Abstract
This study aimed to investigate if the absorption of the human African trypanosomiasis agent eflornithine was stereospecific and dose dependent after oral administration. Male Sprague-Dawley rats were administered single doses of racemic eflornithine hydrochloride as an oral solution (750, 1,500, 2,000, or 3,000 mg/kg of body weight) or intravenously (375 or 1,000 mg/kg of body weight). Sparse blood samples were obtained for determination of eflornithine enantiomers by liquid chromatography with evaporative light-scattering detection (lower limit of quantification [LLOQ], 83 μM for 300 μl plasma). The full plasma concentration-time profile of racemic eflornithine following frequent sampling was determined for another group of rats, using a high-performance liquid chromatography-UV method (LLOQ, 5 μM for 50 μl plasma). Pharmacokinetic data were analyzed in NONMEM for the combined racemic and enantiomeric concentrations. Upon intravenous administration, the plasma concentration-time profile of eflornithine was biphasic, with marginal differences in enantiomer kinetics (mean clearances of 14.5 and 12.6 ml/min/kg for l- and d-eflornithine, respectively). The complex absorption kinetics were modeled with a number of transit compartments to account for delayed absorption, transferring the drug into an absorption compartment from which the rate of influx was saturable. The mean bioavailabilities for l- and d-eflornithine were 41% and 62%, respectively, in the dose range of 750 to 2,000 mg/kg of body weight, with suggested increases to 47% and 83%, respectively, after a dose of 3,000 mg/kg of body weight. Eflornithine exhibited enantioselective absorption, with the more potent l-isomer being less favored, a finding which may help to explain why clinical attempts to develop an oral treatment have hitherto failed. The mechanistic explanation for the stereoselective absorption remains unclear.
Human African trypanosomiasis (HAT), also known as sleeping sickness, is fatal if left untreated. The disease is spread in 36 countries throughout sub-Saharan Africa (13). Sixty million people are exposed to Trypanosoma-infected tsetse flies and are at risk of developing the disease. HAT was largely under control in the mid-20th century but has since reemerged (5). By 1997, an estimated 450,000 people were infected (13). Although this number has decreased in recent years, thanks to coordinated campaigns (1), it remains a neglected disease, with numerous patients not receiving treatment (2).
The human disease is caused by one of the subspecies of the kinetoplastid protozoan Trypanosoma brucei. T. b. rhodesiense is responsible for an acute form of the disease, while T. b. gambiense (causing 97% of reported cases) causes a chronic form in West and Central Africa (5, 6). There are two stages of HAT, namely, an early or hemolymphatic stage and a late or encephalitic stage. The early stage causes a variety of nonspecific symptoms, such as fever, headache, malaise, and weakness. As the disease progresses, specific organ dysfunction, e.g., tachycardia, heart failure, endocrine disturbances, and liver, spleen, and eye involvement, occurs (13). In the late stage, there is a wide spectrum of possible features that may occur, such as psychiatric disturbances, sleep disorders, motor system disorders, sensory syndromes, and abnormal reflexes. In the terminal stage, the patient develops seizures, incontinence, cerebral edema, progressive mental deterioration, and finally, death.
Only two drugs, melarsoprol and eflornithine, are registered for the treatment of late-stage HAT. There is an increasing resistance to melarsoprol, a toxic arsenical compound requiring intravenous injections (4, 8, 14, 21). Eflornithine (d,l-α-difluoromethylornithine [DFMO]) was initially synthesized as an antitumor agent, although it was never registered for that use. It is mainly effective against T. b. gambiense-infected patients. Eflornithine is efficacious compared to melarsoprol but requires four (100 mg/kg of body weight) daily infusions for a duration of 7 to 14 days. This complex mode of administration requires hospital-like settings, with the consequence that many patients living in rural areas are left untreated. It can be expected that a less complex mode of administration would enable more patients to be treated.
Results of oral eflornithine treatment in late-stage infected patients have been discouraging. Oral regimens with the same or similar doses to those for intravenous use (100 to 125 mg/kg of body weight, given four times daily) resulted in eflornithine racemic plasma concentrations of approximately half those observed after intravenous administration (16). Moreover, it was suggested that plasma eflornithine concentrations did not increase proportionally to the dose when the dose was increased from 100 to 125 mg/kg of body weight (60 to 70% of the expected increase), although this was not statistically significant. Relapse was observed in 6 of the 25 patients on oral treatment. When the drug is administered intravenously, racemic cerebrospinal fluid concentrations above 50 μM have been associated with a high cure rate, but this concentration appeared to be insufficient during oral treatment. Increasing the oral dose may improve the cure rate, but gastrointestinal side effects are likely to occur and possibly be dose-limiting (10, 16).
