Abstract
The risk of malaria for travelers varies from region to region and depends on the intensity of transmission, the duration of the stay in the area of endemicity, the style of travel, and the efficacy of preventive measures. The decision to recommend chemoprophylaxis to travelers to areas with a low risk of malarial infection is especially difficult because the risk of infection must be balanced with the risk of experiencing side effects. If the risk of side effects by far exceeds the risk of infection, the traveler needs information on measures against mosquito bites and advice on prompt diagnosis and self-treatment. The risk is difficult to quantify, and the absolute risk for travelers to most areas is not known, especially because the populations at risk are unknown. We propose here that the best approximation of the risk to the traveler to a specific area is to use the risk to the indigenous population as a guideline for the risk to the traveler, and we provide examples on how risk in the indigenous population can be used for the estimation of risk of malarial infection for travelers. Special groups are long-term visitors and residents, who often perceive risk differently, cease using chemoprophylaxis, and rely on self-diagnosis and treatment. For long-term visitors, the problem of fake drugs needs to be discussed. Strategies for chemoprophylaxis and self-treatment of pregnant women and small children are discussed. So far, malaria prophylaxis is recommended to prevent Plasmodium falciparum infections, and primaquine prophylaxis against persistent Plasmodium vivax and Plasmodium ovale infections in travelers is not recommended.
INTRODUCTION
The prevention of malaria in nonimmune persons visiting areas where malaria is endemic is difficult due to a lack of data on the risk of infection for the traveler, disagreement between professionals and national guidelines on optimum strategies, and the risk of side effects from prophylaxis.
An estimated 80 to 90 million travelers visit areas where malaria is endemic annually. Not all travelers have a similar risk. The risk of acquiring malaria depends on many factors including the type and intensity of malaria transmission at the destination, the duration and style of travel, the prevention measures used, and individual characteristics (3, 7, 28). Malaria prevention advice should be evidence based, using sound epidemiological data when available. Travelers to areas where malaria is endemic need (i) information on the disease, its mode of transmission, incubation period, and symptoms; (ii) advice on measures against mosquito bites; (iii) chemoprophylaxis for high-risk areas such as sub-Saharan Africa; and (iv) advice regarding prompt diagnosis and self-treatment of malaria if appropriate.
The decision to use chemoprophylaxis depends on a risk-benefit analysis weighing the risk of malaria against the risk of possible adverse drug reactions. The risk of infection for travelers is difficult to quantify, and new at-risk areas may emerge (16). Even though malaria imported to countries where malaria is not endemic is a notifiable disease, the population at risk, i.e., the exact number of travelers to a specific destination, is often impossible to ascertain. It can be useful, therefore, to use data on malaria endemicity in the indigenous population and then extrapolate the risk to travelers (31). Several studies quantified the rate of adverse events in short-term travelers using malaria chemoprophylaxis, but the methodologies used are rarely comparable so that incidence rates among studies can differ enormously. Data on the long-term use and tolerability of drugs for chemoprophylaxis are lacking. There is also a lack of data on the safety and efficacy of strategies for other at-risk groups such as pregnant/lactating women and small children.
In this paper, we attempt to identify traveler types who are particularly at risk of malaria and to suggest evidence-based strategies for the various risk groups.
RISK OF MALARIAL INFECTION
One of the most difficult questions in malaria prophylaxis today is how to advise travelers who visit low-risk areas, which are often areas with unstable transmission and a changing malaria epidemiology. Few studies reported the absolute risk in specific areas, but these data are necessary to allow a rational decision on whether to recommend chemoprophylaxis or not balanced against the risk of side effects. Travelers should not be exposed to a substantial risk of adverse events from malaria chemoprophylaxis in areas where the risk of malarial infection is very low.
Can we quantify the risk of contracting malaria and weigh this against the risk of adverse events from chemoprophylaxis?
A recent study from Sweden found the risk of malaria to travelers to be 302 per 100,000 visitors to western Africa, 46 per 100,000 visitors to South Africa, 7.2 per 100,000 visitors to South America, and 2 per 100,000 visitors to Thailand (2). Studies from travelers returning to the United Kingdom two decades ago found that 1 in 77 or 1,300 per 100,000 persons visiting friends and relatives in Ghana contracted malaria, falling to 1 per 100,000 travelers to South America (25). A study of malaria in travelers returning to Denmark found that 158 per 100,000 travelers visiting The Gambia, 76 per 100,000 travelers visiting Indonesia, and 1.7 per 100,000 travelers visiting Thailand contracted malaria (17). A declining risk of malaria in travelers to South America has also been reported (4).
