Abstract
Campylobacter is a major cause of acute bacterial diarrhea in humans worldwide. This study was aimed at summarizing the current understanding of host mechanisms involved in the defense against Campylobacter by evaluating data available from three sources: (i) epidemiological observations, (ii) observations of patients, and (iii) experimental observations including observations of animal models and human volunteer studies. Analysis of available data clearly indicates that an effective immune system is crucial for the host defense against Campylobacter infection. Innate, cell-mediated, and humoral immune responses are induced during Campylobacter infection, but the relative importance of these mechanisms in conferring protective immunity against reinfection is unclear. Frequent exposure to Campylobacter does lead to the induction of short-term protection against disease but most probably not against colonization. Recent progress in the development of more suitable animal models for studying Campylobacter infection has opened up possibilities to study the importance of innate and adaptive immunity during infection and in protection against reinfection. In addition, advances in genomics and proteomics technologies will enable more detailed molecular studies. Such studies combined with better integration of host and pathogen research driven by epidemiological findings may truly advance our understanding of Campylobacter infection in humans.
INTRODUCTION
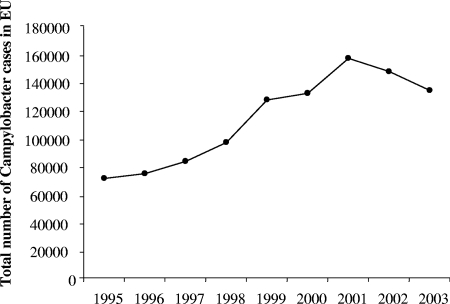
Campylobacter is a major cause of acute bacterial diarrhea in humans worldwide (3). The incidence of human campylobacteriosis increased exponentially during the last decade of the 20th century (183), although part of this increase can be attributed to better detection of Campylobacter and better diagnosis. At the start of the 21st century, this increase has stopped, as shown by data for the total number of Campylobacter cases in the European Union until 2003 (Fig. 1). In humans, the clinical symptoms of campylobacteriosis are watery or bloody diarrhea, abdominal cramps, and nausea (151). In a small subgroup of patients, the acute phase is followed by serious sequelae: Guillain-Barré syndrome (GBS) and reactive arthritis (78, 86). Acute diarrhea, Campylobacter-related mortality, and residual effects of GBS are the main determinants contributing to this disease burden (79). Campylobacteriosis in humans is induced mainly by Campylobacter jejuni (about 90% of cases), and the remaining fraction is induced predominantly by Campylobacter coli. Campylobacter is part of the normal intestinal flora of birds, and humans are not the reservoir for infection. As a result, poultry is a major source of infection. The estimation of incidence of Campylobacter enteritis in the population is usually based on confirmed cases corrected for several factors like the proportion of patients consulting a physician and the number of this group submitting a stool sample for Campylobacter isolation. Because the infection is usually self-limiting, the true population incidence is estimated to be 8 to 30 times higher than confirmed cases, depending on the country (148, 166, 179).
FIG. 1.
Total number of confirmed Campylobacter cases in the European Union until 2003. Data after 2003 are not shown because several new European Union (EU) member states with a high reported incidence of campylobacteriosis joined the European Union (Table 1).
The estimated rate of campylobacteriosis (number of cases/100,000 individuals) differs strongly around the world, with New Zealand as the country with the highest rate (396/100,000 persons), compared to, e.g., the United States (reported as being 12.7/100,000 persons by FoodNet in 2005) (13, 44). The excessive rate in New Zealand seems to be real, but it remains unexplained. New Zealand's campylobacteriosis epidemic reached a new peak in May 2006, with the annualized national notification rate exceeding 400 per 100,000 individuals for the first time, the highest national rate reported in the literature (12). Differences among countries should be considered with care, as surveillance and reporting systems may differ markedly from country to country. For example, differences among countries within the European Union have been reported: the Czech Republic reported an incidence of 303/100,000 individuals, whereas other countries did not report a single case (Table 1). It is highly unlikely that these differences are real. There is little information about mortality due to campylobacteriosis. It is estimated that in The Netherlands (population of about 16,000,000 individuals), with an estimated incidence of campylobacteriosis of about 59,000 cases, around 25 people die of Campylobacter infection every year (92). Most Campylobacter infections occur as sporadic cases, and outbreaks are rare or are not recognized. The few reported outbreaks are most commonly associated with raw milk or water (57, 98, 139, 153, 159). This is surprising for a food-borne pathogen, although it is known that the dose of Campylobacter present in food is highly variable. Better monitoring of possible outbreaks is essential to increase our understanding of the epidemiology of campylobacteriosis in humans. Such outbreaks also provide a unique opportunity to study host responses to Campylobacter, for instance, by measuring immune parameters in cases and exposed controls. Although poultry is a major source of infection, it is estimated that in The Netherlands, only 20% to 40% of all laboratory-confirmed cases are attributable to the consumption of undercooked chicken (80). This percentage is in agreement with estimates from Belgium (40%), where the withdrawal of poultry meat from the market following dioxin contamination of chicken feed resulted in a clear decline in human campylobacteriosis incidences (171). Other risk factors include drinking raw milk or contaminated water, traveling abroad, and contact with pets. However, a large proportion of all infections (i.e., approximately 50%) cannot be attributed to any of the known risk factors, indicating that other sources exist. Although humans are also most probably exposed to Campylobacter from currently unknown sources, the exposure of humans to poultry meat is the best-understood source, and consequently, most effort is put into Campylobacter control strategies along the poultry meat production chain. However, extensive control strategies with the overall aim to reduce Campylobacter contamination on poultry meat have been only partly successful. We conclude that humans will be continuously exposed to Campylobacter from poultry meat, from sources where interventions cannot be implemented (contact with pets), and from unknown sources. As exposure will not be equally distributed in the population (pet owners and professionally exposed humans) and the population will not be equally susceptible (children and the elderly), we urgently need more understanding of the pathogen-host interaction. Only with this knowledge can science-based risk assessments be performed and science-based intervention strategies be developed. Since the exposure of the population to Campylobacter cannot be prevented, it is crucial to understand the risks involved with exposure and to identify groups in the population that are more at risk. Currently available risk assessment models do not explicitly take into account that individuals display differential susceptibility to infection. A better understanding of the pathogenic mechanisms of Campylobacter and, importantly, of the host factors involved in the defense against Campylobacter infection may lead to the identification of risk factors in the population. It is conceivable that the efficacy of some of these host factors in the defense against Campylobacter is genetically determined. Studying these host factors could contribute both to novel intervention strategies and to the development of more realistic risk assessment models that incorporate such host susceptibility factors and/or more targeted intervention strategies.
TABLE 1.
Reported campylobacteriosis cases in humans and incidence of cases in Europe in 2005a
| Country | No. of confirmed cases | No. of confirmed cases/100,000 individuals |
|---|---|---|
| Austria | 5,065 | 61.7 |
| Belgium | 6,879 | 65.8 |
| Cyprus | 0 | 0 |
| Czech Republic | 30,268 | 302.7 |
| Denmark | 3,677 | 68 |
| Estonia | 124 | 9.2 |
| Finland | 4,002 | 76.4 |
| France | 2,049 | 3.3 |
| Germany | 62,114 | 75.3 |
| Greece | —b | — |
| Hungary | 8,288 | 82.1 |
| Ireland | 1,794 | 43.7 |
| Italy | — | — |
| Latvia | 0 | 0 |
| Lithuania | 694 | 20.3 |
| Luxembourg | 194 | 42.6 |
| Malta | 91 | 22.6 |
| The Netherlands | 3,761 | 46.2 |
| Poland | 47 | 0.1 |
| Portugal | — | — |
| Slovakia | 2,204 | 40.9 |
| Slovenia | 0 | 0 |
| Spain | 5,513 | 12.8 |
| Sweden | 5,969 | 66.2 |
| United Kingdom | 52,686 | 88.5 |
| European Union total | 195,419 | 51.6 |
| Iceland | 128 | 43.6 |
| Norway | 2,631 | 57.1 |
| Total | 198,178 | 51.7 |
Adapted from reference 54.
—, no cases reported.
The scope of this review is to summarize available data on host factors involved in the response to Campylobacter jejuni and how these factors can increase our understanding of host-pathogen interactions. In other diseases, the majority of such factors are elucidated by studying infection in murine models with well-defined genetic mutations in host defense mechanisms. However, Campylobacter does not induce disease in wild-type mice, and rodent models that mimic human disease have been lacking. Recent progress in the generation of gene-deleted mice has now resulted in the in the development of murine models, which have contributed to our understanding of the defense against Campylobacter and will be valuable for further studying host responses to Campylobacter infection (discussed below) (63, 106, 177).
This study was aimed at summarizing the current understanding of host mechanisms involved in the defense against Campylobacter by evaluating data available from three sources: (i) epidemiological observations, (ii) observations of patients, and (iii) experimental observation including observations of animal models and human volunteer studies.
PATHOLOGY AND PATHOPHYSIOLOGY OF CAMPYLOBACTER INFECTION
Humans are orally exposed to Campylobacter. During passage through the acidic environment of the stomach, a large proportion of the ingested dose may be killed, depending on the buffering capacity of the food. The remaining bacteria can survive and are able to adhere to intestinal epithelial cells or to the mucus overlying these cells and replicate in the intestine. In infected individuals, this can result either in asymptomatic colonization status, i.e., bacteria are present in the intestine but do not induce disease (41, 45), or in diarrheal illness. Campylobacter is highly infectious, and infective doses as low as 500 to 800 CFU have been reported (23, 140). A probability of 2% for any CFU to establish infection was calculated in a volunteer experiment (23, 160).
The colonization status in humans is reminiscent of that found in various rodents, mammals, and birds. Chickens can be colonized with as many as 109 CFU C. jejuni per gram cecal contents (43), and colonized mice can shed up to 106 CFU per mg feces (18). Studies of children in developing countries have shown that rates of asymptomatic carriage of Campylobacter in children are around 15% (108, 131), suggesting that some acquired immunity is induced from multiple exposures during early childhood. Wheeler et al. reported a rate of asymptomatic carriage of 0.7% in a population study involving adults in the United Kingdom (179). This indicates that bacterial clearance is inefficient and raises questions about how effective the immune response is in clearing all bacteria. The difference between humans and rodents is that in the latter, Campylobacter fails to cause diarrheal illness, indicating that animals lack specific factors, e.g., receptors, necessary for Campylobacter to cause disease, that effective immune mechanisms are present in animals that prevent the development of clinical disease, or that disease-causing host responses are absent.
After colonization of the intestine, clinical disease may occur. Based on clinical syndromes found in patients, two mechanisms by which Campylobacter can induce disease were postulated (85): (i) adherence of Campylobacter to the intestine and the production of toxins (173), which alter the fluid resorption capacity of the intestine, resulting in secretory diarrhea, and (ii) bacterial invasion and replication within the intestinal mucosa accompanied by an inflammatory response resulting in blood-containing, inflammatory diarrhea.
In immunocompetent individuals, disease is restricted to the intestine, although bacteremia has been observed. The reported incidence for bacteremia ranges from 1.5 to 8 in 1,000 individuals (89, 152). Occasionally, passage through the intestinal mucosa and migration to extraintestinal sites via the lymphatic system result in systemic disease. However, it is important to note that systemic disease is very rare in immunocompetent individuals.
