158-163. [DOI] [PubMed] [Google Scholar]
- 234.Uchiyama, T., K. Arai, T. Yamamoto-Tabata, K. Hirai, K. Kishimoto, Y. Nakamura, and T. Hattori. 2005. Generalized myositis mimicking polymyositis associated with chronic active Epstein-Barr virus infection. J. Neurol. 252:519-525. [DOI] [PubMed] [Google Scholar]
- 235.Uparanukraw, P., and N. Morakote. 1997. Detection of circulating Trichinella spiralis larvae by polymerase chain reaction. Parasitol. Res. 83:52-56. [DOI] [PubMed] [Google Scholar]
- 236.van den Berge, M., T. de Marie, S. Kuipers, A. R. Jansz, and B. Bravenboer. 2005. Psoas abscess: report of a series and review of the literature. Neth. J. Med. 63:413-416. [PubMed] [Google Scholar]
- 237.Van den Enden, E., M. Praet, R. Joos, A. Van Gompel, and P. Gigasse. 1995. Eosinophilic myositis resulting from sarcocystosis. J. Trop. Med. Hyg. 98:273-276. [PubMed] [Google Scholar]
- 238.van Deuren, M., C. Neeleman, K. J. Assmann, J. F. Wetzels, and J. W. van der Meer. 1998. Rhabdomyolysis during the subacute stage of meningococcal sepsis. Clin. Infect. Dis. 26:214-215. [DOI] [PubMed] [Google Scholar]
- 239.Vartivarian, S. E., P. E. Coudron, and S. M. Markowitz. 1987. Disseminated coccidioidomycosis. Unusual manifestations in a cardiac transplantation patient. Am. J. Med. 83:949-952. [DOI] [PubMed] [Google Scholar]
- 240.Vicidomini, S., G. Cancrini, S. Gabrielli, R. Naspetti, and A. Bartoloni. 2007. Muscular cystic hydatidosis: case report. BMC Infect. Dis. 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Voloshin, D. K., D. Lacomis, and D. McMahon. 1995. Disseminated histoplasmosis presenting as myositis and fasciitis in a patient with dermatomyositis. Muscle Nerve 18:531-535. [DOI] [PubMed] [Google Scholar]
- 242.Wadia, N. H. 1995. Disseminated cystericercosis: pseudohypertrophic muscular type, p. 119-130. In F. C. Rose (ed.), Recent advances in tropical neurology, 1st ed. Elsevier, Amsterdam, The Netherlands.
- 243.Wagner, J. D., C. D. Prevel, and R. Elluru. 1996. Histoplasma capsulatum necrotizing myofascitis of the upper extremity. Ann. Plast. Surg. 36:330-333. [DOI] [PubMed] [Google Scholar]
- 244.Wagner, J. G., P. M. Schlievert, A. P. Assimacopoulos, J. A. Stoehr, P. J. Carson, and K. Komadina. 1996. Acute group G streptococcal myositis associated with streptococcal toxic shock syndrome: case report and review. Clin. Infect. Dis. 23:1159-1161. [DOI] [PubMed] [Google Scholar]
- 245.Wallin, M. T., and J. F. Kurtzke. 2004. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 63:1559-1564. [DOI] [PubMed] [Google Scholar]
- 246.Walling, D. M., and W. G. Kaelin, Jr. 1991. Pyomyositis in patients with diabetes mellitus. Rev. Infect. Dis. 13:797-802. [DOI] [PubMed] [Google Scholar]
- 247.Walsh, S. S., W. J. Robson, and C. A. Hart. 1988. Acute transient myositis due to Toxocara. Arch. Dis. Child. 63:1087-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Walsh, T. R., J. R. Reilly, E. Hanley, M. Webster, A. Peitzman, and D. L. Steed. 1992. Changing etiology of iliopsoas abscess. Am. J. Surg. 163:413-416. [DOI] [PubMed] [Google Scholar]
- 249.Wang, C., S. Schwaitzberg, E. Berliner, D. A. Zarin, and J. Lau. 2003. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch. Surg. 138:272-279. [DOI] [PubMed] [Google Scholar]
- 250.Wang, J. Y., L. N. Lee, P. R. Hsueh, J. Y. Shih, Y. L. Chang, P. C. Yang, and K. T. Luh. 2003. Tuberculous myositis: a rare but existing clinical entity. Rheumatology 42:836-840. [DOI] [PubMed] [Google Scholar]
