Abstract
The in vivo alkylation of heme by the antimalarial trioxaquine DU1301 afforded covalent heme-drug adducts that were detected in the spleens of Plasmodium sp.-infected mice. This result indicates that the alkylation capacities of trioxaquines in mammals infected with Plasmodium strains are similar to that of artemisinin, a natural antimalarial trioxane-containing drug.
Artemisinin and its derivatives have emerged as antimalarial drugs over the past three decades. With a pharmacophore based on a 1,2,4-trioxane, these drugs are highly active against multidrug-resistant Plasmodium strains and are nontoxic, even for children and pregnant women. In addition, no clinically relevant parasite resistance has been reported so far (7, 24, 25). Many synthetic peroxides have been synthesized as artemisinin models in the last 15 to 20 years (for recent reviews, see reference 8, references therein, and references 15 and 22). Among them, trioxaquines have a unique structure, containing both an aminoquinoline moiety (as in chloroquine) and a synthetic 1,2,4-trioxane entity (as an artemisinin mimic) (2, 4). As previously described (4), trioxaquine DU1301 has been obtained by convergent synthesis from 7-chloro-4-(2-aminoethylamino)quinoline and a 1,2,4-trioxane derived from ascaridole (20, 10). Trioxaquines are able to cure Plasmodium-infected mice treated orally at 15 to 20 mg/kg of body weight/day for 4 days (as demonstrated by the “4-day suppressive test”) (see reference 16). Trioxaquines are being developed by Palumed, and one of them (PA1103-SAR116242) is currently under regulatory preclinical development with Sanofi-Aventis (reference 13 and references therein).
The mechanism of action of antimalarial 1,2,4-trioxanes has been intensively studied during the last two decades. The crucial role of heme digestion-aggregation processes in infected erythrocytes has led to investigations of the possible interaction of heme with artemisinin. In the initial work, it was reported that heme-catalyzed peroxide cleavage is responsible for the alkylation of heme and specific parasite proteins (1, 6). Such iron(II)-mediated cleavage of the endoperoxide function generates, in particular, an alkyl radical centered at position C-4 of artemisinin or synthetic trioxanes (9, 17). The concentration of free iron ions in living cells is close to zero, whereas the hemoglobin concentration in red blood cells is 5 mM (corresponding to a heme concentration of 20 mM). In addition, the extensive ingestion of host hemoglobin into the food vacuoles of Plasmodium parasites leads to vacuolar heme concentrations of about 400 mM (21). Bearing in mind the important role of free heme in infected erythrocytes, we reported previously that artemisinin is efficiently activated by heme in vitro and also in vivo, leading to the alkylation of the heme macrocycle (18, 19). Covalent heme-drug adducts resulting from alkylation by the drug were detected in spleens and urine samples from Plasmodium-infected mice (18). Yet, the exact role of heme-artemisinin adducts in the death of the parasite will probably remain a matter of debate. Nevertheless, it is now well established that artemisinin alkylates heme with its C-4-centered radical, and as underlined in a recent publication, free heme or hemoglobin heme alkylations are “possibly the only malaria-parasite-relevant fully characterized alkylation reactions reported so far for [artemisinin]” (23).
Trioxaquine DU1301 was designed as a potential heme-alkylating agent, and such an alkylating ability has been evidenced previously in vitro (11). To confirm that the formation of heme-trioxaquine adducts is a biologically relevant process, we examined the in vivo alkylating ability of DU1301, a representative member of this new class of antimalarial drugs, in parasite-infected mice.
To address the question of the in vivo alkylating properties of trioxaquines, Plasmodium vinckei petteri-infected mice were treated orally with DU1301, a trioxaquine prototype (Fig. 1). A set of three Swiss female albino mice (25 to 30 g) were intraperitoneally inoculated with erythrocytes parasitized by P. vinckei petteri. When the level of parasitemia (evaluated microscopically on Giemsa-stained thin blood smears) was higher than 40% (after 3 days), mice were treated orally with a single dose of trioxaquine DU1301 (2.5 mg diluted in 100 μl of dimethyl sulfoxide [DMSO], corresponding to a dose of 100 mg/kg). As control experiments, a set of three healthy mice received the same dose of DU1301 and a set of infected mice received pure DMSO (no treatment). Mice were sacrificed 2 h after treatment, and organs (spleens, livers, and kidneys) were collected.