Clinical disease is characterized by acute diarrhea accompanied by intense abdominal pain. Campylobacteriosis is an inflammatory enteritis that is initially found in the small bowel and later affects the colon and the rectum (23). The incubation time is 1 to 7 days (mean, 3 days), which is longer than the incubation times of most other intestinal pathogens. The diarrhea can be either watery or, in almost one-third of the cases, bloody (79, 151, 174), indicating that the extents of intestinal inflammation vary among individuals. Inflammatory diarrhea points to a role for polymorphonuclear leukocytes (PMN) in pathology and suggests that infection can lead to extensive intestinal damage either as a direct result of bacterial toxins or as a result of the inflammatory infiltrate. It has been shown that this is in part related to differences in properties of the infecting strain (23, 60). Usually, diarrhea begins to ease after 3 to 4 days, but Campylobacter can be found in the feces for several weeks (89). Using a highly sensitive culture-based detection assay, Kapperud et al. observed carriage in 16% of individuals during convalescence, with a median carriage time of 31 days (89). Although a large proportion of the patients feel nauseous, only about 15% of patients vomit (151, 174). In 30% of patients, the disease does not start with diarrhea but with a prodrome of influenza virus-like symptoms such as fever, headache, dizziness, and myalgia (reviewed in reference 151), indicating that there is some systemic, probably immune-mediated, effect of local infection. Patients that suffer from such a prodrome tend to have more serious disease than patients without the prodrome, but the reasons for this are currently unknown (reviewed in reference 151).
In most immunocompetent individuals, campylobacteriosis is a self-limiting disease, and treatment with antimicrobials reduces the period of fecal shedding but does not have a large impact on the duration of disease symptoms (4, 105, 180). However, when given early, some clinical benefit has been observed (126, 147). When patients suffer from recurrent or systemic Campylobacter infection, antimicrobial treatment is indicated. However, an increase in antimicrobial resistance, especially fluoroquinolone resistance, in both human and animal isolates has been observed over the last decade (93, 167).
SEQUELAE OF CAMPYLOBACTER INFECTION
While Campylobacter enteritis is usually self-limiting and the disease is resolved within 1 week in the majority of cases, some individuals develop sequelae after the acute phase. Approximately 1 in 1,000 infected individuals develops GBS, a serious autoimmune-mediated neurological disorder that can cause symptoms ranging from weakness of extremities to complete paralysis and respiratory insufficiency (reviewed in reference 116). Mortality rates due to GBS in the industrialized world are 2% to 3%, although the majority of patients recover completely within 6 to 12 months (182). In The Netherlands, the health burden for Campylobacter-associated GBS was estimated at 164 disability-adjusted life years in 2004 (92). Miller-Fisher syndrome, a subvariant of GBS that affects predominantly the nerves that govern eye movement, has also been associated with Campylobacter infection (138, 187).
GBS is thought to occur because of molecular mimicry between lipooligosaccharide, a component of the cell envelope of Campylobacter, and sugar moieties on nerve gangliosides (6, 9, 117, 189). Antibodies that are raised during infection with Campylobacter serotypes containing such ganglioside mimics can cross-react with gangliosides in some individuals, leading to the demyelinization of nerves and the degeneration of axons (for a review, see reference 181). Evidence suggests that both strain properties and host properties play a role in determining the development of GBS. For instance, serotype HS:19 was overrepresented in Japanese GBS patients (97, 188) but not in United Kingdom patients (138), indicating a role for host factors. In addition, although ganglioside-mimicking structures were found more frequently in neuropathy-associated Campylobacter strains than in strains isolated from patients with diarrhea (7), strains that contain these ganglioside mimics are also often found in patients with uncomplicated enteritis (117). Recently, it was shown that specific types of the lipooligosaccharide biosynthesis gene locus are important for the expression of ganglioside mimics and the induction of antiganglioside antibodies (73). Taken together, these data suggest that although the presence of ganglioside mimics is important, it is not the only factor that determines the development of GBS. Currently, the role of host genetic factors in determining if GBS evolves upon infection with Campylobacter strains with ganglioside mimics is studied extensively. A complete review of all factors associated with the development of GBS is beyond the scope of this review (for reviews on this issue, see references 86 and 116), but some of the genetic factors that have recently been associated with the development or severity of GBS are listed in Table 2. Not only Campylobacter but also other pathogens have been associated with the development of GBS. However, most of the genetic studies on susceptibility to GBS are performed with GBS patients, irrespective of the causative pathogen. Therefore, host factors that determine susceptibility to GBS may shed more light on processes involved in breaking tolerance to self-antigens than on susceptibility to diarrheal illness. Further studies are needed to investigate if similar mechanisms are also involved in determining susceptibility to Campylobacter-induced diarrhea.
TABLE 2.
Genetic factors studied in association with the development of GBS or Campylobacter-associated GBS
| Gene studied | Association with GBS | Association with Campylobacter-induced GBSa | Reference |
|---|---|---|---|
| MMP9 | Yes, severity | NS | 68 |
| TNFA | Yes, severity | NS | 68 |
| IL-10 | No | NS | 68 |
| IL-10 | No | NS | 68 |
| CD1 | Yes, incidence | NS | 37 |
| MBL2 | Yes, severity | NS | 70 |
| HLA class II | Yes, severity | NS | 71 |
| HLA-DRB1 | No | Nonsignificant association (trend) | 103 |
| HLA B54 | Yes, incidence | Yes, incidence | 95 |
| HLA-Cw1 | No | Yes, incidence | 95 |
| FCGR2A | Yes, incidence/severity | NS | 165 |
| FCGR3A | No | NS | 165 |
| FCGR3B | No | NS | 165 |
| FCGR3B | Yes, severity | NS | 170 |
| FCGR3B | Yes, severity | NS | 169 |
| IL-10 | Yes, incidence | NS | 115 |
| FAS/CD95 | Yes, GM1 antibodies | NS | 69 |
| CD14 | No | No | 56 |
| TLR4 | No | No | 56 |
| APOE | No | NS | 136 |
| TNFA | NS | Yes, incidence | 102 |
NS, not studied.
Other immune-mediated sequelae of Campylobacter infection include reactive arthritis (22, 101, 164) and Reiter syndrome, an inflammatory disease with either conjunctival or urethral inflammation (91). Symptoms of reactive arthritis usually occur around 14 days after infection (range, 3 days to 6 weeks), and the estimated incidence of reactive arthritis in community outbreaks ranges from 0 to 7% (53, 92, 109, 111). Reactive arthritis is associated with HLA-B27, and various gastrointestinal pathogens can lead to its development (149). The symptoms appear to be similar regardless of the associated bacterial infection, indicating a role for factors common to a range of pathogens (149). Usually, these joint symptoms resolve completely. There are also a few case reports of Campylobacter-associated hemolytic-uremic syndrome, which is a well-known sequela of infection with verocytotoxin (Shiga toxin)-producing Escherichia coli strains (151). Campylobacter strains have also been isolated from patients with inflammatory bowel disease (IBD) such as Crohn's disease and have been associated with flare-ups of IBD, although a causal link between the two is still under debate (21, 67, 178). A recent registry-based study in Denmark revealed very strong associations between Campylobacter infection and the development of IBD, but this association still needs to be confirmed (81). A link between infection by enteric pathogens, including Campylobacter, and irritable bowel syndrome was also observed (52, 81, 156, 162). These enteric infections result in damage to the mucosa and disruption of the native gut flora, which could lead to prolonged bowel dysfunction (156). In a small-scale patient study, a correlation between persistently changed bowel habits following Campylobacter infection and the in vitro toxicity of the infecting strain was observed (162). There is laboratory evidence for a number of Campylobacter toxins (reviewed in reference 173), although, to date, the only toxin cloned, sequenced, and identified from genome sequences is cytolethal distending toxin (CDT), and no direct role for this toxin in the etiology of irritable bowel syndrome has so far been demonstrated.
ROLE OF HUMAN IMMUNITY IN CAMPYLOBACTER DISEASE
Epidemiological Observations
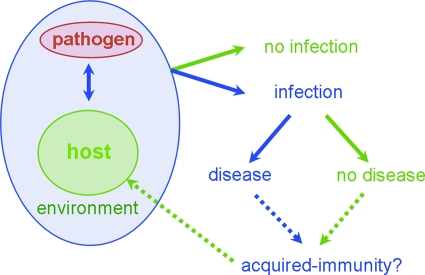
Many Campylobacter types are encountered by the human host, but these types will probably lead to disease in only a minority of cases. Apparently, not every encounter results in the development of disease. Both bacterial virulence factors and host susceptibility factors are thought be involved in determining if disease develops. In addition, environmental factors such as the matrix in which Campylobacter is consumed and the acidity of the stomach are involved (Fig. 2). Exposure is obviously a critical factor in the development of disease, and although hypothetical, a higher incidence in rural areas than in urban areas is often explained as a result of higher exposure in rural areas (58, 74, 161). In accordance with this hypothesis, it was found that in rural areas, like in developing countries, the age distribution was shifted to younger ages than in urban areas (58, 74). However, it is believed that frequent exposure can also result in the development of a certain level of basal immunity to Campylobacter (see also the section on developing countries below). Such responses probably do not lead to protection against a broad range of serotypes. Epidemiological support for this assumption came from data reported recently by Miller et al., which showed that infections with common and rare types of Campylobacter occur in different age groups, where the rare types are overrepresented in the older age groups (110). This indicates that basal immunity to commonly encountered serotypes occurs but that a broad level of protection against all serotypes does not develop. However, since even rare serotypes will have structures in common with common serotypes, this observation warrants further investigation.
FIG. 2.
Schematic representation of host-pathogen interactions in campylobacteriosis. Pathogen-host encounters in a certain environment can either lead to infection or not. Infected individuals can then remain asymptomatic or go on to develop disease. To what extent this leads to the induction of protective immunity is currently unknown.
The fact that not every individual displays the same susceptibility to Campylobacter infection can also be concluded from a range of other epidemiological observations. When outbreak data are analyzed, it is clear that not every person exposed to a certain dose of Campylobacter either will be colonized or will develop disease. These differences can be associated with nonspecific factors such as stomach content and, related to this, the acidity of the stomach. Indeed, the use of proton pump inhibitors in the month prior to Campylobacter infection was shown to increase the risk of clinical disease by as much as 10-fold (121). However, innate and specific immune factors may also play a role in determining the susceptibility of an individual to Campylobacter infection.
In developing countries, the incidence of Campylobacter enteritis peaks in children and declines clearly after childhood. In industrialized countries, Campylobacter disease peaks in children as well, but the steep decline does not occur but peaks again at a young adult age and declines gradually afterwards (66). The course of disease is generally more severe; i.e., infection is more often accompanied by bloody diarrhea (64, 127). In addition, it is thought that after the peak in childhood, in the developing world, asymptomatic infections are more common than in the industrialized world. In the developing world, children are frequently exposed to Campylobacter infection early in life due to contaminated drinking water and close contact with animals and therefore have elevated Campylobacter-specific antibody levels compared to those of children in the United States (25, 30, 107). In Thailand, bloody diarrhea was most often associated with disease in the first year of life, suggesting an association with primary infection (158). However, the occurrence of asymptomatic carriage in developing (and industrialized) countries (127) suggests that any immunity acquired following exposure protects against disease rather than colonization.
