Abstract
The pharmacokinetics of miltefosine in leishmaniasis patients are, to a great extent, unknown. We examined and characterized the pharmacokinetics of miltefosine in a group of patients with Old World (Leishmania major) cutaneous leishmaniasis. Miltefosine plasma concentrations were determined in samples taken during and up to 5 months after the end of treatment from 31 Dutch military personnel who contracted cutaneous leishmaniasis in Afghanistan and were treated with 150 mg miltefosine/day for 28 days. Samples were analyzed with a validated liquid chromatography-tandem mass spectrometry assay with a lower limit of quantification (LLOQ) of 4 ng/ml. Population pharmacokinetic modeling was performed with nonlinear mixed-effect modeling, using NONMEM. The pharmacokinetics of miltefosine could best be described by an open two-compartment disposition model, with a first elimination half-life of 7.05 days and a terminal elimination half-life of 30.9 days. The median concentration in the last week of treatment (days 22 to 28) was 30,800 ng/ml. The maximum duration of follow-up was 202 days after the start of treatment. All analyzed samples contained a concentration above the LLOQ. Miltefosine is eliminated from the body much slower than previously thought and is therefore still detectable in human plasma samples taken 5 to 6 months after the end of treatment. The presence of subtherapeutic miltefosine concentrations in the blood beyond 5 months after treatment might contribute to the selection of resistant parasites, and moreover, the measures for preventing the teratogenic risks of miltefosine treatment should be reconsidered.
Leishmaniasis is an infectious disease caused by protozoan parasites of the genus Leishmania. The parasite is transmitted to and between humans and other mammals by female sand flies. Once inside the mammalian body, the Leishmania parasite multiplies within the macrophage and infects cells of different tissues, depending on the subspecies of Leishmania involved. The clinical manifestations of the leishmaniases are therefore diverse, are linked to the geographical distribution of the different subspecies, and can roughly be divided into cutaneous, mucocutaneous, and visceral leishmaniasis (11). The parasites responsible for cutaneous leishmaniasis in the Middle East and the Indian subcontinent are the Leishmania tropica and Leishmania major subspecies, also known as Old World parasites, which replicate in the skin tissue and cause ulcerative skin lesions.
Miltefosine (hexadecylphosphocholine; marketed by Zentaris GmbH as Impavido) is a new oral drug to treat leishmaniasis, with relatively high efficacy rates reported for treatment of New World cutaneous (21, 22), mucocutaneous (23), and visceral leishmaniasis (4, 5, 13, 27, 30). Only a little information has recently become available on miltefosine for treatment of Old World cutaneous leishmaniasis (17, 24). In vitro data showed that not all subspecies of the Leishmania parasite have the same sensitivity to miltefosine; L. major parasites were the least sensitive to miltefosine (10).
The pharmacokinetics of miltefosine are to a great extent unknown and scarcely published. The manufacturer provided some preclinical and clinical pharmacokinetic data in the registration documents of miltefosine, which were published in a review by Berman (2). Protein binding is approximately 95% in human plasma. Miltefosine is metabolized mainly by phospholipase D, releasing choline, choline-containing metabolites, and hexadecanol, which are likely to enter the intermediary metabolism. The terminal elimination half-life is reported to be 150 to 200 h in adults. Only <0.2% of the applied dose is excreted unchanged in the urine (20).
In relation to the recent increase of international military activity in Southwest and Central Asia (e.g., Iraq, Kuwait, and Afghanistan), Old World cutaneous leishmaniasis has become an increasing problem in returning military personnel (7, 32). During deployment in northern Afghanistan, a total of 172 Dutch military personnel and 3 civilians embedded with the armed forces were infected with L. major. Initial treatment consisted of intralesional antimony (SbV) injections, sometimes preceded by cryotherapy with liquid nitrogen. For second-line treatment of insufficient responses or for primary treatment of extensive disease, systemic treatment was indicated. Mainly due to the large number of patients, logistic problems, the difficulties in keeping the military patients in operational service when parenteral medication needed to be given, and potential side effects, it was decided that administration of systemic antimony was not an optimal choice. Therefore, miltefosine was offered as a systemic treatment, even though at that time there were few data on its efficacy for Old World cutaneous leishmaniasis.