Eflornithine elicits its pharmacological effect by irreversible inhibition of ornithine decarboxylase, blocking polyamine biosynthesis in trypanosomes (3, 18). It has also been shown that compared to d-eflornithine, the l-enantiomer exhibits >20 times higher affinity for the target enzyme ornithine decarboxylase in mammalian cells (18). The l-isomer also appears to be more potent in cultured T. brucei parasites (R. Brun, Swiss Tropical Institute, personal communication). Taken together, these data suggest that the l-form is the principal trypanostatic moiety clinically.
Eflornithine human racemic pharmacokinetics are characterized by an oral bioavailability of approximately 50%, mainly renal elimination (>80%) of low extraction, no reported metabolites, negligible plasma protein binding, and a multiphasic plasma concentration-time profile, probably contributing to highly varying estimates of half-life ranging from 2 to 30 h (7, 10, 12, 16). There are no published data on the pharmacokinetics of eflornithine based on a stereoselective method of bioanalysis. The presence of enantioselective pharmacokinetics should be considered in investigating the possibility of developing an efficacious oral treatment.
The aim of this work was to investigate if the oral absorption of racemic eflornithine was stereospecific and dose dependent in the rat. The secondary aim was to characterize the pharmacokinetic profile of eflornithine enantiomers and to investigate the absorption kinetics of eflornithine.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (B&K Universal AB, Sollentuna, Sweden) weighing 260 to 320 g were acclimatized for at least 5 days after arrival at a certified animal facility (Experimental Biomedicine at Göteborg University, Göteborg, Sweden). The animals were housed under controlled environmental conditions (12-h light-dark cycle at 25 to 27°C and 60 to 65% humidity). Four rats were kept in each cage prior to surgery and thereafter were kept separately. Food (B&K Feeds) and tap water were available ad libitum prior to and after surgery until 6 hours prior to drug administration. All experiments were performed during the light phase of the cycle. The study was approved by the Ethics Committee for Animal Experiments, Göteborg, Sweden (255/2005).
Chemicals for in vivo experiments.
Eflornithine hydrochloride was obtained from WHO/TDR (Geneva, Switzerland). Isoflurane (Forene; Abbot Scandinavia AB, Solna, Sweden) and heparin (Leo Pharma AB, Malmö, Sweden) were obtained from Apoteket AB (Sweden).
dl-4-Amino-3-hydroxybutyric acid, dl-norvaline, nitriloacetic acid, and mercaptoethanol were obtained from Sigma-Aldrich (St. Louis, MO), and ethanol was obtained from Kemetyl (Haninge, Sweden). All chemicals were of analytical grade, and all solvents were of high-performance liquid chromatography (HPLC) grade.
Animal surgery.
The animals were anesthetized by inhalation of isoflurane (3.7 to 4.0% in air). In rats receiving eflornithine orally, the left jugular vein was catheterized using MRE040 1.02-mm-outer-diameter (OD), 0.64-mm-inner-diameter (ID) tubing (AgnThos, Lidingö, Sweden) prefilled with 100 IU/ml of heparin in saline solution. In rats receiving intravenous administration, both the left jugular vein and the right carotid artery were catheterized, the latter by using PE-50 0.96-mm OD, 0.58-mm-ID tubing (AgnThos, Lidingö, Sweden) prefilled with 100 IU/ml of heparin in saline solution. All catheters were tunneled subcutaneously to emerge at the back of the neck. Catheters were kept patent with heparinized saline solution (20 IU/ml) between sampling to prevent blood clotting. All animals were allowed to recover for at least 16 h after surgery.
Drug formulation.
Solutions for oral administration were prepared by dissolving an appropriate amount of eflornithine hydrochloride powder in saline solution. Eflornithine solutions for intravenous administration were prepared likewise but were pH adjusted to 7.2 with sodium hydroxide.
Experimental design.
A total of 69 rats were administered eflornithine hydrochloride either orally (n = 52) or intravenously (n = 17). Selections of dose levels were based on allometric scaling and the lower limit of quantification (LLOQ) for the enantioselective assay. Single oral doses of 750, 1,500, 2,000 or 3,000 mg/kg of body weight of racemic eflornithine hydrochloride were administered by gavage (10 ml/kg). Single intravenous eflornithine doses of 375 or 1,000 mg/kg of body weight were administered as a short-term infusion (3 min) via the jugular vein catheter (3.3 ml/kg) (Table 1).
TABLE 1.