Observations of abattoir workers in Sweden (41, 45) support the idea that frequent exposure to Campylobacter induces protection against disease. Recently employed and presumably immunologically naïve workers suffered many more episodes of Campylobacter diarrhea than workers who were employed for many years. Consistent with the observation in the developing world, the latter group of workers regularly succumbed to asymptomatic infection with Campylobacter (41, 45). These data indicate that humans can develop immunity to Campylobacter disease, but probably not to colonization, although this immunity seems to be short-lived, and data suggest that frequent exposure to multiple serotypes/immunotypes may be necessary to boost this immunity.
In conclusion, these epidemiological observations indicate that differences in immune responses are observed in various individuals and due to differences in exposure, but to what extent they are determined by host factors, or if they are related to frequency of exposure, remains to be established. It is also clear that the acidity of the stomach is a crucial early defense mechanism against Campylobacter, although this is not specific for Campylobacter and has also been observed for other pathogens such as Salmonella (50, 51).
Observations of Patients
Certain groups of patients are more susceptible to Campylobacter disease than the general population. Two groups of patients that are particularly susceptible are those with hypo- or agammaglobulinemia, who suffer from defects in humoral immunity, and those with AIDS, who suffer from a defect in cell-mediated immunity (134, 151). Such patients often experience more severe clinical disease that is more frequently accompanied by bacteremia. The incidence of Campylobacter disease in AIDS patients was shown to be 40-fold higher than that in the general population (155). Chronic carriage and recurrent infection are also more frequently found in these highly susceptible patients, and repeated courses of antimicrobial treatment are often indicated. Severe Campylobacter infection is found in AIDS patients both in the industrialized world and in developing countries (47).
The genetic causes of the above-mentioned immunoglobulin deficiencies can be a result of a whole range of primary or acquired immune deficiencies (reviewed in reference 61). These patients are susceptible not only to Campylobacter but also to a whole range of other pathogens. The most frequent cause of hypogammaglobulinemia is common variable immunodeficiency, a heterogeneous disease that occurs in approximately 1:50,000 to 1:100,000 Caucasians. Mutations in the gene encoding ICOS, an inducible T-cell costimulatormolecule essential for proper B-cell activation, is one genetic cause of common variable immunodeficiency (75). Agammaglobulinemia is a very rare but serious recessive X-linked disease that is usually caused by a mutation in Bruton tyrosine kinase, an enzyme essential for B-cell maturation (163, 172).
From those observations, it can be concluded that various (genetically determined) immune-related host factors are involved in susceptibility to Campylobacter infection, although it has to be taken into account that all the above-mentioned diseases lead to severe immune defects resulting in susceptibility to a whole range of pathogens. Since hypogammaglobulinemic/agammaglobulinemic patients and AIDS patients are subject to prolonged symptoms and repeated infection, these data do suggest a role for humoral and T-cell immunity in limiting the infection (8, 134). However, they do not explain the susceptibility specifically to Campylobacter infection, because such patients are also susceptible to a whole range of other pathogens. This is in sharp contrast to studies of patients with enhanced susceptibility to Salmonella and Mycobacterium spp., where “the human model” clearly points to specific host mechanisms that are involved in the defense against these pathogens (38, 128).
Innate Immunity to Campylobacter
Upon ingestion, Campylobacter has to first pass the acidic environment of the stomach. This is clearly an effective barrier, since patients that use proton pump inhibitors are more susceptible to Campylobacter infection (51, 121). In the intestine, Campylobacter has evolved strategies to circumvent the induction of innate immunity. For instance, Toll-like receptor 5 (TLR5), the pattern recognition receptor for flagellin, is not stimulated by Campylobacter due to the structure of its flagellin (5, 175). Also, TLR9, the receptor for CpG dinucleotides, is not efficiently stimulated (49). However, mice deficient in MyD88, a crucial signaling molecule downstream of TLRs, have recently been shown to be susceptible to Campylobacter infection (177), indicating that TLR pathways are important for the defense against disease. This is confirmed by the fact that NF-κB-regulated transcription is readily activated in in vitro models (88) and apparently necessary for defense, since NF-κB-gene deleted mice display enhanced susceptibility to infection (63). So although Campylobacter can circumvent the activation of innate immunity via TLR5 and TLR9, innate immune mechanisms are essential for host defense. Recently, it was shown that innate responses to Campylobacter are at least partly mediated by the intracellular pattern recognition receptor NOD1 (190) and that natural resistance-associated macrophage protein, a gene involved in macrophage activation, also plays a role in susceptibility to campylobacteriosis (177).
Fucosylated sugars present in breast milk were shown to inhibit the in vitro and in vivo binding of Campylobacter to the intestinal mucosa and inhibit diarrhea (28, 112, 143). In addition, C. jejuni is serum sensitive, highlighting the importance of complement-mediated killing (28). The role of PMN-mediated killing of opsonized bacteria was shown to be variable (133). As discussed below (see “In Vitro Models of Infection”), a wide range of studies have shown that Campylobacter is able to induce a proinflammatory response. Whether a strong proinflammatory response is also induced in vivo is still under study.
Humoral Immunity to Campylobacter
Most people infected with Campylobacter develop humoral responses to a number of Campylobacter antigens. Experimental studies have shown the specificities and kinetics of immune responses during infection of primates and human volunteers (24, 145). In humans, circulating antibodies are first detectable 6 to 7 days after the onset of illness and rise rapidly shortly afterwards (reviewed in reference 124). Specific serum immunoglobulin A (IgA) levels peak 7 to 10 days after the onset of symptoms. Specific serum IgG levels peak after 3 to 4 weeks. Serum IgA levels decline rapidly after the onset of illness, whereas IgM and especially IgG levels remain high for a longer time (26, 40, 157). Antibody decay profiles for patients show that serum IgA levels declined to baseline levels within 2.5 months after infection (157), with a similar trend for salivary IgA levels (40). Serum and salivary IgG levels declined within 4.5 months after acute infection but remained elevated for prolonged periods of time, although large individual variation was apparent (40, 157). It is obviously more difficult to assess the kinetics of local, mucosal responses to infection, so there are fewer data on the subject. Specific antibodies have been detected in feces and urine during natural infection (99), and specific secretory IgA was detected in jejunal fluid from volunteer infections (24).
Antibody specificity studies have identified a number of Campylobacter antigens recognized during infection. Not surprisingly, many of the features highlighted as potential virulence factors, and which are on the cell surface, are immunogenic. A major, immunodominant antigen of Campylobacter is flagellin, the subunit protein of flagella (118, 119).
A number of other proteins, including major outer membrane proteins, have also been identified as being immunogenic, although their natures and roles are often unknown. The periplasmic/membrane-associated proteins PEB1 (28 kDa) and PEB3 (30 kDa) were found to be strongly immunogenic; 15/19 convalescent-phase sera were found to recognize them in enzyme-linked immunosorbent assays (132). Panigrahi et al. (129) identified a number of proteins that were expressed, or overexpressed, only in vivo. Two of these, with molecular masses of 47 and 84 kDa, were found to elicit strong serum IgG responses in humans following infection, including sera from volunteers who were immune to C. jejuni infection when rechallenged. Capsular polysaccharide antigens, the basis of the Penner serotyping scheme, are also immunogenic, eliciting both type-specific and cross-reactive responses (114, 144). The CDT produced by Campylobacter is also immunogenic in human infections, eliciting toxin-neutralizing antibodies (1). Interestingly, chickens do not develop neutralizing antibodies against CDT, indicating host specificity in the immune response to Campylobacter (1). Until we know the true correlates of protective immunity to campylobacteriosis, the role of these antibodies in conferring protective immunity is difficult to establish.
Role of Humoral Immunity in Protection
As described above, epidemiological data indicate that humoral immunity is crucial for the development of protection against Campylobacter disease. Consistent with this, patients with defects in immunoglobulin production are more susceptible to infection. The first humoral immune mechanism encountered by Campylobacter during infection is secretory IgA (sIgA), and various studies have shown that the presence of Campylobacter-specific sIgA and serum IgA correlates with protection against disease (108, 142). Also, studies of breastfed infants point to a protective role of sIgA against infection. In a Mexican study where children were monitored from birth to the age of 2 years, breastfeeding decreased the incidence of diarrhea caused by C. jejuni, and this decrease was associated with the presence of Campylobacter-specific sIgA in breast milk (142). Breast milk containing sIgA against Campylobacter flagellin proteins also decreased the incidence of Campylobacter-induced diarrhea in babies. In addition, there is also a description of one immunocompromised patient in which oral sIgA administration resolved a recurrent Campylobacter infection (77).
Even though all these data point to an important role for sIgA in protection against Campylobacter disease, it is surprising that there are no studies to suggest that patients with IgA deficiency (35) are more susceptible to Campylobacter infection than the general population. IgA deficiency is the most common primary immunodeficiency found in humans, and it is estimated to occur at a frequency of 1:333 to 1:700 in Caucasians (46). The genetic cause underlying IgA deficiency is unknown, but from these data, it can be concluded that other compensatory mechanisms are activated in the absence of IgA and that IgA is probably important but not crucial for the host defense against Campylobacter. In addition, the presence of sIgA in a mother's breast milk is probably accompanied by the transplacental transfer of maternal IgG to the baby during pregnancy, indicating that effects observed in breastfeeding studies could also be related to IgG.
A protective role of IgM against Campylobacter infection was suggested by the observation that in hypo- or agammaglobulinemic patients who suffered from severe Campylobacter infection, the infusion of a pentaglobin preparation, which contained Campylobacter-specific IgM, completely resolved the infection, whereas immunoglobulin preparations that contained only IgG did not (31). Although this observation was made for a few of patients, it does point to a role for IgM in protection. This also fits with the assumption that increased IgM production is one of the general immune compensation mechanisms in patients with IgA deficiency. In addition, there is an active secretion mechanism for IgM at mucosal surfaces (34), and IgM antibodies can fix complement almost 200 times more efficiently than IgG (32). In contrast to Campylobacter-specific IgG, IgM can also enhance reactive oxygen intermediate production and bactericidal activity of PMN (10).
From the finding that patients with hypo- or agammaglobulinemia are more susceptible to Campylobacter infection, it is clear that IgG also plays an important role in protection against disease. IgG levels remain high for a longer time than do IgA and IgM levels after infection (40, 157). Chronic raw milk consumers have high IgG levels and seem to be protected against Campylobacter disease (27). Similarly, children in developing countries develop IgG responses very early in life and are then protected against bloody diarrhea (25, 30), indicating that IgG is also involved in protection against disease.
Cellular Immunity
Systemic and recurrent Campylobacter infections in patients with human immunodeficiency virus or AIDS, who have a significant reduction in the level of CD4+ T cells, point to an important role of cell-mediated immunity in the defense against Campylobacter infection, although B-cell responses and antibody production can also be impaired in AIDS patients. There has been one report on the cellular immunity of a patient who suffered from severe Campylobacter infection. Peripheral blood mononuclear cells of this patient proliferated in response to the homologous strain (19). In addition, the rapid induction of proinflammatory cytokine production was observed in the serum of this patient. Recently, both viable and killed Campylobacter preparations were shown to induce the maturation of dendritic cells in vitro and the induction of various proinflammatory cytokines (83), indicating that Campylobacter induces both innate and specific cell-mediated immune responses.