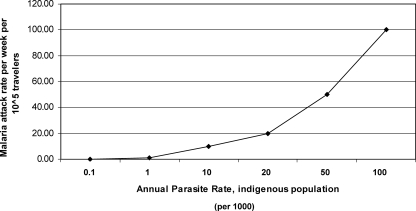
Table 1 shows the risk of malaria for short-term travelers at different levels of endemicity in the indigenous population and the rate of mortality for travelers, assuming a case fatality rate of 2%. The relationship between endemicity measured by the annual parasite rate in the indigenous population and the estimated attack rates in nonimmune travelers is shown in Fig. 1. We assume that the risk of malaria in travelers and that in the indigenous population are the same, which may not always be true. The risk in the indigenous population should therefore be seen as a maximal-risk situation, with a lower risk for travelers staying in houses with air conditioning. An endemicity level of 20 cases per 1,000 indigenous people would result in an estimated 1 death per 100,000 travelers per year without chemoprophylaxis. This would increase to 8 deaths per 100,000 travelers at an endemicity level of 100 cases per 1,000 indigenous persons. It should be noted that the rate of 8 deaths per 100,000 persons per year is very close to the annual risk of being killed in traffic in Europe, which is approximately 1 death per 11,500 inhabitants per year (http://epp.eurostat.ec.europa.eu/).
TABLE 1.
Risk of malaria in short-term travelers at different levels of endemicity in the indigenous population and mortality from malaria in short-term travelersa
| Annual incidence of malaria cases in local population | Example of area of endemicityb | Incidence per wk per 100,000 travelers | Incidence per 2 wk per 100,000 travelers without prophylaxis | Incidence per 2 wk per 100,000 travelers with prophylaxis assuming 90% efficacy of prophylaxis | Mortality per 100,000 travelers per 2 wk without prophylaxis | Mortality per 100,000 travelers per 2 wk with prophylaxis |
|---|---|---|---|---|---|---|
| 1 per 1,000 | Mexico, parts of South America, Vietnam (except Binh Province) | 1.9 | 3.8 | 0.4 | ||
| 10 per 1,000 | Parts of Vietnam (Binh Phuoc province) | 19.2 | 38.5 | 3.8 | ||
| 20 per 1,000 | Parts of India (Assam, Gujarat, Orissa, Rajastan) | 38.4 | 76.8 | 7.7 | 1 | |
| 50 per 1,000 | Parts of South Africa | 96.1 | 192.3 | 19.2 | 4 | |
| 100 per 1,000 | Western Africa | 192.3 | 384.6 | 38.5 | 8 | 0.5 |
Assuming a case fatality rate of 2%. Short-term travel is considered to be travel in a region of endemicity lasting 2 weeks or less.
The areas mentioned serve only as examples of areas with different levels of endemicity in the indigenous population. Risk in southeastern Asia is very unevenly distributed within each country and should be assessed at district levels based on the travelers' planned route and using malaria maps (30).
FIG. 1.
Spatial relationship between annual parasite rates in the indigenous population and estimated attack rates in nonimmune travelers visiting the same area. It is assumed that the annual parasite rate reflects the risk of malaria infection and that each individual is infected only once during the 12-month period, and it is assumed that an infection in a nonimmune traveler is always symptomatic.
RISK OF ADVERSE EVENTS FROM MALARIA CHEMOPROPHYLAXIS
The risk of being infected with malaria should be balanced with the risk of adverse events from malaria chemoprophylaxis. Adverse events can be divided into common events, usually mild and affecting high percentages of users, and rare events, which are seen much less frequently and which are often recognized only after millions of patients have used the drug. Rare events are usually not discovered in phase III trials prior to licensing of a drug and rely on postmarketing surveillance after licensing, which makes the true incidence very uncertain, as unreported events are likely.
There is only one double-blind, randomized controlled trial that compared all current malaria-prophylactic regimens and associated adverse events in 623 travelers randomized to atovaquone-proguanil (Malarone), mefloquine (Mephaquin or Lariam), doxycycline, and chloroquine plus proguanil (as Savarine) (30). Forty-five percent of chloroquine and proguanil users, 42% of mefloquine users, 33% of doxycycline users, and 32% of atovaquone-proguanil users reported mild to moderate adverse events. Severe adverse events (that interfered with daily activity) were reported for 11% of mefloquine users, 12% of chloroquine plus proguanil users, 6% of doxycycline users, and 7% of atovaquone and proguanil users. It should be emphasized that an adverse event is not necessarily attributable to the antimalarial drug but reflects all intercurrent events experienced during the use of the drug. A recent detailed review (9) showed that despite widespread reports on the adverse effects of mefloquine, controlled studies (involving >5,000 subjects) found a low incidence of serious adverse events. Studies of minor adverse events have, however, highlighted the neuropsychiatric/neuropsychological profile of this antimalarial drug, showing an excess of such events in women.
PROPHYLAXIS FOR SHORT-TERM VISITORS
We will not discuss the proper selection of drugs or dosage for chemoprophylaxis for a given area here, as this information was reported elsewhere (6, 31, 40).