Experimental design for the study
| Route of administration | Racemic dose (mg/kg of body wt) | No. of rats
|
|
|---|---|---|---|
| Chiral analysis (1 to 3 plasma samples/rat) | Racemic analysis (8 to 16 plasma samples/rat) | ||
| Oral | 750 | 10 | 4 |
| 1,500 | 9 | 4 | |
| 2,000 | 7 | 4 | |
| 3,000 | 10 | 4 | |
| Intravenous | 375 | 5 | 4 |
| 1,000 | 5 | 3 | |
Blood samples were drawn from the jugular vein catheter or carotid artery catheter after oral or intravenous doses, respectively. Sample volumes were replaced with an equal volume of saline solution, and catheters were flushed with heparinized saline solution (20 IU/ml) after each sampling occasion.
One to three blood samples (700 μl) per rat were obtained up to 13 h after dosing and analyzed with chiral quantification. In another group of rats, frequent (8 to 16 samples per rat) blood samples (130 μl per sample) were drawn up to 25 h after dosing and quantified by the racemic method (Table 1). Plasma was separated by centrifugation for 8 min at 12,000 × g within 30 min after blood collection and kept at −22°C until analysis.
Chiral quantification of eflornithine.
d- and l-difluoromethylornithine was extracted from plasma by solid-phase extraction and separated on a Chirobiotic TAG (250 mm × 4.6 mm) column with isocratic LC and evaporative light-scattering detection, as previously described, using identical instrumentation and chromatographic conditions (15). The chiral method was developed for determination of eflornithine enantiomers in 1,000-μl human plasma samples but was used in this study for 300-μl rat plasma samples. Rat plasma samples (300 μl) were mixed with milliQ water (700 μl) and run with calibrators prepared in human plasma (1,000 μl), ranging from 25 μM to 1,000 μM for each enantiomer. The dilution of plasma samples did not significantly affect the accuracy or precision of the method. No significant differences were observed in slopes or intercepts in regressing nondiluted and diluted calibration curves (t test; P > 0.99).
No matrix differences were observed in analyzing quality control (QC) samples (n = 5) at two levels (250 and 2,500 μM) prepared in rat and human plasma. The LLOQ was set to 83 μM (300 μl). Duplicates of two QC levels (750 and 7,500 μM) were analyzed during analytical runs of rat plasma samples to ensure that accuracy and precision were within acceptable limits.
Racemic quantification of eflornithine.
Racemic eflornithine was quantified using precolumn derivatization, followed by LC and UV detection according to a published method (20), modified as described below. The LC system consisted of a 48-well-plate Prospekt 2 autoinjector (SparkHolland, Emmen, Holland), two interconnected Schimadzu LC10AD pumps (Schimadzu, Kyoto, Japan), and a Schimadzu SPD 10-A UV-Vis detector, set at 330 nm. Data acquisition was performed using Chromatographic Station for Windows, version 1.2.3 (Dataapex, Prague, The Czech Republic).
Plasma samples (50 μl) were precipitated with ice-cold methanol (270 μl) containing an internal standard (dl-4-amino-3-hydroxybutyric acid) at a concentration of 0.155 μM. The samples were placed on a vortex mixer for approximately 10 s, centrifuged for 10 min at 12,000 × g, and thereafter kept at −37°C for 10 minutes. The supernatants were transferred to new tubes and evaporated to dryness at 65°C under a gentle stream of air. The dried samples were redissolved in 50 μl phosphate buffer (0.1 M; pH 7.5). The derivatization mixture was prepared daily by mixing o-phthalaldehyde (20 mg), ethanol (1 ml), nitriloacetic acid (4 mg), mercaptoethanol (100 μl), and 10 ml phosphate buffer (0.1 M; pH 7.5). Prior to injection, the samples were mixed by adding two 50-μl volumes of derivatization mixture (i.e., o-phthalaldehyde) to the 48-well-plate autoinjector. The temperature for the autoinjector was kept constant at 20°C.
The plasma samples containing the derivatization mixture were programmed to stand in the autoinjector for 2.00 min prior to injection. Eflornithine was separated on a Chromolith Performance RP-18e 100-mm by 4.6-mm-ID column (VWR International, Darmstadt, Germany) protected by a ChromSep Guard SS 10-mm by 2-mm-ID column (Varian, Palo Alto, CA), using a gradient program.
Mobile phase A consisted of 92% phosphate buffer (0.1 M; pH 7.5), 5% methanol, and 3% acetonitrile. Mobile phase B consisted of 80% methanol, 10% acetonitrile, and 10% milliQ water. The flow rate was set to 2 ml/min, using the following gradient program: t = 0 to 6.75 min, linear decrease of A from 80% to 40%; t = 6.75 to 8.0 min, 40% A; and t = 8.0 to 10 min, linear increase of A from 40% to 80%.