There are also indications that Campylobacter extracts induce the in vitro expansion of γ/δ T cells obtained from healthy controls. This cell type has been implicated in mucosal immune responses. These cells respond to nonprotein components in the Campylobacter extract (168). Since it is not known whether γ/δ T-cell expansion also occurs in vivo, the significance of this observation in relation to protection against Campylobacter infection is unknown.
Although cell-mediated immunity appears to be important in the defense against Campylobacter, the available data do not point to specific candidate host factors that could be studied in humans.
LESSONS LEARNED FROM EXPERIMENTAL INFECTION
In Vitro Models of Infection
Study of the mechanisms of Campylobacter infection and pathogenesis is complicated by the lack of simple animal models that mimic human infection. In vitro cell culture methods provide a useful alternative to investigate the interactions between Campylobacter and the host epithelium that occur during infection. In the genomics era, there is an increasing use of in vitro cell culture techniques to determine the potential role of different genes in infection and pathogenesis. In vitro studies on host-pathogen interactions often use cells of epithelial origin. These can be nonpolarized (HeLa, HEp-2, and INT407) or polarized (Caco-2, HT29, and T84) cells. Polarized cell lines have an apical surface facing the luminal side and a basolateral side interfacing with the lamina propia and mimic the in vivo situation. Both sides differ biochemically with respect to transport functions and cellular localization of surface components such as TLRs (11, 72, 130). The use of polarized models is useful for studying microbial effects on transport, transcytosis mechanisms, and cell invasion (113). Nonpolarized models can also be used for studying bacterial virulence. Such studies have elucidated receptors, signaling pathways, and internalization mechanisms (55, 59, 96).
Invasion assays using in vitro cell culture models allow many parameters to be independently adjusted to achieve optimal results. Incubation time and assay volume, which can affect the results, are standard variables, while the number of internalized bacteria strongly depends on the type of cell line and Campylobacter strain used, the number of bacteria added per cell, and the concentration of antibiotics used to kill noninternalized bacteria (65). Although the mechanism of invasion is currently being unraveled, the fate of internalized Campylobacter, and whether they are able to replicate intracellularly, is still unknown (for a recent review, see reference 184). More recently, it was shown that Campylobacter was able to prevent targeting to lysosomes in epithelial cells, whereas it was targeted to lysosomes and rapidly killed by macrophages (176). These data indicate that the invasive properties of various Campylobacter strains are not fully understood. They also show a considerable range in invasive abilities among strains. However, evidence on the in vitro invasive ability of a strain and the development of disease symptoms (bacteremic/bloody diarrhea, etc.) has been conflicting (48, 60, 94, 120, 123) (see also http://www.medvetnet.org/pdf/Reports/Workpackage8.pdf), and some of the observed correlations may have been due to in vivo passage and not virulence properties per se (123). The toxicity of various strains has also been studied in cell culture systems, and those studies revealed that Campylobacter-induced toxicity varies from strain to strain (reviewed in reference 173).
Several studies have investigated host cell cytokine and chemokine responses to Campylobacter infection in cell culture models using human epithelial or macrophage cell lines. A number of studies showed that Campylobacter induces proinflammatory cytokines such as interleukin-8 (IL-8), IL-1, and tumor necrosis factor and chemokines such as CCL2 and CCL4 (2, 14, 88, 104). In addition, the production of Th1 cytokine gamma interferon, regulatory cytokine IL-10, and Th2 cytokine IL-4 has been observed (2). These responses appear to be dependent on NF-κB and AP-1 activation (84, 88), although one study suggested NF-κB-independent activation of proinflammatory cytokine production (88). Interestingly, viable Campylobacter cells are more potent at inducing proinflammatory cytokines than bacterial sonicates or supernatants (2, 14), suggesting that an active Campylobacter process is involved in these responses. Consistent with this, Campylobacter mutants with a reduced ability to adhere to epithelial cells are less potent inducers of proinflammatory responses (82). Furthermore, Campylobacter-induced IL-8 production is dependent on de novo protein synthesis (82, 175).
Animal Models of Infection
Murine models with defined deletions in components of innate or adaptive immunity are crucial in identifying genetic factors involved in the host defense against infection. However, progress in our understanding of Campylobacter infection and disease has been seriously hampered by the lack of an appropriate animal model, which makes studies in the above-mentioned gene-deleted mice impossible. Whereas most animals can be colonized with Campylobacter, gastroenteritis does not occur (reviewed in reference 122). Mice are not naturally colonized with Campylobacter, but in an experimental setting, colonization can be established. Campylobacter vaccination experiments have also been performed using such models, and protection against colonization with a homologous strain could be induced. Some authors have been able to induce gastrointestinal disease in infant mice (90). In these mice, intraperitoneal injection with C. jejuni produced self-limiting diarrhea, but since infant mice do not have a fully developed immune system, they are not suitable for studying “normal” Campylobacter disease or vaccine-induced protection. Also, in athymic, germ-free, nude mice, transient diarrhea was observed (186). Because these models display severe defects in the capacity to raise innate and adaptive immunity, they are not suitable for measuring immune responses to Campylobacter. For that reason, an intranasal challenge model in mice has been developed (16). Although this is not the natural infection route, intranasal infection of mice with Campylobacter results in systemic disease and death of a high proportion of mice. Various clinical isolates were differentially virulent in this model, and also, vaccine-induced protection could be measured. However, as no diarrhea has been reported, the relevance of this model for human disease is debatable, and extensive follow-up studies have not been performed.
More recently, it was shown that NF-κB-deficient mice, which have a defect in the induction of the production of proinflammatory cytokines such as tumor necrosis factor alpha, IL-12, IL-1, and IL-6, develop gastroenteritis when infected with Campylobacter (63). Recently, two novel murine Campylobacter models were described, one using IL-10 gene-deleted mice (106) and one using MyD88 gene-deleted mice (177). The latter model, which is again a model of severely immunocompromised mice, also revealed a role for the gene encoding natural resistance-associated macrophage protein in determining resistance to campylobacteriosis, suggesting that in this model, macrophage activation and intracellular survival may contribute to pathology (177).
Diarrheal disease in young weanling ferrets (20, 62) and in some nonhuman primates (145) has been reported, although few laboratories have the facilities to maintain these models. A removable intestinal tie adult rabbit diarrhea model was also reported (185). The model involves surgery and is of questionable relevance to human disease, so it has not been used extensively. Although these models can shed light on the virulence of Campylobacter and the pathogenesis of the disease, they do not contribute to our understanding of the host factors involved in determining susceptibility to infection. In addition, ferret models may be complicated by the fact that ferrets are often fed on chicks and, as a result, could be relatively resistant to Campylobacter infection. A New World monkey Aotus nancymae model was recently reported (87), which, if it proved to be reproducible in different laboratories and was able to demonstrate colonization and invasive differences among strains, could help to improve our understanding of C. jejuni virulence properties and the interaction of the organism with the host.
A large amount of work has been done using chicken models of infection. The avian gut is considered to be the natural environment of C. jejuni. Although disease has been reported (144), the organism is generally regarded as being a commensal pathogen. Therefore, although inappropriate for determining pathogenesis mechanisms, the chicken is a suitable model for determining colonization factors and in vivo survival mechanisms of thermophilic campylobacters (42). Furthermore, as the reduction of C. jejuni numbers in poultry is seen as a way to reduce the number of human cases (125), there have been a number of published reports focusing on avian host factors. Studies have characterized antibody responses to infection (39), and in vitro studies using avian cells have identified cell-mediated immune responses (154). Such studies have shown that maternally derived antibodies can protect against colonization (146) and identified a genetic basis for susceptibility to colonization (33). Those studies highlight the importance of host factors in determining the outcome of infection. Furthermore, comparison of responses among hosts with different pathologies and patterns of colonization can help to elucidate pathogenesis and virulence mechanisms of the bacterium and so aid in the development of control strategies.
Consistent with observations of patients, these studies show that severe immune defects in mice also led to enhanced susceptibility to infection. However, research using animal models has not yet led to the identification of clearly defined, specific immune mechanisms that are crucial for the host defense against Campylobacter. The recent progress in gene-deleted mice holds promise for future studies.
Human Volunteer Studies
With the lack of an appropriate animal model for Campylobacter infection, infection of human volunteers has been important in increasing our understanding of colonization and disease induction. These studies have shown that there is a clear dose-response relation between the number of ingested bacteria and colonization of the patients and that Campylobacter is highly infectious (23, 24). Surprisingly, no clear dose-response relation between the number of ingested bacteria and the development of clinical disease could be demonstrated in these studies. This is in sharp contrast to the data from a raw-milk outbreak, which showed a clear dose response, in presumably immunologically naïve children (159). However, the volunteers in this study were not screened for preexisting immunity to Campylobacter, and this, together with the small study groups, may (partially) explain this finding. The two Campylobacter strains used in these studies induced disease with different severities, indicating that not all Campylobacter strains have similar disease-inducing properties. After the volunteers recovered, some of them were challenged with the homologous strain, and it appeared that primary infection resulted in protection against disease but not against colonization. These data indicate that vaccination against Campylobacter may be feasible, although the high level of variation among Campylobacter strains may hamper this approach.
VACCINE-INDUCED PROTECTION
Currently, there is no vaccine against campylobacteriosis available, but vaccination seems to be a good way to increase basal immunity in the population. Several approaches are followed: the development of (i) live attenuated vaccines, (ii) vaccines based on heat-killed/formalin-killed bacteria with or without mucosal adjuvants, (iii) subunit vaccines delivered together with adjuvants, and (iv) live attenuated Salmonella strains expressing Campylobacter proteins. For example, recA mutants that could be used as live attenuated vaccines have been developed (76). Formalin- or heat-killed bacterial preparations or combinations of the two have been used as oral vaccines, with or without E. coli heat-labile toxin to enhance mucosal responses. Such vaccine preparations were shown to induce protective immunity in mice, ferrets, and nonhuman primates (15, 17, 36, 141). Subunit vaccines based on FlaA were shown to induce short-term protective immunity in mice (100), and proteomics approaches are currently being used to identify Campylobacter surface proteins that could be included in subunit vaccines (137). Finally, an attenuated Salmonella vaccine expressing Campylobacter PEB1 was shown to induce humoral immunity in mice, with high seroconversion rates (90% to 100%), although these responses were not protective (150). Because of the link between Campylobacter infection and GBS, whole-cell vaccine approaches are seriously hampered. Both live and killed vaccine preparations should be based on Campylobacter strains that cannot induce GBS. A small study with volunteers has shown that none of the volunteers infected with virulent Campylobacter strains or with a killed vaccine preparation developed persistent antiganglioside antibodies (135). However, until we know exactly which bacterial and host properties are involved in the development of GBS, large-scale vaccine trials with whole-cell vaccines are probably not feasible.