FIG. 1.
Reductive activation of trioxaquine DU1301 by iron(II)-heme, leading to the covalent heme-drug adducts 1 and 2. Quin stands for the aminoquinoline fragment, and FeIIIPPIX stands for ferriprotoporphyrin IX.
The detection of heme-DU1301 adducts in mouse organ extracts first required the efficient extraction of these hydrophobic compounds from biological tissues. Each organ was crushed with sand in a mortar. Heme-DU1301 adducts are poorly soluble in common aqueous and organic solvents, and the stacking of heme with quinoline moieties may account for this low solubility (11). Strong π-π interactions between heme and the quinoline ring of antimalarials has been evidenced previously by nuclear magnetic resonance analyses (14). Because of the low solubilities of these heme-DU1301 adducts, pyridine (1.0 to 1.5 ml) was found to be the best solvent for their extraction (by subjecting the tissues to a vortex for 20 min). The organ extracts were analyzed by liquid chromatography-mass spectrometry (LC-MS) without any additional treatment.
The characterization of small amounts of poorly soluble hydrophobic adducts in biological extracts was much more complicated than the usual in vitro characterization of adducts prepared on the bench on a 10- to 100-mg scale (11). Because of the difficulties with the organ extracts, it was essential to optimize the analytical conditions by using chemically prepared adducts (11) in order to have reliable, accurate, and sensitive detection of the in vivo adducts. Analytical separations were performed on a 5-μm C18 Modulo-Cart QS Uptisphere column (250 by 4.6 mm) equipped with a precolumn containing the same packing material. Compounds were eluted under the following conditions: solvent A consisted of methanol-H2O-trifluoroacetic acid, 70/30/0.05; solvent B consisted of methanol-trifluoroacetic acid, 100/0.05; the gradient from an A/B ratio of 100/0 to an A/B ratio of 0/100 over 25 min was followed by 10 min at an A/B ratio of 0/100; the elution rate was 0.5 ml/min; and UV-visible light detection was performed at 340 and 400 nm by using a diode array detector. The volume of pyridine extract injected was 100 μl. Positive-ion electrospray MS was performed on a Q-Trap AB Sciex quadrupole instrument (Fig. 2).
FIG. 2.
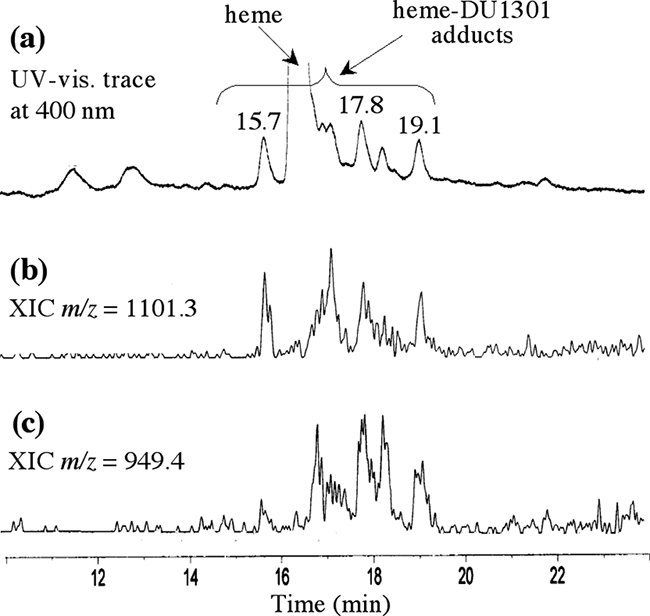
LC-MS analyses of chemically prepared heme-trioxaquine adducts. (a) UV-visible (vis.) trace at 400 nm; (b and c) extracted ionic current (XIC) traces for the indicated m/z.