The typical retention times for the internal standard and eflornithine were 5.2 and 7.0 min, respectively. A calibration curve (n = 8) ranging from 5 to 4,000 μM was used. Calibration standards prepared in rat and human plasmas were compared to ensure that calibrators prepared in human plasma could be used during analysis.
The LLOQ for racemic eflornithine was set to 5 μM, at which level precision and accuracy were <15%. QC samples were prepared at three concentrations (30, 300, and 3,000 μM) and were analyzed in duplicate at each level during the analytical runs to ensure that experimental samples were accurately and precisely determined.
Pharmacokinetic analysis.
The sparse enantiomeric and rich racemic data were modeled simultaneously to obtain pharmacokinetic parameters for eflornithine enantiomers. Racemic concentrations were set to be the sum of d- and l-eflornithine concentrations. Pharmacokinetic parameters that could not be estimated separately for each enantiomer were assumed to be identical for both enantiomers.
Pharmacokinetics was modeled by nonlinear mixed-effect modeling of log-transformed concentrations, using NONMEM, version VI (Globomax, LLC, Hanover, MD). The analysis was conducted using the first-order conditional estimation method with interactions. Interindividual variability was modeled as proportional to the corresponding parameter value. Random residual variability was modeled as proportional to the observed concentrations. Model selection was based on the objective function value (OFV) and visually explored using Census, version 0.998r5a (Novartis Pharma AG, Basel, Switzerland). Discrimination between two nested models was achieved by using the log likelihood ratio where the difference in the OFV between the full and reduced models was asymptotically chi-square distributed. Decreases in the OFV of 3.84 and 6.63 between two nested models (1 degree of freedom) were considered to indicate a statistically superior model (P < 0.05 and P < 0.01, respectively).
For the intravenous data, one-, two-, and three-compartment models with linear or saturable elimination were evaluated. The intravenous infusions (3 min) were modeled as a bolus dose. Dose was tested as a covariate for the model parameters. During model evaluation, it was assumed that the pharmacokinetics of the two enantiomers could be described by the same structural model, albeit with differing parameter values. The selected structural model and its estimated parameters after intravenous dosing were then used as fixed parameters to model the oral data.
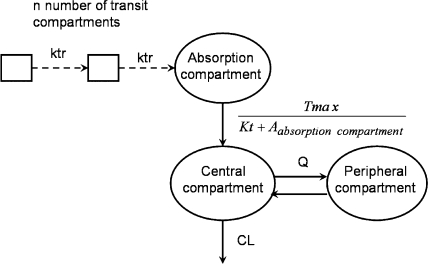
Absorption rates after oral administration were modeled and evaluated as either zero, first order, mixed zero-first order, sequential zero-first order, or Michaelis-Menten (hyperbola function) kinetics. The delay in absorption was initially modeled using a lag-time model and, subsequently, by using a chain of transit compartments delivering the drug to the absorption compartment (Fig. 1). The number of transit compartments was estimated in NONMEM by using the Stirling approximation for a small number of transit compartments (17, 19). The average duration of the absorption delay was characterized by the mean transit time to the absorption compartment. Bioavailability was modeled as the fraction of administered dose reaching the absorption compartment.
FIG. 1.
Structural model for d- and l-eflornithine after intravenous and oral administration. The transit compartment absorption model consisted of a number of transit compartments (n) and the transit compartment rate (ktr) delivering the drug to the absorption compartment. The transfer of drug from the absorption compartment to the central compartment was modeled with a Michaelis-Menten-type function, using the parameters Tmax, Kt, and the amount of drug in the absorption compartment (Aabsorption compartment). The volumes of the central and peripheral compartments, clearance (CL), and intercompartment clearance (Q) were obtained from the intravenous data.
Dose was evaluated as a covariate explaining variability in the pharmacokinetic parameters. To mimic the tendency toward increased oral bioavailability at higher doses, linear, nonlinear, and categorical relationships between dose and oral bioavailability were investigated.