Short-term travelers are persons visiting an area where malaria is endemic for weeks or a few months. This group includes most tourists, and the main reason for travel is business and leisure. The travelers usually have little prior knowledge of disease, including malaria risk at their destination. Because of the incubation period of malaria, many short-term travelers who acquire the disease will be diagnosed after return. The actual profile of imported species varies among countries, reflecting the primarily geographic origin of the infection. Malaria deaths are due almost exclusively to infection with Plasmodium falciparum (1).
At present, the key problem for travel health practitioners is when to advise the use of chemoprophylaxis for travelers to low-risk areas of endemicity. Based on the estimates of risk for malaria and adverse events, we propose that the prescription of chemoprophylaxis in areas with an endemicity level in the indigenous population below 10 cases per 1,000 population per year is not justified (Table 1). To prevent one case at this level of transmission, 2,600 travelers should be prescribed chemoprophylaxis, and to prevent one fatality, assuming a 2% case fatality rate, 130,000 travelers should be prescribed chemoprophylaxis (Table 2). For comparison, the proper use of impregnated bed nets can reduce the risk of infection by 50% without causing adverse events in users (1). However, it should be noted that clinical malaria cases in the local population may reflect a lower attack rate than that for visitors if there is substantial clinical immunity in the local population. Therefore, the level of endemicity should be determined by the parasite rate and not the rate of reported clinical malaria cases in the indigenous population.
TABLE 2.
Numbers of travelers needed to take chemoprophylaxis to prevent one case of malaria and to prevent one fatal case of malaria at different levels of endemicity
| Annual incidence of cases of malaria in local population | No. of travelers needed
|
|||
|---|---|---|---|---|
| Taking prophylaxis to prevent 1 casea | Taking prophylaxis to prevent 1 deatha,b | With severe side effects to prevent 1 malaria casec | With severe side effects to prevent 1 deathc | |
| 1 per 1,000 | 26,000 | 1,300,000 | 2,600 | 130,000 |
| 10 per 1,000 | 2,600 | 130,000 | 260 | 13,000 |
| 20 per 1,000 | 1,300 | 65,000 | 130 | 6,500 |
| 50 per 1,000 | 520 | 26,000 | 52 | 2,600 |
| 100 per 1,000 | 260 | 13,000 | 26 | 1,300 |
Assuming 2 weeks of travel.
Assuming 2% mortality.
Severe side effects in 10% of cases.
Standby emergency treatment (SBET), where the traveler is prepared to self-treat a suspected case of malaria if he or she has symptoms suggestive of malaria and is out of reach of medical attention, is recommended by many authorities as a strategy for short-term travelers to low-risk destinations (6, 24, 40). However, SBET is not indicated for travelers on trips shorter than the incubation period for malaria, which is a minimum of 6 days for P. falciparum malaria.
PROPHYLAXIS FOR LONG-TERM VISITORS AND FREQUENT VISITORS
The risk of malaria cumulates over time, but the increased risk cannot be attributed to longer exposure alone (25). Long-term travelers, defined as persons staying permanently in an area for 6 months or longer, and frequent travelers to regions of endemicity behave differently from short-term visitors. Long-term travelers frequently discontinue chemoprophylaxis prematurely because they perceive the risk to be lower than expected. They also believe that they can effectively manage an infection, and they worry about side effects from the long-term use of malaria chemoprophylaxis (8, 37). The use of counterfeit drugs (fake drugs containing no or subtherapeutic doses of active compounds) is particularly an issue for long-term travelers, who often buy supplies of dubious quality, and expatriates should be encouraged to bring adequate quantities of antimalarial medications with them (37). The consequences for travelers using counterfeit drugs are far-reaching: high levels of mortality due to untreated P. falciparum infection, subtherapeutic dosages leading to inadequate prophylactic doses, a risk of increased adverse events due to excessive dosage or potentially toxic contaminants, and, finally, a loss of faith in genuine medicines.
The health personnel advising long-term travelers should understand these aspects and accept that these are important issues that must be addressed.
The key to managing malaria prevention in long-term and frequent travelers is to provide the travelers with a knowledge and understanding of malaria so that they can take more responsibility for their own health compared to the short-term traveler.
Long-term travelers to high-risk areas should take malaria chemoprophylaxis, even if this is necessary over several years. Mefloquine is the best-documented drug for long-term travelers and, if well tolerated, can be used for prolonged periods; i.e., there is no upper time limits for the use of mefloquine (34). It has a simple weekly dosing schedule that encourages adherence (39), and toxic accumulation in the body does not occur during long-term use (14). Doxycycline (a tetracycline compound) is used for the treatment of skin infections for months, but side effects, especially vaginal candidiasis in women, is a problem for long-term users. A third option is the use of atovaquone-proguanil (13). The CDC has no upper limit on the duration of intake of atovaquone-proguanil (6), and recent United Kingdom guidelines consider atovaquone-proguanil to be safe for continuous use of up to 12 months (36).
This means that long-term travelers must have knowledge about malaria as a disease, including key symptoms such as fever, the adequate use of SBET, the problems of counterfeit drugs, and the need to identify reliable health care facilities in case of emergency.