FUTURE DIRECTIONS
What can we learn from all available information? All data described above clearly indicate that an effective immune system is crucial in the host defense against Campylobacter infection. However, which specific components of the host response are important is still largely unclear. In fact, there are many more open questions than clear answers. For instance, even though serological responses to Campylobacter infection have been studied and reveal that a good antibody response is essential, it is still not clear whether IgG, IgA, IgM, or combinations of the three are necessary.
Various approaches can be used to get answers to these basic questions. Murine models with defined deletions in components of innate or adaptive immunity, which have greatly aided the identification of genetic factors involved in the host defense against other pathogens, may yet be useful for our understanding of Campylobacter pathogenesis. Novel developments in such animal model systems may therefore open up possibilities for answering basic questions. Even though these models rely on the use of severely immunocompromised mice, the transfer of sera and lymphocytes obtained after the infection of immunocompetent mice may be used to elucidate the immune mechanisms involved in protection against campylobacteriosis. Combined with measurement of both serum and saliva antibodies in human infections, this approach may shed light on this issue. This again highlights the importance of the development of protocols which can be followed when suspected outbreaks occur. Such naturally occurring events should be exploited more effectively to advance research into host-pathogen interactions in campylobacteriosis.
Another approach that could be taken is to perform human genetic studies. Infectious diseases have clearly posed a strong evolutionary pressure on the selection of immune genes. To what extent Campylobacter infection has also played a role in this process is unclear. Analysis of common polymorphisms in genes involved in gastric acid production, humoral immunity, innate immunity, and cell-mediated immunity could shed light on the roles of various processes in the defense against Campylobacter infection. However, it is also clear that it will not be so easy to select candidate genes for such studies.
A third approach that could be taken is to allow research into both host and pathogen factors to be much more driven by epidemiological findings. Age-related differences in acquiring infection with common and rare Campylobacter variants are an example of how this could be done. One could also envisage that similar studies can be performed with patients who do or do not use proton pump inhibitors. This may be used to elucidate whether enhanced susceptibility in these patients is related either to a higher effective dose or infection with less virulent strains. Also, the role of other identified risk factors for disease could be studied.
Finally, recent technical advances in host-pathogen interaction research now enable detailed molecular studies into the interaction of Campylobacter and the host. Large-scale microarray analysis can be performed either in vivo or in vitro, and the host response to Campylobacter infection can be analyzed in detail. Proteomics approaches to study host-pathogen interactions are also currently being developed. Such detailed molecular studies combined with better integration of host and pathogen research driven by epidemiological findings may truly advance our understanding of Campylobacter infection in humans.
Acknowledgments
This review is presented on behalf of all members of Workpackage 30, whom we thank for their comments and support. Members of WP30 (alphabetically) are Thomas Alter, Dang Doung Bang, Shaun Cawthraw, Aurora Echeita, Steen Ethelberg, Rafal Gierczyniski, Riny Janssen, Karen Krogfelt, Ida Luzzi, Jean-Yves Madec, Andy Lawson, Eva Moller Nielsen, Kare Molback, Noel McCarthy, Diane Newell, Robert Owen, Eva Olsson Engvall, Wilfrid van Pelt, Anne Ridley, Katell Rivoal, Fimme Jan van der Wal, and Jaap Wagenaar. We also thank Trudy Wassenaar, Arie Havelaar, Rob de Jonge, Barbara Hoebee, Sarah O'Brien, and Julian Ketley for critical comments.
This work was funded as an activity of Med-Vet-Net, a European Network of Excellence within the EU 6th Framework Programme.
REFERENCES
- 1.Abuoun, M., G. Manning, S. A. Cawthraw, A. Ridley, I. H. Ahmed, T. M. Wassenaar, and D. G. Newell. 2005. Cytolethal distending toxin (CDT)-negative Campylobacter jejuni strains and anti-CDT neutralizing antibodies are induced during human infection but not during colonization in chickens. Infect. Immun. 73:3053-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Salloom, F. S., A. Al Mahmeed, A. Ismaeel, G. A. Botta, and M. Bakhiet. 2003. Campylobacter-stimulated INT407 cells produce dissociated cytokine profiles. J. Infect. 47:217-224. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders, B. J., B. A. Lauer, J. W. Paisley, and L. B. Reller. 1982. Double-blind placebo controlled trial of erythromycin for treatment of Campylobacter enteritis. Lancet i:131-132. [DOI] [PubMed] [Google Scholar]
- 5.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang, C. W., B. C. Jacobs, and J. D. Laman. 2004. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 25:61-66. [DOI] [PubMed] [Google Scholar]
- 7.Ang, C. W., J. D. Laman, H. J. Willison, E. R. Wagner, H. P. Endtz, M. A. De Klerk, A. P. Tio-Gillen, N. Van den Braak, B. C. Jacobs, and P. A. van Doorn. 2002. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barre and Miller Fisher patients. Infect. Immun. 70:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo, F. J., and D. L. Swerdlow. 1995. Bacterial enteric infections in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 1):S84-S93. [DOI] [PubMed] [Google Scholar]
- 9.Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barre syndrome. Biochemistry 33:241-249. [DOI] [PubMed] [Google Scholar]
- 10.Autenrieth, I. B., A. Schwarzkopf, J. H. Ewald, H. Karch, and R. Lissner. 1995. Bactericidal properties of Campylobacter jejuni-specific immunoglobulin M antibodies in commercial immunoglobulin preparations. Antimicrob. Agents Chemother. 39:1965-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backhed, F., and M. Hornef. 2003. Toll-like receptor 4-mediated signaling by epithelial surfaces: necessity or threat? Microbes Infect. 5:951-959. [DOI] [PubMed] [Google Scholar]
- 12.Baker, M., N. Wilson, R. Ikram, S. Chambers, P. Shoemack, and G. Cook. 2006. Regulation of chicken contamination is urgently needed to control New Zealand's serious campylobacteriosis epidemic. N. Z. Med. J. 119:U2264. [PubMed] [Google Scholar]
- 13.Baker, M. G., E. Sneyd, and N. A. Wilson. 2007. Is the major increase in notified campylobacteriosis in New Zealand real? Epidemiol. Infect. 135:163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakhiet, M., F. S. Al Salloom, A. Qareiballa, K. Bindayna, I. Farid, and G. A. Botta. 2004. Induction of alpha and beta chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J. Infect. 48:236-244. [DOI] [PubMed] [Google Scholar]
- 15.Baqar, S., L. A. Applebee, and A. L. Bourgeois. 1995. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect. Immun. 63:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baqar, S., A. L. Bourgeois, L. A. Applebee, A. S. Mourad, M. T. Kleinosky, Z. Mohran, and J. R. Murphy. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baqar, S., A. L. Bourgeois, P. J. Schultheiss, R. I. Walker, D. M. Rollins, R. L. Haberberger, and O. R. Pavlovskis. 1995. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine 13:22-28. [DOI] [PubMed] [Google Scholar]
- 18.Baqar, S., N. D. Pacheco, and F. M. Rollwagen. 1993. Modulation of mucosal immunity against Campylobacter jejuni by orally administered cytokines. Antimicrob. Agents Chemother. 37:2688-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. [DOI] [PubMed] [Google Scholar]
- 20.Bell, J. A., and D. D. Manning. 1990. A domestic ferret model of immunity to Campylobacter jejuni-induced enteric disease. Infect. Immun. 58:1848-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berberian, L. S., Y. Valles-Ayoub, L. K. Gordon, S. R. Targan, and J. Braun. 1994. Expression of a novel autoantibody defined by the VH3-15 gene in inflammatory bowel disease and Campylobacter jejuni enterocolitis. J. Immunol. 153:3756-3763. [PubMed] [Google Scholar]
- 22.Berden, J. H., H. L. Muytjens, and L. B. van de Putte. 1979. Reactive arthritis associated with Campylobacter jejuni enteritis. Br. Med. J. i:380-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 24.Black, R. E., D. M. Perlman, M. L. Clements, M. M. Levine, and M. J. Blaser. 1992. Human volunteer studies with Campylobacter jejuni, p. 207-215. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. ASM Press, Washington, DC.
- 25.Blaser, M. J., R. E. Black, D. J. Duncan, and J. Amer. 1985. Campylobacter jejuni-specific serum antibodies are elevated in healthy Bangladeshi children. J. Clin. Microbiol. 21:164-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaser, M. J., and D. J. Duncan. 1984. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect. Immun. 44:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaser, M. J., E. Sazie, and L. P. Williams, Jr. 1987. The influence of immunity on raw milk-associated Campylobacter infection. JAMA 257:43-46. [PubMed] [Google Scholar]
- 28.Blaser, M. J., P. F. Smith, and P. F. Kohler. 1985. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J. Infect. Dis. 151:227-235. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Blaser, M. J., D. N. Taylor, and P. Echeverria. 1986. Immune response to Campylobacter jejuni in a rural community in Thailand. J. Infect. Dis. 153:249-254. [DOI] [PubMed] [Google Scholar]
- 31.Borleffs, J. C., J. F. Schellekens, E. Brouwer, and M. Rozenberg-Arska. 1993. Use of an immunoglobulin M containing preparation for treatment of two hypogammaglobulinemic patients with persistent Campylobacter jejuni infection. Eur. J. Clin. Microbiol. Infect. Dis. 12:772-775. [DOI] [PubMed] [Google Scholar]
- 32.Borsos, T., and H. J. Rapp. 1965. Complement fixation on cell surfaces by 19S and 7S antibodies. Science 150:505-506. [DOI] [PubMed] [Google Scholar]
- 33.Boyd, Y., E. G. Herbert, K. L. Marston, M. A. Jones, and P. A. Barrow. 2005. Host genes affect intestinal colonisation of newly hatched chickens by Campylobacter jejuni. Immunogenetics 57:248-253. [DOI] [PubMed] [Google Scholar]
- 34.Brandtzaeg, P. 1981. Transport models for secretory IgA and secretory IgM. Clin. Exp. Immunol. 44:221-232. [PMC free article] [PubMed] [Google Scholar]
- 35.Brandtzaeg, P., D. E. Nilssen, T. O. Rognum, and P. S. Thrane. 1991. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol. Clin. N. Am. 20:397-439. [PubMed] [Google Scholar]
- 36.Burr, D. H., D. Rollins, L. H. Lee, D. L. Pattarini, S. S. Walz, J. H. Tian, J. L. Pace, A. L. Bourgeois, and R. I. Walker. 2005. Prevention of disease in ferrets fed an inactivated whole cell Campylobacter jejuni vaccine. Vaccine 23:4315-4321. [DOI] [PubMed] [Google Scholar]
- 37.Caporale, C. M., F. Papola, M. A. Fioroni, A. Aureli, A. Giovannini, F. Notturno, D. Adorno, V. Caporale, and A. Uncini. 2006. Susceptibility to Guillain-Barre syndrome is associated to polymorphisms of CD1 genes. J. Neuroimmunol. 177:112-118. [DOI] [PubMed] [Google Scholar]
- 38.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 39.Cawthraw, S., R. Ayling, P. Nuijten, T. Wassenaar, and D. G. Newell. 1994. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 38:341-349. [PubMed] [Google Scholar]
- 40.Cawthraw, S. A., R. A. Feldman, A. R. Sayers, and D. G. Newell. 2002. Long-term antibody responses following human infection with Campylobacter jejuni. Clin. Exp. Immunol. 130:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cawthraw, S. A., L. Lind, B. Kaijser, and D. G. Newell. 2000. Antibodies, directed towards Campylobacter jejuni antigens, in sera from poultry abattoir workers. Clin. Exp. Immunol. 122:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cawthraw, S. A., S. Park, B. W. Wren, J. M. Ketley, R. Ayling, and D. G. Newell. 1996. The usefulness of the chick colonisation model to investigate potential colonisation factors of campylobacters, p. 649-652. In D. G. Newell, J. M. Ketley, and R. A. Feldman (ed.), Campylobacter, Helicobacter and related organisms. Plenum Press, New York, NY.