The LC-MS analyses of spleen extracts from a Plasmodium-infected mouse treated with trioxaquine DU1301 (100 mg/kg in 100 μl of DMSO administered per os) are summarized in Fig. 3a to c. Currents of ions with m/z 1,101.0 and 949.2, which correspond to adducts 1 and 2, respectively, were detected with retention times of 18.5 to 21.7 min, along with heme (retention time, 16.5 min). Retention times and mass spectra were consistent with those observed with chemically prepared adducts (Fig. 2) (11). Furthermore, the isotopic patterns of signals at m/z 1,101.1 and 949.2 clearly showed the presence of both one iron atom (M − 2 due to 54Fe) and one chlorine atom (M + 2 due to 37Cl) (Fig. 3). This result is fully consistent with the reductive activation of the peroxide bond of trioxaquine DU1301 by iron(II)-heme within infected erythrocytes. This reaction leads to the formation of an oxygen-centered radical that rapidly rearranges to give an alkyl radical (Fig. 1) able to alkylate heme at meso positions, giving rise to the “complete” covalent adduct heme-DU1301, adduct 1. Heme-drug adduct 2, generated by hydrolysis and the loss of the terpene moiety, probably in the spectrometer, was also detected.
FIG. 3.
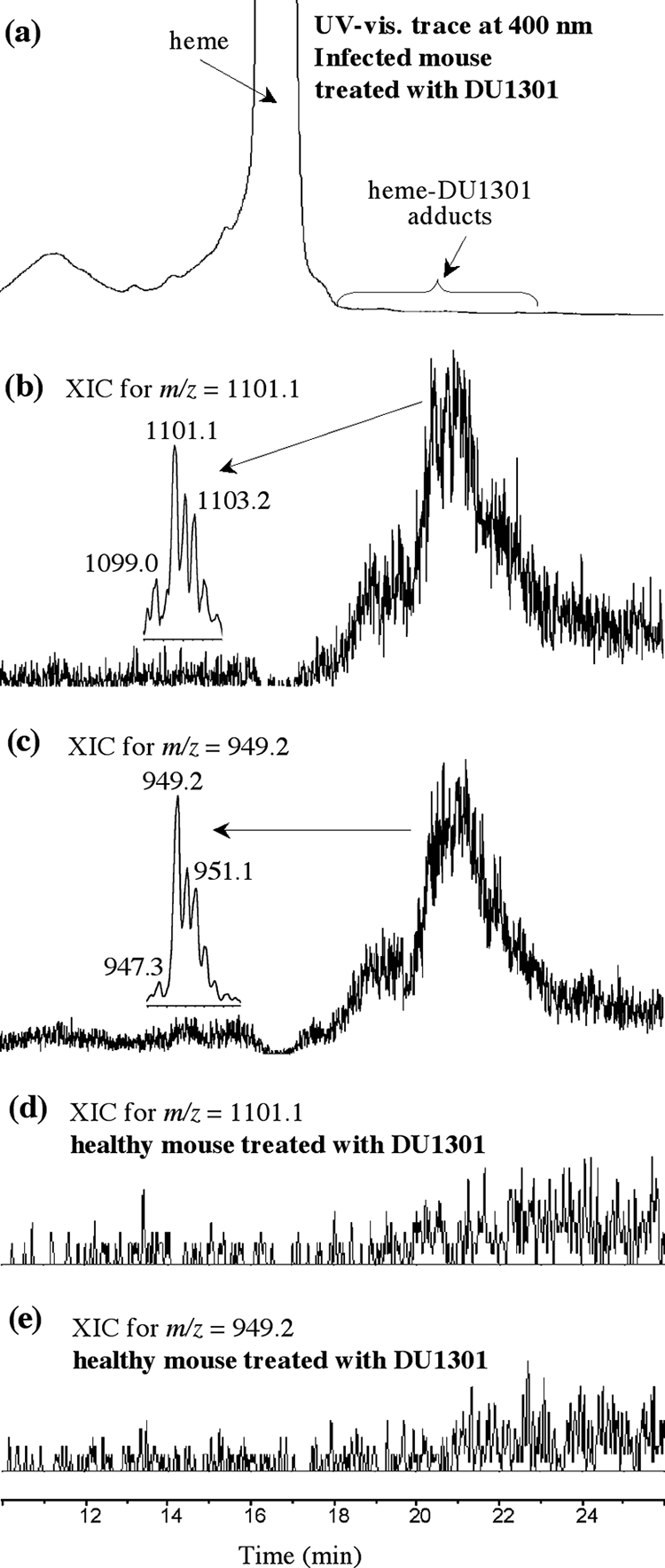
(a to c) LC-MS analyses of the spleen extracts from a mouse infected with P. vinckei petteri and treated orally with DU1301 (single dose of 100 mg/kg). (a) UV-visible (vis.) trace at 400 nm; (b and c) extracted ionic current (XIC) traces for the indicated m/z; (d and e) LC-MS analyses of the spleen extracts from a healthy mouse treated orally with DU1301 (single dose of 100 mg/kg).