Mosquito bite prophylaxis is even more relevant for the long-term traveler, and comprehensive information about screening, impregnated bed nets, coils, repellents, insecticides, and the biting habits of mosquitoes is required. The systemic use of impregnated bed nets can reduce the risk of malaria by 50% (19). In selected cases, the long-term traveler may be trained to use rapid malaria diagnostic kits, but this option needs to be restricted to persons staying in isolated places, and thorough pretravel instruction is essential (31).
Health personnel advising travelers must recognize that this group is unlikely to take chemoprophylaxis continuously for years, and it will increase our credibility if travel health advisors acknowledge this and try to realistically prepare this group for their long-term travel.
Suggestions for advice to long-term travelers and frequent visitors are as follows: (i) inform travelers about disease, symptoms, diagnosis, and need for rapid treatment; (ii) discuss access to qualified medical assistance at destination and the need to identify qualified medical staff before the traveler becomes ill; (iii) inform travelers about alternatives to continuous chemoprophylaxis; (iv) provide detailed advice on methods to prevent mosquito bites; (v) inform travelers about the use of SBET and emphasize the need for medical assessment despite the use of SBET; and (vi) inform travelers about the problems with counterfeit drugs in many countries where malaria is endemic and that drugs for both chemoprophylaxis and SBET should be purchased before arriving at the destination. Rapid diagnostic tests can be recommended for selected travelers but require comprehensive pretravel instruction. The drug of choice for SBET depends on the use of prophylaxis, if any, the expected drug susceptibility pattern at the destination, and the guidelines of the traveler's country of origin, and as a rule, the drug used for treatment should not be the same as that used for prophylaxis. Malaria breakthrough while compliant and on prophylaxis could be due to resistance of the P. falciparum parasite to the chemoprophylactic drug, which is why the drug used for prophylaxis should not be reused for treatment.
PROPHYLAXIS AGAINST PLASMODIUM VIVAX AND PLASMODIUM OVALE
The main goal of malaria chemoprophylaxis is to prevent P. falciparum infection, which is primarily responsible for malaria fatalities. However, both Plasmodium vivax and Plasmodium ovale can cause serious febrile illness in nonimmune patients. The clinical presentation of P. vivax and P. ovale malaria cannot be distinguished from that of P. falciparum malaria (5). The burden of infection is considerable in indigenous populations, and P. vivax is the most prevalent malaria type in southeast Asia and South America and is found in eastern Africa (21). P. vivax is susceptible to most antimalarial drugs, although isolates with reduced sensitivity to chloroquine and primaquine have been found in parts of Indonesia and in Papua New Guinea, Iraq, and Afghanistan (3, 33, 35). There is a decreased susceptibility of P. vivax to sulfadoxine-pyrimethamine in Thailand (26).
A study from Europe of 518 imported cases of P. vivax found that 60% of patients were admitted to the hospital on average 4 days after the start of symptoms, and seven patients had severe complications (hepatosplenomegaly [three patients], spleen rupture [one patient], pancytopenia [one patient], macrohematuria [one patient], and psychosis [one patient]) (23).
P. vivax and P. ovale develop hypnozoites when the host is infected, which may relapse later and cause malaria symptoms long after the traveler has returned home. Hypnozoites are susceptible only to primaquine. One study found that the first P. vivax attack was seen approximately 3 months after leaving the area where malaria is endemic regardless of whether the traveler had taken prophylaxis or not (12). The prevention of relapsing P. vivax infection can be achieved only by presumptive posttravel treatment with a course of primaquine or by using primaquine as a chemoprophylaxis during travel. So far, neither of these options has been extensively used, and primaquine is not registered as primary prophylaxis in most countries. Terminating a stay in an area where P. vivax is endemic with a 2-week course of primaquine without knowing whether the traveler is infected or not is not attractive for practitioners and patients, as primaquine has some side effects, causing primarily methemoglobinemia. The use of primaquine requires that the individuals be tested for glucose-6-phosphate deficiency (G6PD). The recommended adult dose for “antirelapse treatment” based on clinical trials and expert opinion is 30 mg base daily for 14 days, starting upon return from a region where malaria is endemic and taken together with a blood schizonticide based on evidence from the 1950s showing that primaquine's activity against hypnozoites is enhanced when given with chloroquine. The adult dose for primary prophylaxis is 30 mg daily starting 1 day before travel and continuing for 7 days after return (15).
One study used primaquine as prophylaxis alone in an area with a high risk of P. falciparum infections and found breakthroughs in four users (32). Primaquine has only limited activity against P. falciparum blood-stage activity, and if parasites emerge from the liver into the blood, primaquine alone will be insufficient as chemoprophylaxis.
The use of primaquine as the drug of choice for travelers to areas where P. vivax is endemic raises the concern of inducing primaquine resistance, which so far has been found only in Irian Jaya, Papua New Guinea, along with a single report from Iraq and Afghanistan (35).