RESULTS
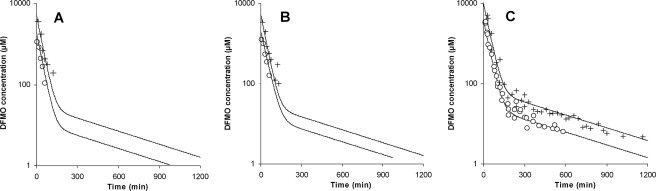
Enantiomeric concentrations were below the LLOQ within 2 h after intravenous drug administration, whereas racemic concentrations of eflornithine could be quantitated for up to 18 h after dosing (Fig. 2). Up to the last sampling point, the sums of the enantiomer concentrations were in agreement with racemic concentrations. The plasma concentration profile was described with a two-compartment model with first-order elimination. Clearance (CL) and central volume were estimated for each enantiomer, whereas intercompartment clearance (Q) and peripheral volume were set to be identical for both isomers (Fig. 2; Table 2). Interindividual variability was estimated for CLd-eflornithine and CLl-eflornithine. Although the OFV did not decrease significantly in estimating CL and central volume of distribution individually for each enantiomer compared with their estimation as shared parameters, they were nevertheless estimated individually in consideration of diagnostic plots as well as the further employment of the estimates as fixed parameters for fitting of the oral plasma concentration-time data. The estimated population CLl-eflornithine was 15% higher than the estimated population CLd-eflornithine, with dose having no effect on CL. The diagnostic plots and parameter estimates for the intravenous data are shown in Fig. 3 and Table 3.
FIG. 2.
Experimental values and population predicted values (black lines) for l-eflornithine (A), d-eflornithine (B), and racemic eflornithine (C) after intravenous administration of 375 (open circles) and 1,000 (crosses) mg/kg of body weight of racemic eflornithine to laboratory rats (n = 17). The data were modeled simultaneously to obtain pharmacokinetic parameters for l- and d-enantiomers, based on a two-compartment disposition model with linear elimination.
TABLE 2.
Population pharmacokinetic parameter estimates for l- and d-eflornithine in the rat after intravenous administrationa
| Parameter | Value for l-eflornithine
|
R-eflornithine estimate (RSE) | Value for d-eflornithine
|
||
|---|---|---|---|---|---|
| Estimate (RSE) | IIV (RSE) | Estimate (RSE) | IIV (RSE) | ||
| CL (ml/min/kg of body wt) | 14.5 (8.5) | 14.9 (48) | 12.6 (5.9) | 18.2 (35) | |
| Vcentral (liters/kg of body wt) | 0.404 (13) | 0.401 (8.5) | |||
| Q (ml/min/kg of body wt) | 1.2 (11) | ||||
| Vperipheral (liters/kg of body wt) | 0.446 (15) | ||||
| Random residual variability (σ) (% coefficient of variation) | 19.9 (17) | ||||
R-eflornithine represents parameters that were set to be identical for l- and d-eflornithine. CL, clearance; Vcentral, central volume of distribution; Q, intercompartment clearance; IIV, interindividual variability; RSE, relative standard error [(standard error/mean) × 100]; σ, additive residual error.
FIG. 3.
Observed data versus population predictions and individual predictions for l-eflornithine (white squares), d-eflornithine (gray triangles), and racemic eflornithine (black circles) after intravenous administration. Concentrations are on a logarithmic scale.
TABLE 3.
Population pharmacokinetic parameter estimates for l- and d-eflornithine after oral administration of racemic eflornithinea
| Parameter | l-Eflornithine estimate (RSE) | Value for R-eflornithine
|
d-Eflornithine estimate (RSE) | |
|---|---|---|---|---|
| Estimate (RSE) | IIV (RSE) | |||
| Tmax (μmol/min/kg of body wt) | 11.1 (17) | 14.5 (10) | ||
| Kt (μmol/kg of body wt) | 1,560 (25) | 784 (28) | ||
| MTT (min) | 88 (12) | 38 (29) | ||
| n | 1.424 (21) | |||
| F (%) at 750 to 2,000 mg/kg of body wt | 41 (6.5) | 10.0 (37) | 62.3 (9.5) | |
| F (%) at 3,000 mg/kg of body wt | 47 (7.5) | 82.7 (7.5) | ||
| Random residual variability (σ) (% coefficient of variation) | 27.7 (18) | |||
R-eflornithine represents parameters that were set to be identical for l- and d-eflornithine. CL, Q, and central and peripheral volumes of distribution were fixed to the values obtained in fitting the intravenous data. MTT, mean transit time to the absorption compartment; n, number of transit compartments before reaching the absorption compartment; F, absolute oral bioavailability; IIV, interindividual variability; σ, additive residual error; RSE, relative standard error [(standard error/mean) × 100].
In contrast to intravenous administration, where l- and d-eflornithine concentrations were comparable, plasma concentrations after oral administration of l-eflornithine were lower than those after oral administration of d-eflornithine. The population mean bioavailability values for l- and d-eflornithine, in the dose range of 750 to 2,000 mg/kg of body weight, were 41% and 62%, respectively, with no apparent dose dependency. At the highest dose level, the population bioavailability values for l- and d- eflornithine were estimated to be higher, at 47% and 83%, respectively. Including the highest dose level as a categorical parameter for oral bioavailability for both d- and l-eflornithine significantly decreased the OFV (P < 0.01; ΔOFV, −11.4).