Adducts 1 and 2 were both detected in all infected mice treated with DU1301. In the control experiments, these heme-trioxaquine adducts were undetectable in all spleen extracts from healthy mice treated under the same conditions as infected mice (Fig. 3d and e) and in all spleen extracts from untreated Plasmodium-infected mice (which received only excipient) (data not shown).
Heme-DU1301 adducts remained below detectable levels in the kidneys and urine samples from Plasmodium-infected mice that had been treated with trioxaquine. However, it should be considered that the spleen, an organ devoted to the elimination of damaged red blood cells, is the first candidate for the accumulation of heme-drug adducts. Further metabolism of these adducts is a dynamic process, whereas their distribution may be responsible for the fact that they remained below the detection limit in kidneys and urine samples.
The alkylation of heme by DU1301 occurred only in infected mice treated at pharmacologically relevant doses. The presence of these heme-drug adducts should therefore be considered as evidence of the alkylating capacities of these trioxane-containing drugs triggered by the presence of the parasite in mice. The detection of heme-DU1301 adducts within a Plasmodium-infected mammal indicates that heme alkylation by DU1301 is an efficient reaction that occurs in vivo and may be considered to be a key element concerning the mechanism of action of this antimalarial agent. This result underlines the importance of the alkylating abilities of trioxane-based antimalarial drugs and also confirms that artemisinin and trioxaquines share the same heme-alkylating properties. In addition, since artemisinin and the citrate salt of DU1301 are active against the same stages of synchronized P. falciparum parasites (ring, trophozoite, and gametocyte stages) (2), these pharmacological features strongly suggest that this common alkylating reactivity may be one of the key factors of their antimalarial activities.
In addition, aminoquinolines (such as chloroquine) are considered to inhibit the formation of hemozoin by π-π stacking (21, 3, 5). It has been reported recently that trioxaquine DU1301 efficiently prevents the in vitro formation of β-hematin at a lower concentration than chloroquine itself, whereas the synthetic trioxane precursor of DU1301 does not inhibit the dimerization of hemin (12). On the other hand, heme-artemisinin adducts also inhibit β-hematin dimerization and are themselves unable to dimerize (12). These results suggest that, besides the alkylating ability of the peroxide moiety, trioxaquines may have the ability to prevent heme aggregation within the parasite either (i) by the stacking of their quinoline fragment with heme or (ii) by the formation of unpolymerizable heme-drug adducts. Thus, trioxaquines should be considered as hybrid antimalarial molecules with a dual mode of action: heme alkylation and the inhibition of heme polymerization.
Acknowledgments
F.B.-E.G. is indebted to the EU-AntiMal program for a Ph.D. fellowship. CNRS, INSERM, and ANR are acknowledged for financial support.
This study was approved by the French Institutional Animal Experimentation Ethics Committee (approval no. MP/R/06/33/11/07).
Guy Lavigne (LCC-CNRS, Toulouse) is acknowledged for English editing.