It was difficult to extrapolate the dosage from the studies on miltefosine for visceral leishmaniasis in India, since dosage was dictated mainly by the upper limit of the tolerable dose and less by the pharmacokinetic properties of miltefosine. Previous empirical experience showed that a total daily dose of >150 mg may cause severe gastrointestinal side effects (30).
To reduce the paucity of pharmacokinetic data on miltefosine, our objective was to characterize and to describe the pharmacokinetics of miltefosine in our group of Old World cutaneous leishmaniasis patients, making use of our recently developed and validated sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for the quantification of miltefosine in human plasma samples (9).
MATERIALS AND METHODS
Study population.
Dutch patients with cutaneous leishmaniasis were presented at the Academic Medical Center (AMC), Amsterdam, The Netherlands. All Leishmania infections were contracted in northern Afghanistan and were confirmed by microscopy and/or positive PCR/nucleic acid sequence-based amplification (NASBA) from intralesional puncture biopsies (31). Patients were Dutch military personnel or embedded Dutch civilians quartered near Mazar-e-Sharif, Afghanistan, as part of the ISAF Election Support Force.
Patients with uncomplicated lesions received first-line treatment with weekly series of intralesional injections of pentavalent antimony (Pentostam) (100 mg SbV/ml; 0.1 to 0.3 ml; three times every other day), sometimes in combination with cryotherapy using liquid nitrogen. Patients with extensive disease, insufficient response to the intralesional therapy, or resurgence of the parasite after the intralesional therapy were treated with miltefosine. This treatment was always started in the AMC. Pregnancy and desire to have children were obviously exclusion criteria, since miltefosine is considered teratogenic (2). Patients were highly encouraged to use contraceptives.
Protocol.
This was an open-label, nonrandomized study. Patients were examined by a multidisciplinary team consisting of an infectious disease physician and a dermatologist, both specialized in tropical medicine. The patients were examined before treatment, on days 1, 2, and 3 of the first week of treatment, and then once every week of treatment. All patients were treated with oral miltefosine (Impavido; Zentaris GmbH, Frankfurt, Germany) at 50 mg three times daily, for a total of 28 days. Patients were advised to take the medication together with a meal or snack to prevent gastrointestinal side effects. After discontinuation of treatment, patients were repeatedly reexamined for up to 6 months after the end of treatment. Written informed consent was obtained from each patient before enrollment.
Sample collection and analysis.
On the first day of treatment (day 1), patients stayed in the AMC for 6 h after the first dose (taken at time zero), with the aim to obtain blood samples 2, 4, and 6 h after the first dose. Further blood samples were taken on an outpatient basis at several time points during treatment. After treatment, blood samples were taken irregularly until 5 months posttreatment, with the intention to take a blood sample every 2 to 4 weeks while allowing for variability in the number of samples taken per patient and the sampling time points.
Blood samples sufficient to provide a minimum of 4 ml plasma for the measurement of miltefosine concentrations were obtained by direct venipuncture, collected into EDTA tubes, and centrifuged at approximately 23,100 × g at 4°C for 15 min. The plasma fraction was transferred to another tube, and plasma samples were stored at −20°C or below until plasma drug concentrations were determined.
Plasma concentrations of miltefosine were determined by a recently developed LC-MS/MS assay (9). The assay consisted of a solid-phase extraction on Bond Elut PH cartridges (Varian Inc., Bergen op Zoom, The Netherlands) containing 100 mg sorbent, using 250 μl of a plasma sample. Cartridges were conditioned with 1 ml of acetonitrile and 1 ml of 0.9 M acetic acid in water (pH 4.5). After sample loading and washing with 50% (vol/vol) methanol in water, miltefosine was eluted with 0.1% (vol/vol) triethylamine in methanol.