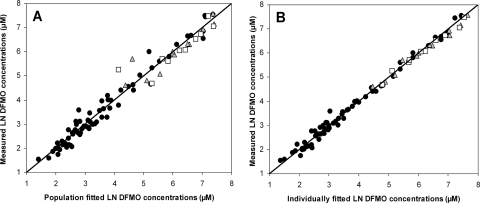
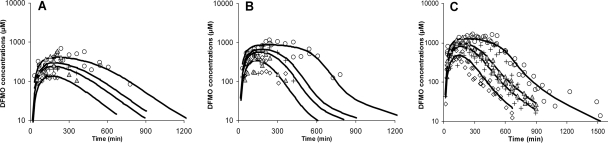
The absorption delay and an increasing time to reach maximum concentration with dose were best described, among the models evaluated, by a transit model followed by a Michaelis-Menten function determining the absorption rate. Using a transit model instead of a lag time before absorption occurred reduced the OFV significantly (P < 0.01; ΔOFV, −148). Parameters for the transit model could not be obtained individually for each enantiomer and were therefore assumed to be identical for both eflornithine enantiomers. Interindividual variability values for mean transit time and bioavailability could not be estimated separately for d- and l-eflornithine and were therefore assumed to be identical. Population parameters and population predicted concentration profiles are shown in Table 3 and Fig. 4, respectively. Estimates of maximum absorption rates (Tmax) were similar for the two enantiomers, whereas the estimate for the amount of drug in the absorption compartment when the absorption rate was half of the maximum rate (Kt) was twofold higher for l-eflornithine than that for d-eflornithine. The selected structural model adequately described the plasma concentration profiles for d-, l-, and racemic eflornithine (Fig. 5).
FIG. 4.
Experimental values and mean population predicted values (black lines) for l-eflornithine (A), d-eflornithine (B), and racemic eflornithine (C) after oral administration of racemic eflornithine hydrochloride to Sprague-Dawley rats at doses of 750 (diamonds), 1,500 (crosses), 2,000 (triangles), and 3,000 (circles) mg/kg of body weight. l-, d-, and racemic eflornithine data were fitted simultaneously to obtain absorption kinetic parameters for each eflornithine enantiomer, using a two-compartment disposition model with parameters fixed from the intravenous data. The absorption data were described with a transit model transferring drug to an absorption compartment, from which the systemic absorption rate was characterized by a Michaelis-Menten-type function.
FIG. 5.
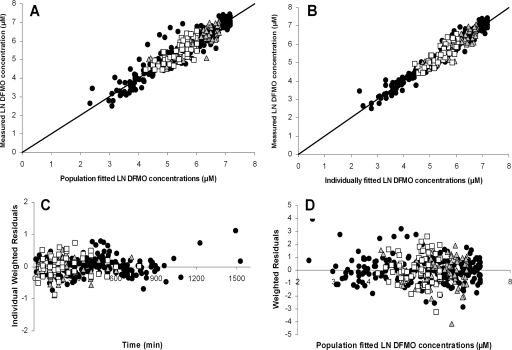
Basic goodness-of-fit plots for the absorption model with fixed disposition parameter values. The measured d-eflornithine (gray triangles), l-eflornithine (white squares), and racemic eflornithine (black circles) concentrations were plotted versus the population fitted concentrations (A) and the individually fitted concentrations (B). Individually weighted residuals were plotted versus time (C), and weighted residuals were plotted versus population fitted eflornithine concentrations (D).
DISCUSSION
The HAT drug eflornithine is efficacious following the complex mode of intravenous infusions in patients but has so far exhibited disappointing results when administered orally. The present study is the first to describe the stereoselective pharmacokinetics of eflornithine and clearly demonstrates that, in the rat, intestinal absorption following oral administration of racemic eflornithine is stereoselective, with the more potent l-enantiomer being less well absorbed than d-eflornithine. Taken together with a 15% higher CL for l-eflornithine than that for the d-enantiomer, this results in systemic exposure of the l-isomer being only half that of the less active d-form after a single oral dose. Our results indicate that in order to achieve a comparable systemic exposure to the l-isomer after oral administration to that obtained with intravenous infusions, an approximately threefold higher dose is required. The present results, although containing single-dose data only, may therefore contribute to our understanding of why oral eflornithine has been inefficacious in the treatment of late-stage sleeping sickness.