As long as the absolute risk of infection by P. vivax and P. ovale is not known, the best strategy is still to inform travelers of the risk of a late-onset attack of P. vivax or P. ovale after return and cessation of chemoprophylaxis.
MALARIA PREVENTION FOR PREGNANT AND BREASTFEEDING WOMEN
A significant proportion of travelers are women with childbearing potential who need evidence-based advice on the use of antimalarials in the periconception period, during pregnancy, and when breastfeeding. Malaria during pregnancy is hazardous for the mother, the fetus, and the neonate and is an important cause of maternal and child morbidity and mortality (11). Plasmodium falciparum is responsible for the main burden of malarial disease in pregnant women (20). Other malaria species do not parasitize placental blood to the same extent and hence have less impact (11). The clinical features of P. falciparum malaria in pregnancy depend to a large extent on the immune status of the woman, which in turn is determined by her prior exposure to malaria. Nonimmune travelers have little or no immunity and are prone to episodes of severe malaria, leading to stillbirths, spontaneous abortions, or even maternal death. Mosquito bite protection is essential, and research shows that N,N-diethyl-3-methylbenzamide is effective and safe and has a low risk of accumulation in the fetus (20). Pyrethroid insecticide-treated nets are safe and have been shown to substantially reduce the risk of placental malaria (18, 22). Bed nets are particularly indicated in rooms with no air conditioning.
Malaria chemoprophylaxis in pregnancy is complex. Due to ethical and safety restrictions, few antimalarial drugs have been evaluated for pregnant travelers, and there is also a dearth of information on drug disposition in pregnant woman (Table 3). Chloroquine can be used, but widespread resistance limits this option. Doxycycline and primaquine are contraindicated. Due to insufficient data, atovaquone-proguanil is not recommended, although proguanil is considered to be safe during pregnancy, and no teratogenicity has been observed in animal studies using atovaquone. Mefloquine is an option for pregnant women who cannot defer travel and who need chemoprophylaxis for areas where chloroquine-resistant malaria is endemic. Some authorities now allow the use of mefloquine in all trimesters, and others advise against using the drug in the first trimester apart from exceptional circumstances. A recent trial of chloroquine prophylaxis for P. vivax malaria in pregnant women in Thailand found no effect on maternal anemia or birth weight (38). However, in areas with predominantly P. vivax malaria, infection in pregnancy contributes to maternal morbidity and mortality (27).
TABLE 3.
Antimalarials for chemoprophylaxis and SBET in pregnancy
| Antimalarial | Recommendation for chemoprophylaxis in pregnancy | Recommendation for emergency self-treatment in pregnancy | Description |
|---|---|---|---|
| Atovaquone-proguanil | No data; should not be used | No data; should be used only if no other options are available | Not recommended due to lack of safety data; inadvertent use in pregnancy probably safe, but few data are available |
| Chloroquine (hydroxychloroquine) | Can be used | Can be used | Regarded as safe |
| Proguanil | Can be used | Not used for treatment | Supplement with folic acid is recommended |
| Doxycycline | Contraindicated | Contraindicated | May cause bone malformation and discolored teeth |
| Mefloquine | Can be used after the first trimester; some authorities (WHO and CDC) allow the use of mefloquine in the first trimester if the risk of malaria is high and travel cannot be deferred | Can be used after 16th gestational wk or if no other options are available | Regarded as safe after 16th gestational wk based on postmarketing surveillance; inadvertent use periconception or during pregnancy is not considered an indication for termination |
| Artemisinins | Not used for prophylaxis | Few data; can be used only if no other options are available | One small study found no adverse impact on the pregnant mother or the fetusa |
| Quinine | Not used for prophylaxis | Can be used | Drug of choice for P. falciparum malaria; combination with clindamycin is recommended |
| Primaquine | Contraindicated | Treatment of P. vivax hypnozoites should be deferred until after pregnancy | Use of primaquine in pregnancy would necessitate G6PD testing for mother and fetus |
See reference 10.
With regard to breastfeeding, chloroquine, hydroxychloroquine, and mefloquine are considered to be compatible with breastfeeding, and atovaquone-proguanil can be used if the breastfed infant weighs more than 5 kg. Proguanil is excreted into human milk in small quantities, and in a rat study, atovaquone concentrations in milk were 30% of the concurrent atovaquone concentrations in maternal plasma. Infants who are breastfed do not receive adequate concentrations of any antimalarial drugs and require their own chemoprophylaxis (29).