The eluate containing the analyte was kept in autosampler vials at a temperature of 10°C and injected directly on a Gemini C18 column (150 mm by 2.0-mm inner diameter; 5-μm particle size) (Phenomenex, Torrance, CA) in combination with a guard column (Gemini C18 precolumn; 4.0 mm by 2.0-mm inner diameter) (Phenomenex), both of which were operated at ambient temperature. The analyte was eluted from the analytical column by using an isocratic elution with a mixture of 10 mM aqueous ammonium hydroxide and 10 mM ammonium hydroxide in methanol (at a ratio of 5:95), with a total run time of 7 min.
Detection was performed by MS/MS with electrospray ionization, using an API 2000 mass spectrometry system (Sciex, Thornhill, Ontario, Canada) with Analyst software (version 1.2). Miltefosine was monitored in the positive-ion mode, with the following transition of precursor ([MH]+) to product ion: m/z 408.4 to 124.8.
The quantifiable range of the assay was 4 to 2,000 ng/ml miltefosine in plasma. The assay was validated over this range according to FDA guidelines for the validation of bioanalytical assays (9). At the lowest level (4 ng/ml), the intra-assay precision was lower than 10.7%, the interassay precision was 10.6%, and accuracies were between 95.1 and 109%. At higher concentrations, the assay performed even better in terms of precision and accuracy (9).
Samples with a concentration above the upper limit of quantification were diluted in drug-free human control EDTA-plasma to fit the calibration curve. Along with study samples, a calibration curve was prepared and analyzed in duplicate, together with a set of quality control samples at low, mid, and high levels, prepared and analyzed in triplicate.
Pharmacokinetic data analysis.
Population pharmacokinetic modeling was performed with the nonlinear mixed-effects modeling program NONMEM, version VI (GloboMax LLC, Hanover, MD), using the first-order conditional estimation procedure with interaction between interindividual variability and residual error components.
The adequacy of the tested models was evaluated using statistical and graphical methods. The minimal value of the objective function (equal to minus twice the log likelihood) provided by NONMEM was used as a goodness-of-fit characteristic to discriminate between nested models, using the log likelihood ratio test. Standard errors for all parameters were calculated with the COVARIANCE option in NONMEM, and individual Bayesian pharmacokinetic parameters were obtained using the POSTHOC option (1). The R-based model building aid Xpose (version 4) and Perl speaks NONMEM (PsN) were used for graphical model evaluation (14, 16). Among others, plots of observed versus predicted concentrations and plots of conditional weighted residuals were used for graphical inspection of the goodness of fit. Conditional weighted residuals for model diagnosis were determined as described by Hooker et al. (12). Piraña (an interface to NONMEM, PsN, and our cluster) was used for run deployment and analysis (15).
Interindividual variability in the pharmacokinetic parameters was estimated with an exponential model. For instance, variability in clearance was described by the equation CL/Fi = θ1 × exp(ηi), where CL/Fi represents the clearance of the ith individual, θ1 is the typical value of clearance, and ηi is the interindividual random effect with a mean of 0 and a variance of ω2. Residual variability was modeled with a proportional error model.
Single and multicompartmental models, with and without first-order absorption and with linear elimination from the central compartment, were evaluated. Absorption rate (ka), clearance (elimination clearance [CL] or intercompartmental clearance [Q]), and volume of distribution (V) were the primary pharmacokinetic parameters estimated. Secondary parameters, such as elimination half-life, were estimated from these primary parameters. Bioavailability (F) was unknown, and therefore, parameters relative to the bioavailability were estimated (CL/F, V/F, etc.).
Using the final covariate model, a visual predictive check was performed by simulating 2,000 subjects to assess the predictive performance of the final model. The visual predictive check, including 90% confidence interval and median, was generated and visually assessed using an R script.
RESULTS
Patients.