Differing bioavailabilities of the enantiomers are attributed to stereoselective absorption rather than first-pass metabolism. In both rats and humans, >80% of the drug is excreted renally as unchanged drug (7, 11). Together with a low total CL (approximately 90 ml/min and 4 ml/min in humans and rats, respectively), this suggests hepatic first-pass metabolism to be of minor importance for eflornithine bioavailability.
The mechanistic causes for the stereoselective intestinal absorption in the rat are unclear. Eflornithine has a molecular structure similar to those of natural amino acids and could be subject to active intestinal uptake. However, whereas one would generally expect the transport of l-isomers to be favored biologically, the bioavailability of this enantiomer is lower.
Since eflornithine is a relatively small and very polar molecule, paracellular intestinal absorption of eflornithine may occur, but whether such an absorption process would be stereoselective is unclear.
For humans, the available information on the oral absorption of eflornithine is contradictory. In one clinical study, the fraction absorbed was constant with the dose (12), whereas other studies, using higher doses, suggested that eflornithine may display saturable, dose-dependent absorption (10, 16). In rats, oral bioavailability of l-eflornithine was about 40 to 50% for all doses, with no evidence of dose-limiting bioavailability, whereas a slight increase in absolute oral bioavailability was observed following the highest dose. Efforts to model such a putative change in bioavailability with dose, using linear or power models over the four dose levels studied, did not improve fits. In the end, bioavailability was allowed to be estimated separately following the highest dose, although there is no immediate reason why a small but abrupt increase would occur at the highest dose. Our selected dose levels were high and may have resulted in higher intestinal drug concentrations than those in a clinical situation. Still, resulting plasma concentrations and the overall, racemic bioavailability (mean for l- and d-enantiomers) were not dissimilar to those seen clinically, suggesting that the rat is a suitable model for studying the absorption of eflornithine. The laboratory rat has previously been found to be a good model for prediction of the oral fraction absorbed in humans (9). For further investigation of eflornithine absorption kinetics, lower dose levels given to rats would be of interest but would require a more sensitive stereoselective analytical method.
The atypical plasma concentration-time profile after oral dosing was adequately described with a transit model leading to an absorption rate described by a Michaelis-Menten function. The mechanistic explanation for this absorption profile cannot be explained by the dissolution rate because the drug was given as a solution. The successful characterization of the time profile by a Michaelis-Menten input function suggests that eflornithine is absorbed by active processes but is in conflict with the increased fraction absorbed at the highest dose level. Changes in the physiology of the gastrointestinal tract along its different segments, such as motility, luminal metabolism, epithelial expression of transporters, surface area, blood flow, etc., can result in variable permeability and absorption rates along the gastrointestinal tract. The time to reach maximum concentration increased with the dose from approximately 1.5 to 6 h, suggesting that the actual site of absorption varied with the dose due to the consequence of drug transfer along the gastrointestinal tract. This transfer of drug along the gastrointestinal tract may have given an absorption profile that could be described with a Michaelis-Menten-type function. Such a passage of the drug along the gastrointestinal tract with variable permeability and windows for active transport could contribute to the atypical absorption profile of eflornithine.
A sensitive stereoselective assay for eflornithine is hampered by the lack of chromophores and fluorophores. Realizing that even at the high doses employed we would have some difficulty in separately quantitating the enantiomers in an adequate number of samples for a sufficient duration of time with the analytical method at hand, we chose to support the enantiomeric data by including rich racemic concentration-time data determined with separate groups of rats. Only by combining racemic and chiral data could the pharmacokinetic parameters for the eflornithine enantiomers be estimated. There were insufficient stereospecific data after oral administration to allow estimation of separate interindividual variability values for l- and d-eflornithine, and therefore they were set to be identical. After intravenous administration, enantiospecific interindividual variability could be estimated for CL only.
In conclusion, the stereoselective eflornithine pharmacokinetics after oral and intravenous single dosing in the rat were determined by an approach based on the simultaneous modeling of sparse enantiomeric combined with rich racemic drug concentration-time data. Eflornithine was found to exhibit enantioselective absorption after oral dosing, with the more potent l-isomer being less well absorbed. The stereospecific difference in absorption may explain why attempts to develop an oral treatment for eflornithine have so far failed.
Acknowledgments
We acknowledge Veronika Sjöstrand and Eva Albers for their early contributions to this study and Göran Oresten from Sorbent AB for donating SPE columns used in the chiral analysis. The provision of eflornithine by WHO/TDR is gratefully acknowledged.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Anonymous. 2006. Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly. Epidemiol. Rec. 81:71-80. [PubMed] [Google Scholar]
- 2.Anonymous. August 2006. African trypanosomiasis (sleeping sickness). World Health Organization, Geneva, Switzerland.