MALARIA PREVENTION FOR SMALL CHILDREN
Imported malaria case numbers in children are increasing with the rise in travel among children and changing profiles of immigrants, particularly settled immigrants visiting friends and relatives in countries where malaria is endemic. The use of N,N-diethyl-3-methylbenzamide containing insect repellents is recommended for children older than 2 months of age, and an alternative repellent, picaridin, can be recommended for children older than 2 years of age. Chloroquine is safe for children of all ages and weights, but the use of this option is delimited by widespread resistance to the drug. Mefloquine can be used for children >5 kg and atovaquone-proguanil prophylaxis (as pediatric tablets) can be used for children >5 kg according to new CDC guidelines (6). The manufacturer, the World Health Organization (40), and some European authorities sanction the use of this combination only for children weighing more that 11 kg. Artemether-lumefantrine (Coartem), an artemisinin combination in dispersible tablet form with cherry flavor, will be available in 2008 for the treatment of infants (Novartis, Switzerland) but cannot be used for prophylaxis. Doxycycline is indicated for children aged >8 years (and allowed for children over 12 years of age in the United Kingdom). Table 4 shows the currently recommended doses for malaria prophylaxis in children.
TABLE 4.
Antimalarial chemoprophylaxis for children
| Antimalarial | Indication for chemoprophylaxis | Dosing | Description |
|---|---|---|---|
| Atovaquone-proguanil | >5 kg body wt per CDCa | Daily | Palatable |
| >11 kg body wt per manufacturer and some European countries | Pediatric tablets | Expensive | |
| Chloroquine (hydroxychloroquine) | All ages and weights | 5 mg base/kg weekly | Limited use due to resistance |
| Proguanil | All ages and weights | 3 mg/kg/day | Only in combination with chloroquine |
| Doxycycline | Children >8 yr old | 1.5 mg salt/kg daily | Contraindicated for small children |
| Mefloquine | Children >5 kg | 5 mg/kg weekly | Bitter taste |
| Primaquine | Children >4 yr old per WHO | 0.5 mg/kg base | G6PD testing essential |
| CDC specifies no lower age limit | Daily | Last choice |
New recommendations for 2007.
When possible, deferral of travel is recommended for pregnant and breastfeeding women and also for young children.
CONCLUSIONS
The risk of malarial infection can be based to some extent on the annual incidence data in the indigenous population. The risk of malaria infection should be balanced with the risk of severe and serious adverse events from the use of chemoprophylaxis (Table 5). We propose that travelers to areas with an annual incidence of malaria in the indigenous population of below 10 cases per 1,000 individuals should not be advised to use chemoprophylaxis but should rely on preventing mosquito bites alone and on the carriage of standby emergency self-treatment. Travelers need to be aware of the risks and time frames of late-onset malaria, particularly P. vivax malaria. Pregnant or breastfeeding mothers and travelers with small children require expert advice on malaria prevention and choice of drug and should defer travel to high-risk areas. Long-term travelers must take more responsibility and receive comprehensive advice on all aspects of malaria prevention and treatment. This group needs thorough pretravel advice with particular emphasis on the prevention of mosquito bites, seasonal prophylaxis, if appropriate, the use of and potential risks from long-term use of chemoprophylaxis, advice on SBET, and the possible use of rapid diagnostic tests. Recommendations on antimalaria strategies and choice of drug differ among countries because precise and up-to-date data are lacking for many areas and because there is a lack of international consensus.
TABLE 5.
Examples of travel itineraries, risk estimation, and suggested advice for adults and nonpregnant travelers
| Country | Travel itinerary | Risk estimation | Recommendation |
|---|---|---|---|
| Vietnam | 3 wk traveling from Hanoi to Ho Chi Minh City primarily along the coast | Very limited; the only area of Vietnam where the annual parasite rate exceeds 10 per 1,000 individuals per yr is in the central highlands bordering Laos | No chemoprophylaxis; use impregnated bed nets and repellants; carry SBET |
| South Africa | 3 days in Kruger National Park and 2 wk traveling in the rest of the country down to Cape Town | Risk in Kruger National Park but not the remaining part of the visit; chloroquine resistance reported | Chemoprophylaxis recommended in Kruger National Park, and doxycycline, mefloquine, or atovaquone-proguanil (most practical option for short-term use) can be used |
| Mexico | Visiting Yucatan including Palenque and San Cristobal de la Casas for 2 wk | Low-risk area | No chemoprophylaxis; use impregnated bed nets and repellants |
| Tanzania | Pregnant student who planned a 3-mo stay in rural Tanzania found out she was pregnant gestational wk 12 6 wk before leaving | High-risk area; chemoprophylaxis highly needed; chloroquine resistance widespread | Doxycycline is contraindicated in pregnancy, and data are lacking on the safety of atovaquone-proguanil; mefloquine is considered safe after gestational wk 16 |
| Ghana | 12-wk-old infant; Ghanean family living in Europe had their first child 3 mo ago and want to visit their family in Ghana living in the rural areas north of Kumasi | High-risk area; recommend deferral of travel; chemoprophylaxis and mosquito bite protection essential | Doxycycline contraindicated; mefloquine possible for infants weighing >5 kg; tablets can be cut and crushed; atovaquone-proguanil is recommended by some authorities for children >5 kg |
REFERENCES
- 1.Arguin, P., and P. Schlagenhauf. 2008. Malaria deaths in travelers, p. 323-331. In P. Schlagenhauf-Lawlor (ed.), Travelers' malaria, 2nd ed. BC Decker, Hamilton, Ontario, Canada.