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Asawamahasakda, W., I. Ittarat, Y.-M. Pu, H. Ziffer, and S. R. Meshnick. 1994. Reaction of antimalarial endoperoxide with specific parasite proteins. Antimicrob. Agents Chemother. 38:1854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit-Vical, F., J. Lelièvre, A. Berry, C. Deymier, O. Dechy-Cabaret, J. Cazelles, C. Loup, A. Robert, J.-F. Magnaval, and B. Meunier. 2007. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob. Agents Chemother. 51:1463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dechy-Cabaret, O., F. Benoit-Vical, C. Loup, A. Robert, H. Gornitzka, A. Bonhoure, H. Vial, J.-F. Magnaval, J.-P. Séguéla, and B. Meunier. 2004. Synthesis and antimalarial activity of trioxaquine derivatives. Chem. Eur. J. 10:1625-1636. [DOI] [PubMed] [Google Scholar]
- 5.Egan, T. J., and H. M. Marques. 1999. The role of haem in the activity of chloroquine and related antimalarial drugs. Coord. Chem. Rev. 190-192:493-517. [Google Scholar]
- 6.Hong, Y.-L., Y.-Z. Yang, and S. R. Meshnick. 1994. The interaction of artemisinin with malarial hemozoin. Mol. Biochem. Parasitol. 63:121-128. [DOI] [PubMed] [Google Scholar]
- 7.Ittarat, W., A. L. Pickard, P. Rattanasinganchan, P. Wilairatana, S. Looareesuwan, K. Emery, J. Low, R. Udomsangpetch, and S. R. Meshnick. 2003. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am. J. Trop. Med. Hyg. 68:147-152. [PubMed] [Google Scholar]
- 8.Jefford, C. W. 2007. New developments in synthetic peroxidic drugs as artemisinin mimics. Drug Discov. Today 12:487-495. [DOI] [PubMed] [Google Scholar]
- 9.Jefford, C. W., F. Favarger, V. H. Maria da Graca, and Y. Jacquier. 1995. The decomposition of cis-fused cyclopenteno-1,2,4-trioxanes by ferrous salts and some oxophilic reagents. Helv. Chim. Acta 78:452-458. [Google Scholar]
- 10.Jefford, C. W., A. Jaber, and J. Boukouvalas. 1989. The regio- and stereo-controlled synthesis of cis-p-menth-3-ene-1,2-diol by means of a 1,2,4-trioxane intermediate. J. Chem. Soc. Chem. Commun. 1989:1916-1918. [Google Scholar]
- 11.Laurent, S. A.-L., C. Loup, S. Mourgues, A. Robert, and B. Meunier. 2005. Heme alkylation by artesunic acid and trioxaquine DU1301, two antimalarial trioxanes. Chembiochem 6:653-658. [DOI] [PubMed] [Google Scholar]
- 12.Loup, C., J. Lelièvre, F. Benoit-Vical, and B. Meunier. 2007. Trioxaquines and heme-artemisinin adducts inhibit the in vitro formation of hemozoin better than chloroquine. Antimicrob. Agents Chemother. 51:3768-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meunier, B. 2008. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 41:69-77. [DOI] [PubMed] [Google Scholar]
- 14.Moreau, S., B. Perly, C. Chachaty, and C. Deleuze. 1985. A nuclear magnetic resonance study of the interactions of antimalarial drugs with porphyrins. Biochim. Biophys. Acta 840:107-116. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill, P. M., and G. H. Posner. 2004. A medicinal perspective on artemisinin and related endoperoxides. J. Med. Chem. 47:2945-2964. [DOI] [PubMed] [Google Scholar]
- 16.Peters, W., J. H. Portus, and B. L. Robinson. 1975. Chemotherapy of rodent malaria. XXII. Value of drug-resistant strains of Plasmodium berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 69:155-171. [PubMed] [Google Scholar]
- 17.Posner, G. H., C. H. Ho, D. Wang, L. Gerena, W. K. Milhous, S. R. Meshnick, and W. Asawamahasakda. 1994. Mechanism-based design, synthesis, and in vitro antimalarial testing of new 4-methylated trioxanes structurally related to artemisinin: the importance of a carbon-centered radical for antimalarial activity. J. Med. Chem. 37:1256-1258. [DOI] [PubMed] [Google Scholar]
- 18.Robert, A., F. Benoit-Vical, C. Claparols, and B. Meunier. 2005. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 102:13676-13680. (Erratum, 103:3943, 2006.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert, A., and B. Meunier. 1997. Characterization of the first covalent adduct between artemisinin and a heme model. J. Am. Chem. Soc. 119:5968-5969. [Google Scholar]
- 20.Schenck, G. O., and K. Ziegler. 1944. Die Synthese des Ascaridols. Naturwissenschaften 32:157. [Google Scholar]
- 21.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russel, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, Y., Y. Dong, and J. L. Vennerstrom. 2004. Synthetic peroxides as antimalarials. Med. Res. Rev. 24:425-448. [DOI] [PubMed] [Google Scholar]
- 23.Tang, Y., Y. Dong, X. Wang, K. Sriraghavan, J. K. Wood, and J. L. Vennerstrom. 2005. Dispiro-1,2,4-trioxane analogues of a prototype dispiro-1,2,4-trioxolane: mechanistic comparators for artemisinin in the context of reaction pathways with iron(II). J. Org. Chem. 70:5103-5110. [DOI] [PubMed] [Google Scholar]
- 24.White, N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330-334. [DOI] [PubMed] [Google Scholar]
- 25.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]