- 3.Bacchi, C. J., H. C. Nathan, S. H. Hutner, P. P. McCann, and A. Sjoerdsma. 1980. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science 210:332-334. [DOI] [PubMed] [Google Scholar]
- 4.Balasegaram, M., S. Harris, F. Checchi, S. Ghorashian, C. Hamel, and U. Karunakara. 2006. Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo. Bull. W. H. O. 84:783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett, M. P. 2006. The rise and fall of sleeping sickness. Lancet 367:1377-1378. [DOI] [PubMed] [Google Scholar]
- 6.Barrett, M. P., D. W. Boykin, R. Brun, and R. R. Tidwell. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 152:1155-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burri, C., and R. Brun. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90(Suppl. 1):S49-S52. [DOI] [PubMed] [Google Scholar]
- 8.Burri, C., and J. Keiser. 2001. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Trop. Med. Int. Health 6:412-420. [DOI] [PubMed] [Google Scholar]
- 9.Chiou, W. L., and A. Barve. 1998. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharm. Res. 15:1792-1795. [DOI] [PubMed] [Google Scholar]
- 10.Griffin, C. A., M. Slavik, S. C. Chien, J. Hermann, G. Thompson, O. Blanc, G. D. Luk, S. B. Baylin, and M. D. Abeloff. 1987. Phase I trial and pharmacokinetic study of intravenous and oral alpha-difluoromethylornithine. Investig. New Drugs 5:177-186. [DOI] [PubMed] [Google Scholar]
- 11.Grove, J., J. R. Fozard, and P. S. Mamont. 1981. Assay of alpha-difluoromethylornithine in body fluids and tissues by automatic amino-acid analysis. J. Chromatogr. 223:409-416. [DOI] [PubMed] [Google Scholar]
- 12.Haegele, K. D., R. G. Alken, J. Grove, P. J. Schechter, and J. Koch-Weser. 1981. Kinetics of alpha-difluoromethylornithine: an irreversible inhibitor of ornithine decarboxylase. Clin. Pharmacol. Ther. 30:210-217. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy, P. G. 2006. Human African trypanosomiasis: in and out of Africa. Neurology 66:962-963. [DOI] [PubMed] [Google Scholar]
- 14.Kibona, S. N., L. Matemba, J. S. Kaboya, and G. W. Lubega. 2006. Drug-resistance of Trypanosoma b. rhodesiense isolates from Tanzania. Trop. Med. Int. Health 11:144-155. [DOI] [PubMed] [Google Scholar]
- 15.Malm, M., and Y. Bergqvist. 2007. Determination of eflornithine enantiomers in plasma, by solid-phase extraction and liquid chromatography with evaporative light-scattering detection. J. Chromatogr. B 846:98-104. [DOI] [PubMed] [Google Scholar]
- 16.Na-Bangchang, K., F. Doua, J. Konsil, W. Hanpitakpong, B. Kamanikom, and F. Kuzoe. 2004. The pharmacokinetics of eflornithine (alpha-difluoromethylornithine) in patients with late-stage T.b. gambiense sleeping sickness. Eur. J. Clin. Pharmacol. 60:269-278. [DOI] [PubMed] [Google Scholar]
- 17.Osterberg, O., R. M. Savic, M. O. Karlsson, U. S. Simonsson, J. P. Norgaard, J. V. Walle, and H. Agerso. 2006. Pharmacokinetics of desmopressin administrated as an oral lyophilisate dosage form in children with primary nocturnal enuresis and healthy adults. J. Clin. Pharmacol. 46:1204-1211. [DOI] [PubMed] [Google Scholar]
- 18.Qu, N., N. A. Ignatenko, P. Yamauchi, D. E. Stringer, C. Levenson, P. Shannon, S. Perrin, and E. W. Gerner. 2003. Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine. Biochem. J. 375:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savic, R. M., D. M. Jonker, T. Kerbusch, and M. O. Karlsson. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 34:711-726. [DOI] [PubMed] [Google Scholar]
- 20.Smithers, J. 1988. A precolumn derivatization high-performance liquid chromatographic (HPLC) procedure for the quantitation of difluoromethylornithine in plasma. Pharm. Res. 5:684-686. [DOI] [PubMed] [Google Scholar]
- 21.Welburn, S. C., and M. Odiit. 2002. Recent developments in human African trypanosomiasis. Curr. Opin. Infect. Dis. 15:477-484. [DOI] [PubMed] [Google Scholar]