- 2.Askling, H. H., J. Nilsson, A. Tegnell, R. Janzon, and K. Ekdahl. 2005. Malaria risk in travelers. Emerg. Infect. Dis. 11:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, R. H., B. Carroll, J. Beran, O. Bouchaud, U. Hellgren, C. Hatz, T. Jelinek, F. Legros, N. Mühlberger, B. Myrvang, H. Siikamäki, L. Visser, et al. 2007. The low and declining risk of malaria in travellers to Latin America: is there still an indication for chemoprophylaxis? Malar. J. 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottieau, E., J. Clerinx, E. Van Den Enden, M. Van Esbroeck, R. Colebunders, A. Van Gompel, and J. Van Den Ende. 2006. Imported non-Plasmodium falciparum malaria: a five-year prospective study in a European referral center. Am. J. Trop. Med. Hyg. 75:133-138. [PubMed] [Google Scholar]
- 6.CDC. 2007. Travellers health, yellow book 2007. CDC, Atlanta, GA. www.cdc.gov.
- 7.Chen, L. H., and J. S. Keystone. 2005. New strategies for the prevention of malaria in travelers. Infect. Dis. Clin. N. Am. 19:185-210. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L. H., M. E. Wilson, and P. Schlagenhauf. 2006. Prevention of malaria in long-term travelers. JAMA 296:2234-2244. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. H., M. E. Wilson, and P. Schlagenhauf. 2007. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA 297:2251-2263. [DOI] [PubMed] [Google Scholar]
- 10.Dellicour, S., S. Hall, D. Chandramohan, and B. Greenwood. 2007. The safety of artemisinins during pregnancy: a pressing question. Malar. J. 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai, M., F. O. ter Kuile, F. Nosten, R. McGready, K. Asamoa, B. Brabin, and R. D. Newman. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7:93-104. [DOI] [PubMed] [Google Scholar]
- 12.Elliott, J. H., D. O'Brien, K. Leder, S. Kitchener, E. Schwartz, L. Weld, G. V. Brown, K. C. Kain, J. Torresi, et al. 2004. Imported Plasmodium vivax malaria: demographic and clinical features in nonimmune travelers. J. Travel Med. 11:213-217. [DOI] [PubMed] [Google Scholar]
- 13.Genderen, P. J., H. R. vanKoene, K. Spong, and D. Overbosch. 2007. The safety and tolerance of atovaquone/proguanil for the long-term prophylaxis of Plasmodium falciparum malaria in non-immune travelers and expatriates. J. Travel Med. 14:92-95. [DOI] [PubMed] [Google Scholar]
- 14.Hellgren, U., V. H. Angel, Y. Bergqvist, J. S. Forero-Gomez, and L. Rombo. 1991. Plasma concentrations of sulfadoxine-pyrimethamine, mefloquine and its main metabolite after regular malaria prophylaxis for two years. Trans. R. Soc. Trop. Med. Hyg. 85:356-357. [DOI] [PubMed] [Google Scholar]
- 15.Hill, D. R., J. K. Baird, M. E. Parise, L. S. Lewis, E. T. Ryan, and A. J. Magill. 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am. J. Trop. Med. Hyg. 75:402-415. [PubMed] [Google Scholar]
- 16.Jelinek, T., R. Behrens, Z. Bisoffi, A. Bjorkmann, J. Gascon, U. Hellgren, E. Petersen, T. Zoller, R. H. Andersen, A. Blaxhult, et al. 2007. Recent cases of falciparum malaria imported to Europe from Goa, India, December 2006-January 2007. Eur. Surveill. 12:E070111.1. [DOI] [PubMed] [Google Scholar]
- 17.Kofoed, K., and E. Petersen. 2003. The efficacy of chemoprophylaxis against malaria with chloroquine plus proguanil, mefloquine and atovaquone plus proguanil in travellers from Denmark. J. Travel Med. 10:150-154. [DOI] [PubMed] [Google Scholar]
- 18.Lengeler, C. 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2:CD000363. [DOI] [PubMed]
- 19.Marbiah, N. T., E. Petersen, K. David, J. Lines, E. Magbity, and D. B. Bradley. 1998. Control of clinical malaria due to Plasmodium falciparum in children in an area of rural Sierra Leone with perennial transmission: a community-wide trial of lambdacyhalothrin-impregnated mosquito nets alone and/or with fortnightly dapsone/pyrimethamine/placebo chemoprophylaxis. Am. J. Trop. Med. Hyg. 58:1-6.9452282 [Google Scholar]
- 20.McGready, R., E. A. Ashley, and F. Nosten. 2004. Malaria and the pregnant traveller. Travel Med. Infect. Dis. 2:127-142. [DOI] [PubMed] [Google Scholar]