- 43.Cawthraw, S. A., T. M. Wassenaar, R. Ayling, and D. G. Newell. 1996. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol. Infect. 117:213-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted trough food-selected sites, United States, 2003. MMBR Morb. Mortal. Wkly. Rep. 53:338-343. [PubMed] [Google Scholar]
- 45.Christenson, B., A. Ringner, C. Blucher, H. Billaudelle, K. N. Gundtoft, G. Eriksson, and M. Bottiger. 1983. An outbreak of campylobacter enteritis among the staff of a poultry abattoir in Sweden. Scand. J. Infect. Dis. 15:167-172. [DOI] [PubMed] [Google Scholar]
- 46.Clark, J. A., P. A. Callicoat, N. A. Brenner, C. A. Bradley, and D. M. Smith, Jr. 1983. Selective IgA deficiency in blood donors. Am. J. Clin. Pathol. 80:210-213. [DOI] [PubMed] [Google Scholar]
- 47.Coker, A. O., R. D. Isokpehi, B. N. Thomas, K. O. Amisu, and C. L. Obi. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coote, J. G., D. E. Stewart-Tull, R. J. Owen, F. J. Bolton, B. L. Siemer, D. Candlish, D. H. Thompson, A. C. Wardlaw, S. L. On, A. Candlish, B. Billcliffe, P. J. Jordan, K. Kristiansen, and P. Borman. 2007. Comparison of virulence-associated in vitro properties of typed strains of Campylobacter jejuni from different sources. J. Med. Microbiol. 56:722-732. [DOI] [PubMed] [Google Scholar]
- 49.Dalpke, A., J. Frank, M. Peter, and K. Heeg. 2006. Activation of Toll-like receptor 9 by DNA from different bacterial species. Infect. Immun. 74:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doorduyn, Y., W. E. Van Den Brandhof, Y. T. van Duynhoven, W. J. Wannet, and W. Van Pelt. 2006. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol. Infect. 134:617-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doorduyn, Y., W. Van Pelt, C. L. Siezen, F. van der Horst, Y. T. van Duynhoven, B. Hoebee, and R. Janssen. 2007. Novel insight in the association between salmonellosis or campylobacteriosis and chronic illness, and the role of host genetics in susceptibility to these diseases. Epidemiol. Infect. [Epub ahead of print.] doi: 10.1017/S095026880700996X. [DOI] [PMC free article] [PubMed]
- 52.Dunlop, S. P., D. Jenkins, K. R. Neal, and R. C. Spiller. 2003. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 125:1651-1659. [DOI] [PubMed] [Google Scholar]
- 53.Eastmond, C. J., J. A. Rennie, and T. M. Reid. 1983. An outbreak of Campylobacter enteritis—a rheumatological followup survey. J. Rheumatol. 10:107-108. [PubMed] [Google Scholar]
- 54.EFSA. 2006. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2005. EFSA J. 94:1-236. [Google Scholar]
- 55.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 56.Emonts, M., R. H. Veenhoven, S. P. Wiertsema, J. J. Houwing-Duistermaat, V. Walraven, R. de Groot, P. W. Hermans, and E. A. Sanders. 2007. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 120:814-823. [DOI] [PubMed] [Google Scholar]
- 57.Engberg, J., P. Gerner-Smidt, F. Scheutz, N. E. Moller, S. L. On, and K. Molbak. 1998. Water-borne Campylobacter jejuni infection in a Danish town—a 6-week continuous source outbreak. Clin. Microbiol. Infect. 4:648-656. [DOI] [PubMed] [Google Scholar]
- 58.Ethelberg, S., J. Simonsen, P. Gerner-Smidt, K. E. Olsen, and K. Molbak. 2005. Spatial distribution and registry-based case-control analysis of Campylobacter infections in Denmark, 1991-2001. Am. J. Epidemiol. 162:1008-1015. [DOI] [PubMed] [Google Scholar]
- 59.Everest, P. H., H. Goossens, J. P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 60.Fauchere, J. L., A. Rosenau, M. Veron, E. N. Moyen, S. Richard, and A. Pfister. 1986. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect. Immun. 54:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer, A. 2004. Human primary immunodeficiency diseases: a perspective. Nat. Immunol. 5:23-30. [DOI] [PubMed] [Google Scholar]
- 62.Fox, J. G., J. I. Ackerman, N. Taylor, M. Claps, and J. C. Murphy. 1987. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am. J. Vet. Res. 48:85-90. [PubMed] [Google Scholar]
- 63.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-139. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 65.Friis, L. M., C. Pin, B. M. Pearson, and J. M. Wells. 2005. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J. Microbiol. Methods 61:145-160. [DOI] [PubMed] [Google Scholar]
- 66.Gauci, A., and A. Ammon. 2007. The First European Communicable Disease Epidemiological Report. European Centre of Disease Prevention and Control, Solna, Sweden.
- 67.Geboes, K. 2001. Crohn's disease, ulcerative colitis or indeterminate colitis—how important is it to differentiate? Acta Gastroenterol. Belg. 64:197-200. [PubMed] [Google Scholar]
- 68.Geleijns, K., M. Emonts, J. D. Laman, W. van Rijs, P. A. van Doorn, P. W. Hermans, and B. C. Jacobs. 2007. Genetic polymorphisms of macrophage-mediators in Guillain-Barre syndrome. J. Neuroimmunol. 190:127-130. [DOI] [PubMed] [Google Scholar]
- 69.Geleijns, K., B. C. Jacobs, W. van Rijs, A. P. Tio-Gillen, J. D. Laman, and P. A. van Doorn. 2004. Functional polymorphisms in LPS receptors CD14 and TLR4 are not associated with disease susceptibility or Campylobacter jejuni infection in Guillain-Barre patients. J. Neuroimmunol. 150:132-138. [DOI] [PubMed] [Google Scholar]
- 70.Geleijns, K., A. Roos, J. J. Houwing-Duistermaat, W. van Rijs, A. P. Tio-Gillen, J. D. Laman, P. A. van Doorn, and B. C. Jacobs. 2006. Mannose-binding lectin contributes to the severity of Guillain-Barre syndrome. J. Immunol. 177:4211-4217. [DOI] [PubMed] [Google Scholar]
- 71.Geleijns, K., G. M. Schreuder, B. C. Jacobs, K. Sintnicolaas, R. van Koningsveld, J. Meulstee, J. D. Laman, and P. A. van Doorn. 2005. HLA class II alleles are not a general susceptibility factor in Guillain-Barre syndrome. Neurology 64:44-49. [DOI] [PubMed] [Google Scholar]
- 72.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 73.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green, C. G., D. Krause, and J. Wylie. 2006. Spatial analysis of Campylobacter infection in the Canadian province of Manitoba. Int. J. Health Geogr. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grimbacher, B., A. Hutloff, M. Schlesier, E. Glocker, K. Warnatz, R. Drager, H. Eibel, B. Fischer, A. A. Schaffer, H. W. Mages, R. A. Kroczek, and H. H. Peter. 2003. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat. Immunol. 4:261-268. [DOI] [PubMed] [Google Scholar]
- 76.Guerry, P., P. M. Pope, D. H. Burr, J. Leifer, S. W. Joseph, and A. L. Bourgeois. 1994. Development and characterization of recA mutants of Campylobacter jejuni for inclusion in attenuated vaccines. Infect. Immun. 62:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammarstrom, V., C. I. Smith, and L. Hammarstrom. 1993. Oral immunoglobulin treatment in Campylobacter jejuni enteritis. Lancet 341:1036. [PubMed] [Google Scholar]
- 78.Hannu, T., L. Mattila, H. Rautelin, P. Pelkonen, P. Lahdenne, A. Siitonen, and M. Leirisalo-Repo. 2002. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 41:312-318. [DOI] [PubMed] [Google Scholar]
- 79.Havelaar, A. H., M. A. de Wit, R. van Koningsveld, and E. van Kempen. 2000. Health burden in The Netherlands due to infection with thermophilic Campylobacter spp. Epidemiol. Infect. 125:505-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Havelaar, A. H., M. Nauta, M. J. Mangen, E. Katsma, M. J. Boogaardt, J. Wagenaar, and the CARMA Projectgroep. 2005. Kosten en baten van Campylobacter bestrijding-integratie van risico-analyse, epidemiologie en economie. RIVM report 250911008. RIVM, Bilthoven, The Netherlands.
- 81.Helms, M., J. Simonsen, and K. Molbak. 2006. Foodborne bacterial infection and hospitalization: a registry-based study. Clin. Infect. Dis. 42:498-506. [DOI] [PubMed] [Google Scholar]
- 82.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu, L., M. D. Bray, M. Osorio, and D. J. Kopecko. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu, L., and T. E. Hickey. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect. Immun. 73:4437-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu, L., and D. J. Kopecko. 2000. Interactions of Campylobacter with eukaryotic cells: gut luminal colonization and mucosal invasion mechanisms, p. 191-215. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM, Washington, DC.
- 86.Hughes, R. A., and D. R. Cornblath. 2005. Guillain-Barre syndrome. Lancet 366:1653-1666. [DOI] [PubMed] [Google Scholar]
- 87.Jones, F. R., S. Baqar, A. Gozalo, G. Nunez, N. Espinoza, S. M. Reyes, M. Salazar, R. Meza, C. K. Porter, and S. E. Walz. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect. Immun. 74:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kapperud, G., J. Lassen, S. M. Ostroff, and S. Aasen. 1992. Clinical features of sporadic Campylobacter infections in Norway. Scand. J. Infect. Dis. 24:741-749. [DOI] [PubMed] [Google Scholar]
- 90.Kazmi, S. U., B. S. Roberson, and N. J. Stern. 1984. Animal-passed, virulence-enhanced Campylobacter jejuni causes enteritis in neonatal mice. Curr. Microbiol. 11:159-164. [Google Scholar]
- 91.Keat, A., and I. Rowe. 1991. Reiter's syndrome and associated arthritides. Rheum. Dis. Clin. N. Am. 17:25-42. [PubMed] [Google Scholar]
- 92.Kemmeren, J. M., M. J. Mangen, Y. T. van Duynhoven, and A. H. Havelaar. 2005. Priority setting of foodborne pathogens. RIVM report 330080001. RIVM, Bilthoven, The Netherlands.