- 251.Wang, W. Y., F. C. Lin, T. Y. Tsao, and J. J. Lu. 2007. Tuberculous myositis: an unusual presentation of extrapulmonary tuberculosis. J. Microbiol. Immunol. Infect. 40:79-82. [PubMed] [Google Scholar]
- 252.Warin, J. F., J. B. Davies, F. K. Sanders, and A. D. Vizoso. 1953. Oxford epidemic of Bornholm disease. Br. Med. J. 1:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Watt, G., S. Saisorn, K. Jongsakul, Y. Sakolvaree, and W. Chaicumpa. 2000. Blinded, placebo-controlled trial of antiparasitic drugs for trichinosis myositis. J. Infect. Dis. 182:371-374. [DOI] [PubMed] [Google Scholar]
- 254.Wells, R. D., and V. S. Bebarta. 2006. Primary iliopsoas abscess caused by community-acquired methicillin-resistant Staphylococcus aureus. Am. J. Emerg. Med. 24:897-898. [DOI] [PubMed] [Google Scholar]
- 255.Wheeler, D. S., W. D. Vazquez, K. K. Vaux, and W. B. Poss. 1998. Streptococcal pyomyositis: case report and review. Pediatr. Emerg. Care 14:411-412. [DOI] [PubMed] [Google Scholar]
- 256.Whitfeld, M. J., S. Kaveh, J. E. Koehler, P. Mead, and T. G. Berger. 1997. Bacillary angiomatosis associated with myositis in a patient infected with human immunodeficiency virus. Clin. Infect. Dis. 24:562-564. [DOI] [PubMed] [Google Scholar]
- 257.Widrow, C. A., S. M. Kellie, B. R. Saltzman, and U. Mathur-Wagh. 1991. Pyomyositis in patients with the human immunodeficiency virus: an unusual form of disseminated bacterial infection. Am. J. Med. 91:129-136. [DOI] [PubMed] [Google Scholar]
- 258.Wong, K. T., and R. Pathmanathan. 1992. High prevalence of human skeletal muscle sarcocystosis in south-east Asia. Trans. R. Soc. Trop. Med. Hyg. 86:631-632. [DOI] [PubMed] [Google Scholar]
- 259.Woo, P. C., J. L. Teng, S. K. Lau, P. N. Lum, K. W. Leung, K. L. Wong, K. W. Li, K. C. Lam, and K. Y. Yuen. 2003. Analysis of a viridans group strain reveals a case of bacteremia due to Lancefield group G alpha-hemolytic Streptococcus dysgalactiae subsp equisimilis in a patient with pyomyositis and reactive arthritis. J. Clin. Microbiol. 41:613-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wortmann, R. L. 2005. Inflammatory diseases of muscle and other myopathies, p. 1309-1335. In E. D. Harris, R. C. Budd, G. S. Firestein, M. C. Genovese, J. S. Sergent, S. Ruddy, and C. B. Sledge (ed.), Kelley's textbook of rheumatology, 7th ed. Elsevier/Saunders, Philadelphia, PA.
- 261.Wrobel, C. J., E. T. Chappell, and W. Taylor. 2001. Clinical presentation, radiological findings, and treatment results of coccidioidomycosis involving the spine: report on 23 cases. J. Neurosurg. 95:33-39. [DOI] [PubMed] [Google Scholar]
- 262.Wysoki, M. G., E. Angeid-Backman, and B. A. Izes. 1997. Iliopsoas myositis mimicking appendicitis: MRI diagnosis. Skeletal Radiol. 26:316-318. [DOI] [PubMed] [Google Scholar]
- 263.Yoshino, M., S. Suzuki, K. Adachi, M. Fukayama, and T. Inamatsu. 2000. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern. Med. 39:431-432. [DOI] [PubMed] [Google Scholar]
- 264.Ytterberg, S. R. 1996. Infectious agents associated with myopathies. Curr. Opin. Rheumatol. 8:507-513. [DOI] [PubMed] [Google Scholar]
- 265.Yuh, W. T., A. E. Schreiber, W. J. Montgomery, and S. Ehara. 1988. Magnetic resonance imaging of pyomyositis. Skeletal Radiol. 17:190-193. [DOI] [PubMed] [Google Scholar]
- 266.Zvolanek, J. R. 1984. Benign acute childhood myositis associated with parainfluenza type 2 infection. Pediatr. Infect. Dis. 3:594-595. [DOI] [PubMed] [Google Scholar]