- 21.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64(1-2 Suppl.):97-106. [DOI] [PubMed] [Google Scholar]
- 22.Msyamboza, K., E. Senga, E. Tetteh-Ashong, P. Kazembe, and B. J. Brabin. 2007. Estimation of effectiveness of interventions for malaria control in pregnancy using the screening method. Int. J. Epidemiol. 36:406-411. [DOI] [PubMed] [Google Scholar]
- 23.Mühlberger, N., T. Jelinek, J. Gascon, M. Probst, T. Zoller, M. Schunk, et al. 2004. Epidemiology and clinical features of vivax malaria imported to Europe: sentinel surveillance data from TropNetEurop. Malar. J. 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osei-Akoto, A., L. Orton, and S. P. Owusu-Ofori. 2005. Atovaquone-proguanil for treating uncomplicated malaria. Cochrane Database Syst. Rev. 4:CD004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips-Howard, P. A., A. Radalowicz, J. Mitchell, and D. J. Bradley. 1990. Risk of malaria in British residents returning from malarious areas. Br. Med. J. 300:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pukrittayakamee, S., M. Imwong, S. Looareesuwan, and N. J. White. 2004. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria. Acta Trop. 89:351-356. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Morales, A. J., E. Sanchez, M. Vargas, C. Piccolo, R. Colina, M. Arria, and C. Franco-Paredes. 2006. Pregnancy outcomes associated with Plasmodium vivax malaria in northeastern Venezuela. Am. J. Trop. Med. Hyg. 74:755-757. [PubMed] [Google Scholar]
- 28.Schlagenhauf, P. (ed.). 2008. Travelers' malaria, p. 1-9, 2nd ed. BC Decker, Hamilton, Ontario, Canada.
- 29.Schlagenhauf, P., A. Tschopp, R. Johnson, H. D. Nothdurft, B. Beck, E. Schwartz, M. Herold, B. Krebs, O. Veit, R. Allwinn, and R. Steffen. 2003. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ 327:1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlagenhauf, P., and M. Funk-Baumann. 2005. PDQ travellers malaria. BC Decker, London, United Kingdom.
- 31.Schlagenhauf, P. 2007. Pregnant? Breastfeeding? Traveling with infants? Malaria prevention challenges for mothers, abstr. PL03.03 P34. Abstr. Proc. 10th Conf. Int. Soc. Travel Med., Vancouver, Canada, 20 to 24 May 2007.
- 32.Schwartz, E., and G. Regev-Yochay. 1999. Primaquine as prophylaxis for malaria for nonimmune travelers: a comparison with mefloquine and doxycycline. Clin. Infect. Dis. 29:1502-1506. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz, I. K., E. M. Lackritz, and L. C. Patchen. 1991. Chloroquine-resistant Plasmodium vivax from Indonesia. N. Engl. J. Med. 324:927. [DOI] [PubMed] [Google Scholar]
- 34.Sonmez, A., A. Harlak, S. Kilic, Z. Polat, L. Hayat, O. Keskin, T. Dogru, M. I. Yilmaz, C. H. Acikel, and I. H. Kocar. 2005. The efficacy and tolerability of doxycycline and mefloquine in malaria prophylaxis of the ISAF troops in Afghanistan. J. Infect. 51:253-258. [DOI] [PubMed] [Google Scholar]
- 35.Spudick, J. M., L. S. Garcia, D. M. Graham, and D. A. Haake. 2005. Diagnostic and therapeutic pitfalls associated with primaquine-tolerant Plasmodium vivax. J. Clin. Microbiol. 43:978-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swales, C. A., P. L. Chiodini, B. A. Bannister, et al. 2007. New guidelines on malaria prevention: a summary. J. Infect. 54:107-110. [DOI] [PubMed] [Google Scholar]
- 37.Toovey, S., F. Moerman, and A. van Gompel. 2007. Special infectious disease risks of expatriates and long-term travelers in tropical countries. Part I: malaria. J. Travel Med. 14:42-49. [DOI] [PubMed] [Google Scholar]
- 38.Villegas, L., R. McGready, M. Htway, M. K. Paw, M. Pimanpanarak, R. Arunjerdja, S. J. Viladpai-Nguen, B. Greenwood, N. J. White, and F. Nosten. 2007. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop. Med. Int. Health 12:209-218. [DOI] [PubMed] [Google Scholar]
- 39.Wells, T. S., T. C. Smith, B. Smith, L. Z. Wang, C. J. Hansen, R. J. Reed, W. E. Goldfinger, T. E. Corbeil, C. N. Spooner, and M. A. Ryan. 2006. Mefloquine use and hospitalizations among US service members, 2002-2004. Am. J. Trop. Med. Hyg. 74:744-749. [PubMed] [Google Scholar]
- 40.WHO. 2007. International travel and health. WHO, Geneva, Switzerland. www.who.int/ITH.