- 93.Kist, M. 2002. Impact and management of Campylobacter in human medicine—European perspective. Int. J. Infect. Dis. 6:S44-S47. [DOI] [PubMed] [Google Scholar]
- 94.Klipstein, F. A., R. F. Engert, H. Short, and E. A. Schenk. 1985. Pathogenic properties of Campylobacter jejuni: assay and correlation with clinical manifestations. Infect. Immun. 50:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koga, M., N. Yuki, K. Kashiwase, K. Tadokoro, T. Juji, and K. Hirata. 1998. Guillain-Barre and Fisher's syndromes subsequent to Campylobacter jejuni enteritis are associated with HLA-B54 and Cw1 independent of anti-ganglioside antibodies. J. Neuroimmunol. 88:62-66. [DOI] [PubMed] [Google Scholar]
- 96.Krause-Gruszczynska, M., M. Rohde, R. Hartig, H. Genth, G. Schmidt, T. Keo, W. Konig, W. G. Miller, M. E. Konkel, and S. Backert. 2007. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell. Microbiol. 9:2431-2444. [DOI] [PubMed] [Google Scholar]
- 97.Kuroki, S., T. Saida, M. Nukina, T. Haruta, M. Yoshioka, Y. Kobayashi, and H. Nakanishi. 1993. Campylobacter jejuni strains from patients with Guillain-Barre syndrome belong mostly to Penner serogroup 19 and contain beta-N-acetylglucosamine residues. Ann. Neurol. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 98.Kuusi, M., J. P. Nuorti, M. L. Hanninen, M. Koskela, V. Jussila, E. Kela, I. Miettinen, and P. Ruutu. 2005. A large outbreak of campylobacteriosis associated with a municipal water supply in Finland. Epidemiol. Infect. 133:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lane, E. M., R. A. Batchelor, A. L. Bourgeois, D. H. Burr, and J. G. Olson. 1987. Urine and faecal IgA response during naturally acquired infection with Campylobacter jejuni. Lancet i:1141. [DOI] [PubMed] [Google Scholar]
- 100.Lee, L. H., E. Burg III, S. Baqar, A. L. Bourgeois, D. H. Burr, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect. Immun. 67:5799-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Locht, H., and K. A. Krogfelt. 2002. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann. Rheum. Dis. 61:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma, J. J., M. Nishimura, H. Mine, S. Kuroki, M. Nukina, M. Ohta, H. Saji, H. Obayashi, H. Kawakami, T. Saida, and T. Uchiyama. 1998. Genetic contribution of the tumor necrosis factor region in Guillain-Barre syndrome. Ann. Neurol. 44:815-818. [DOI] [PubMed] [Google Scholar]
- 103.Ma, J. J., M. Nishimura, H. Mine, S. Kuroki, M. Nukina, M. Ohta, H. Saji, H. Obayashi, T. Saida, H. Kawakami, and T. Uchiyama. 1998. HLA and T-cell receptor gene polymorphisms in Guillain-Barre syndrome. Neurology 51:379-384. [DOI] [PubMed] [Google Scholar]
- 104.MacCallum, A. J., D. Harris, G. Haddock, and P. H. Everest. 2006. Campylobacter jejuni-infected human epithelial cell lines vary in their ability to secrete interleukin-8 compared to in vitro-infected primary human intestinal tissue. Microbiology 152:3661-3665. [DOI] [PubMed] [Google Scholar]
- 105.Mandal, B. K., M. E. Ellis, E. M. Dunbar, and K. Whale. 1984. Double-blind placebo-controlled trial of erythromycin in the treatment of clinical campylobacter infection. J. Antimicrob. Chemother. 13:619-623. [DOI] [PubMed] [Google Scholar]
- 106.Mansfield, L. S., J. A. Bell, D. L. Wilson, A. J. Murphy, H. M. Elsheikha, V. A. Rathinam, B. R. Fierro, J. E. Linz, and V. B. Young. 2007. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect. Immun. 75:1099-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin, P. M., J. Mathiot, J. Ipero, M. Kirimat, A. J. Georges, and M. C. Georges-Courbot. 1989. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect. Immun. 57:2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Megraud, F., G. Boudraa, K. Bessaoud, S. Bensid, F. Dabis, R. Soltana, and M. Touhami. 1990. Incidence of Campylobacter infection in infants in western Algeria and the possible protective role of breast feeding. Epidemiol. Infect. 105:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Melby, K., O. P. Dahl, L. Crisp, and J. L. Penner. 1990. Clinical and serological manifestations in patients during a waterborne epidemic due to Campylobacter jejuni. J. Infect. 21:309-316. [DOI] [PubMed] [Google Scholar]
- 110.Miller, G., G. M. Dunn, T. M. Reid, I. D. Ogden, and N. J. Strachan. 2005. Does age acquired immunity confer selective protection to common serotypes of Campylobacter jejuni? BMC Infect. Dis. 5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Millson, M., M. Bokhout, J. Carlson, L. Spielberg, R. Aldis, A. Borczyk, and H. Lior. 1991. An outbreak of Campylobacter jejuni gastroenteritis linked to meltwater contamination of a municipal well. Can. J. Public Health 82:27-31. [PubMed] [Google Scholar]
- 112.Morrow, A. L., G. M. Ruiz-Palacios, M. Altaye, X. Jiang, M. L. Guerrero, J. K. Meinzen-Derr, T. Farkas, P. Chaturvedi, L. K. Pickering, and D. S. Newburg. 2004. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 145:297-303. [DOI] [PubMed] [Google Scholar]
- 113.Mostov, K. E., M. Verges, and Y. Altschuler. 2000. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12:483-490. [DOI] [PubMed] [Google Scholar]
- 114.Moura, A. C., and M. Mariano. 1997. Lipids from Mycobacterium leprae cell wall suppress T-cell activation in vivo and in vitro. Immunology 92:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myhr, K. M., K. S. Vagnes, T. H. Maroy, J. H. Aarseth, H. I. Nyland, and C. A. Vedeler. 2003. Interleukin-10 promoter polymorphisms in patients with Guillain-Barre syndrome. J. Neuroimmunol. 139:81-83. [DOI] [PubMed] [Google Scholar]
- 116.Nachamkin, I., B. M. Allos, and T. W. Ho. 2000. Campylobacter jejuni infection and the association with Guillain-Barré syndrome, p. 155-178. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 117.Nachamkin, I., H. Ung, A. P. Moran, D. Yoo, M. M. Prendergast, M. A. Nicholson, K. Sheikh, T. Ho, A. K. Asbury, G. M. McKhann, and J. W. Griffin. 1999. Ganglioside GM1 mimicry in Campylobacter strains from sporadic infections in the United States. J. Infect. Dis. 179:1183-1189. [DOI] [PubMed] [Google Scholar]
- 118.Nachamkin, I., and X. H. Yang. 1989. Human antibody response to Campylobacter jejuni flagellin protein and a synthetic N-terminal flagellin peptide. J. Clin. Microbiol. 27:2195-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nachamkin, I., and X. H. Yang. 1992. Local immune responses to the Campylobacter flagellin in acute Campylobacter gastrointestinal infection. J. Clin. Microbiol. 30:509-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nadeau, E., S. Messier, and S. Quessy. 2003. Comparison of Campylobacter isolates from poultry and humans: association between in vitro virulence properties, biotypes, and pulsed-field gel electrophoresis clusters. Appl. Environ. Microbiol. 69:6316-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neal, K. R., H. M. Scott, R. C. Slack, and R. F. Logan. 1996. Omeprazole as a risk factor for campylobacter gastroenteritis: case-control study. BMJ 312:414-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Newell, D. G. 2001. Animal models of Campylobacter jejuni colonization and disease and the lessons to be learned from similar Helicobacter pylori models. Symp. Ser. Soc. Appl. Microbiol. 2001:57S-67S. [DOI] [PubMed] [Google Scholar]
- 123.Newell, D. G., H. McBride, F. Saunders, Y. Dehele, and A. D. Pearson. 1985. The virulence of clinical and environmental isolates of Campylobacter jejuni. J. Hyg. (London) 94:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newell, D. G., and I. Nachamkin. 1992. Immune responses directed against Campylobacter jejuni, p. 201-206. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. ASM Press, Washington, DC.
- 125.Newell, D. G., and J. A. Wagenaar. 2000. Poultry infections and their control at farm level, p. 497-509. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 126.Nolan, C. M., K. E. Johnson, M. B. Coyle, and K. Faler. 1983. Campylobacter jejuni enteritis: efficacy of antimicrobial and antimotility drugs. Am. J. Gastroenterol. 78:621-626. [PubMed] [Google Scholar]
- 127.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-154. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 128.Ottenhoff, T. H., F. A. Verreck, E. G. Lichtenauer-Kaligis, M. A. Hoeve, O. Sanal, and J. T. van Dissel. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat. Genet. 32:97-105. [DOI] [PubMed] [Google Scholar]
- 129.Panigrahi, P., G. Losonsky, L. J. DeTolla, and J. G. Morris, Jr. 1992. Human immune response to Campylobacter jejuni proteins expressed in vivo. Infect. Immun. 60:4938-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 131.Pazzaglia, G., A. L. Bourgeois, K. el Diwany, N. Nour, N. Badran, and R. Hablas. 1991. Campylobacter diarrhoea and an association of recent disease with asymptomatic shedding in Egyptian children. Epidemiol. Infect. 106:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pei, Z. H., R. T. Ellison III, and M. J. Blaser. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 266:16363-16369. [PubMed] [Google Scholar]
- 133.Pennie, R. A., R. D. Pearson, L. J. Barrett, H. Lior, and R. L. Guerrant. 1986. Susceptibility of Campylobacter jejuni to strain-specific bactericidal activity in sera of infected patients. Infect. Immun. 52:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perlman, D. M., N. M. Ampel, R. B. Schifman, D. L. Cohn, C. M. Patton, M. L. Aguirre, W. L. Wang, and M. J. Blaser. 1988. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV). Ann. Intern. Med. 108:540-546. [DOI] [PubMed] [Google Scholar]
- 135.Prendergast, M. M., D. R. Tribble, S. Baqar, D. A. Scott, J. A. Ferris, R. I. Walker, and A. P. Moran. 2004. In vivo phase variation and serologic response to lipooligosaccharide of Campylobacter jejuni in experimental human infection. Infect. Immun. 72:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pritchard, J., R. A. Hughes, J. H. Rees, H. J. Willison, and J. A. Nicoll. 2003. Apolipoprotein E genotypes and clinical outcome in Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatry 74:971-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prokhorova, T. A., P. N. Nielsen, J. Petersen, T. Kofoed, J. S. Crawford, C. Morsczeck, A. Boysen, and P. Schrotz-King. 2006. Novel surface polypeptides of Campylobacter jejuni as traveller's diarrhoea vaccine candidates discovered by proteomics. Vaccine 24:6446-6455. [DOI] [PubMed] [Google Scholar]
- 138.Rees, J. H., S. E. Soudain, N. A. Gregson, and R. A. Hughes. 1995. Campylobacter jejuni infection and Guillain-Barre syndrome. N. Engl. J. Med. 333:1374-1379. [DOI] [PubMed] [Google Scholar]
- 139.Richardson, G., D. R. Thomas, R. M. Smith, L. Nehaul, C. D. Ribeiro, A. G. Brown, and R. L. Salmon. 2007. A community outbreak of Campylobacter jejuni infection from a chlorinated public water supply. Epidemiol. Infect. 135:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robinson, D. A. 1981. Infective dose of Campylobacter jejuni in milk. BMJ (Clin. Res. Ed.) 282:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rollwagen, F. M., N. D. Pacheco, J. D. Clements, O. Pavlovskis, D. M. Rollins, and R. I. Walker. 1993. Killed Campylobacter elicits immune response and protection when administered with an oral adjuvant. Vaccine 11:1316-1320. [DOI] [PubMed] [Google Scholar]
- 142.Ruiz-Palacios, G. M., J. J. Calva, L. K. Pickering, Y. Lopez-Vidal, P. Volkow, H. Pezzarossi, and M. S. West. 1990. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J. Pediatr. 116:707-713. [DOI] [PubMed] [Google Scholar]
- 143.Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112-14120. [DOI] [PubMed] [Google Scholar]
- 144.Ruiz-Palacios, G. M., E. Escamilla, and N. Torres. 1981. Experimental Campylobacter diarrhea in chickens. Infect. Immun. 34:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Russell, R. G., M. J. Blaser, J. I. Sarmiento, and J. Fox. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sahin, O., N. Luo, S. Huang, and Q. Zhang. 2003. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol. 69:5372-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salazar-Lindo, E., R. B. Sack, E. Chea-Woo, B. A. Kay, Z. A. Piscoya, R. Leon-Barua, and A. Yi. 1986. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J. Pediatr. 109:355-360. [DOI] [PubMed] [Google Scholar]
- 148.Samuel, M. C., D. J. Vugia, S. Shallow, R. Marcus, S. Segler, T. McGivern, H. Kassenborg, K. Reilly, M. Kennedy, F. Angulo, and R. V. Tauxe. 2004. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999. Clin. Infect. Dis. 38(Suppl. 3):S165-S174. [DOI] [PubMed] [Google Scholar]
- 149.Schiellerup, P., K. A. Krogfelt, and H. Locht. 2008. A comparison of self-reported joint symptoms following infection with different enteric pathogens: effect of HLA-B27. J. Rheumatol. 35:480-487. [PubMed] [Google Scholar]
- 150.Sizemore, D. R., B. Warner, J. Lawrence, A. Jones, and K. P. Killeen. 2006. Live, attenuated Salmonella typhimurium vectoring Campylobacter antigens. Vaccine 24:3793-3803. [DOI] [PubMed] [Google Scholar]
- 151.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 152.Skirrow, M. B., D. M. Jones, E. Sutcliffe, and J. Benjamin. 1993. Campylobacter bacteraemia in England and Wales, 1981-91. Epidemiol. Infect. 110:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith, A., M. Reacher, W. Smerdon, G. K. Adak, G. Nichols, and R. M. Chalmers. 2006. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992-2003. Epidemiol. Infect. 134:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith, C. K., P. Kaiser, L. Rothwell, T. Humphrey, P. A. Barrow, and M. A. Jones. 2005. Campylobacter jejuni-induced cytokine responses in avian cells. Infect. Immun. 73:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sorvillo, F. J., L. E. Lieb, and S. H. Waterman. 1991. Incidence of campylobacteriosis among patients with AIDS in Los Angeles County. J. Acquir. Immune Defic. Syndr. 4:598-602. [PubMed] [Google Scholar]
- 156.Spiller, R. C. 2007. Role of infection in irritable bowel syndrome. J. Gastroenterol. 42(Suppl. 17):41-47. [DOI] [PubMed] [Google Scholar]
- 157.Strid, M. A., J. Engberg, L. B. Larsen, K. Begtrup, K. Molbak, and K. A. Krogfelt. 2001. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin. Diagn. Lab. Immunol. 8:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor, D. N., P. Echeverria, C. Pitarangsi, J. Seriwatana, L. Bodhidatta, and M. J. Blaser. 1988. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. J. Clin. Microbiol. 26:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Teunis, P., W. Van den Brandhof, M. Nauta, J. Wagenaar, K. H. Van den, and W. Van Pelt.2005. A reconsideration of the Campylobacter dose-response relation. Epidemiol. Infect. 133:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Teunis, P. F., and A. H. Havelaar. 2000. The beta Poisson dose-response model is not a single-hit model. Risk Anal. 20:513-520. [DOI] [PubMed] [Google Scholar]
- 161.Thompson, J. S., F. E. Cahoon, and D. S. Hodge. 1986. Rate of Campylobacter spp. isolation in three regions of Ontario, Canada, from 1978 to 1985. J. Clin. Microbiol. 24:876-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thornley, J. P., D. Jenkins, K. Neal, T. Wright, J. Brough, and R. C. Spiller. 2001. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J. Infect. Dis. 184:606-609. [DOI] [PubMed] [Google Scholar]
- 163.Tsukada, S., D. C. Saffran, D. J. Rawlings, O. Parolini, R. C. Allen, I. Klisak, R. S. Sparkes, H. Kubagawa, T. Mohandas, and S. Quan. 1993. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72:279-290. [DOI] [PubMed] [Google Scholar]
- 164.van de Putte, L. B., J. H. Berden, M. T. Boerbooms, W. H. Muller, J. J. Rasker, A. Reynvaan-Groendijk, and S. M. van der Linden. 1980. Reactive arthritis after Campylobacter jejuni enteritis. J. Rheumatol. 7:531-535. [PubMed] [Google Scholar]
- 165.van der Pol, W. L., L. H. Van den Berg, R. H. Scheepers, J. G. van der Bom, P. A. van Doorn, R. van Koningsveld, M. C. van den Broek, J. H. Wokke, and J. G. van de Winkel. 2000. IgG receptor IIa alleles determine susceptibility and severity of Guillain-Barre syndrome. Neurology 54:1661-1665. [DOI] [PubMed] [Google Scholar]
- 166.Van Pelt, W., M. A. de Wit, W. J. Wannet, E. J. Ligtvoet, M. A. Widdowson, and Y. T. van Duynhoven. 2003. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991-2001. Epidemiol. Infect. 130:431-441. [PMC free article] [PubMed] [Google Scholar]
- 167.Van Pelt, W., W. J. Wannet, A. W. van de Giessen, D. J. Mevius, and Y. T. van Duynhoven. 2004. Trends in gastro-enteritis van 1996-2003. Infect. Bull. 9:335-341. [Google Scholar]
- 168.Van Rhijn, I., L. H. Van den Berg, C. W. Ang, J. Admiraal, and T. Logtenberg. 2003. Expansion of human gammadelta T cells after in vitro stimulation with Campylobacter jejuni. Int. Immunol. 15:373-382. [DOI] [PubMed] [Google Scholar]
- 169.van Sorge, N. M., W. L. van der Pol, M. D. Jansen, K. P. Geleijns, S. Kalmijn, R. A. Hughes, J. H. Rees, J. Pritchard, C. A. Vedeler, K. M. Myhr, C. Shaw, I. N. van Schaik, J. H. Wokke, P. A. van Doorn, B. C. Jacobs, J. G. van de Winkel, and L. H. Van den Berg. 2005. Severity of Guillain-Barre syndrome is associated with Fc gamma receptor III polymorphisms. J. Neuroimmunol. 162:157-164. [DOI] [PubMed] [Google Scholar]
- 170.Vedeler, C. A., G. Raknes, K. M. Myhr, and H. Nyland. 2000. IgG Fc-receptor polymorphisms in Guillain-Barre syndrome. Neurology 55:705-707. [DOI] [PubMed] [Google Scholar]
- 171.Vellinga, A., and F. Van Loock. 2002. The dioxin crisis as experiment to determine poultry-related campylobacter enteritis. Emerg. Infect. Dis. 8:19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vetrie, D., I. Vorechovsky, P. Sideras, J. Holland, A. Davies, F. Flinter, L. Hammarstrom, C. Kinnon, R. Levinsky, and M. Bobrow. 1993. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361:226-233. [DOI] [PubMed] [Google Scholar]
- 173.Wassenaar, T. M. 1997. Toxin production by Campylobacter spp. Clin. Microbiol. Rev. 10:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wassenaar, T. M., M. Kist, and A. de Jong. 2007. Re-analysis of the risks attributed to ciprofloxacin-resistant Campylobacter jejuni infections. Int. J. Antimicrob. Agents 30:195-201. [DOI] [PubMed] [Google Scholar]
- 175.Watson, R. O., and J. E. Galan. 2005. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell. Microbiol. 7:655-665. [DOI] [PubMed] [Google Scholar]
- 176.Watson, R. O., and J. E. Galan. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 4:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Watson, R. O., V. Novik, D. Hofreuter, M. Lara-Tejero, and J. E. Galan. 2007. A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect. Immun. 75:1994-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weber, P., M. Koch, W. R. Heizmann, M. Scheurlen, H. Jenss, and F. Hartmann. 1992. Microbic superinfection in relapse of inflammatory bowel disease. J. Clin. Gastroenterol. 14:302-308. [DOI] [PubMed] [Google Scholar]
- 179.Wheeler, J. G., D. Sethi, J. M. Cowden, P. G. Wall, L. C. Rodrigues, D. S. Tompkins, M. J. Hudson, P. J. Roderick, et al. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Williams, M. D., J. B. Schorling, L. J. Barrett, S. M. Dudley, I. Orgel, W. C. Koch, D. S. Shields, S. M. Thorson, J. A. Lohr, and R. L. Guerrant. 1989. Early treatment of Campylobacter jejuni enteritis. Antimicrob. Agents Chemother. 33:248-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Willison, H. J. 2005. The immunobiology of Guillain-Barre syndromes. J. Peripher. Nerv. Syst. 10:94-112. [DOI] [PubMed] [Google Scholar]
- 182.Willison, H. J., and G. M. O'Hanlon. 2000. Anti-glycosphingolipid antibodies and Guillain-Barre syndrome, p. 241-258. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 183.World Health Organization. 2001. The increasing incidence of human campylobacteriosis, p. 19-25. World Health Organization, Geneva, Switzerland.
- 184.Young, K. T., L. M. Davis, and V. J. Dirita. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665-679. [DOI] [PubMed] [Google Scholar]
- 185.Young, V. B., D. B. Schauer, and J. G. Fox. 2000. Animal models of Campylobacter infection, p. 287-302. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 186.Yrios, J. W., and E. Balish. 1986. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect. Immun. 53:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuki, N. 1997. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barre syndrome and Miller Fisher syndrome. J. Infect. Dis. 176(Suppl. 2):S150-S153. [DOI] [PubMed] [Google Scholar]
- 188.Yuki, N., M. Takahashi, Y. Tagawa, K. Kashiwase, K. Tadokoro, and K. Saito. 1997. Association of Campylobacter jejuni serotype with antiganglioside antibody in Guillain-Barre syndrome and Fisher's syndrome. Ann. Neurol. 42:28-33. [DOI] [PubMed] [Google Scholar]
- 189.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and T. Miyatake. 1993. A bacterium lipopolysaccharide that elicits Guillain-Barre syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zilbauer, M., N. Dorrell, A. Elmi, K. J. Lindley, S. Schuller, H. E. Jones, N. J. Klein, G. Nunez, B. W. Wren, and M. Bajaj-Elliott. 2007. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell. Microbiol. 9:2404-2416. [DOI] [PubMed] [Google Scholar]