Thirty-four patients were treated with miltefosine at the AMC on an outpatient basis. Information from three patients was eventually discarded from the data set because of inconsistent drug concentrations, which could be traced to logistic errors in labeling samples at the sampling site. In the final data set, 382 concentrations were present from 31 patients treated with miltefosine, and their baseline characteristics are described in Table 1. All but one of the patients were male, and all but three had received prior intralesional treatment with pentavalent antimony. Leishmaniasis was confirmed for all patients by microscopy and, in most cases (n = 27), also by positive PCR/NASBA. In all cases that were genotyped (n = 27), the L. major subspecies was found to be the causative parasite.
TABLE 1.
Baseline characteristics of patients treated with miltefosine and included in the pharmacokinetic analysis (n = 31)
| Parameter | Median value (interquartile range) |
|---|---|
| Age (yr) | 24 (23-29) |
| No. of males/no. of females | 30/1 |
| Weight (kg) | 85 (78-89) |
| Height (cm) | 184 (180-188) |
| Dose (mg/kg/day) | 1.76 (1.69-1.92) |
| No. of blood samples per patient | 13 (9-20a) |
| During treatment | 8 (6-13a) |
| After end of treatment | 5 (1-12a) |
| No. of patients with prior treatment with intralesional SbVb/no. of patients naïve to treatment | 30/1 |
Range.
100 mg SbV/ml, given as 0.1 to 0.3 ml three times every other day for 1 to 3 weeks.
The efficacy and toxicity of miltefosine in these patients will be reported in more detail elsewhere. In brief, adverse events included transient, mild-to-moderate gastrointestinal side effects (nausea and vomiting) and fatigue. Only mild elevations of alanine aminotransferase, aspartate aminotransferase, and creatinine (<2× the normal value) were noticed in a small subset of patients. None of these adverse events was a reason for discontinuation of the therapy.
Pharmacokinetic data analysis.
The median number of available samples per patient was 13 (range, 9 to 20), with a maximum duration of follow-up of 202 days. The median number of samples taken during treatment was 8 (range, 6 to 13), and after treatment, this number was 5 (range, 1 to 12). Miltefosine plasma concentrations ranged from 6.75 ng/ml to 51,600 ng/ml. The median concentration in the last week of treatment (days 22 to 28 after the start of treatment) was 30,800 ng/ml. We were able to quantify miltefosine concentrations in samples taken around 5 to 6 months after the end of treatment, and the median concentration in these samples (taken between days 178 and 202 after time zero) was 17.5 ng/ml (range, 6.75 to 27.6 ng/ml). All samples analyzed after the start of treatment contained a concentration of miltefosine above our lower limit of quantification (4 ng/ml).
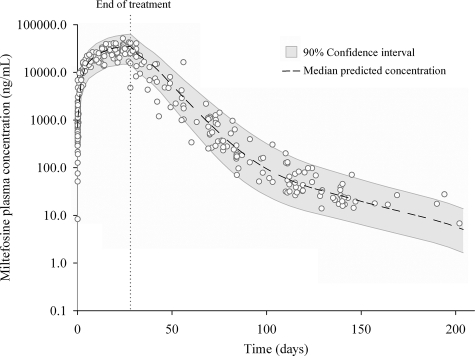
The observed miltefosine plasma concentration-versus-time data are shown in Fig. 1, together with a visual predictive check of the model. From this figure, it already follows that the elimination of miltefosine from the body could not be described by a log-linear function as previously supposed. Disposition was best described by the addition of a second compartment resulting in a very long second and terminal elimination half-life of 30.9 days.
FIG. 1.
Visual predictive check of population pharmacokinetic model for miltefosine. The open dots present data for all analyzed study samples (n = 382) from 31 Dutch military personnel with cutaneous leishmaniasis (L. major) contracted in Afghanistan. All patients were treated with 50 mg of oral miltefosine three times daily for a total of 28 days. The gray area shows the 90% confidence interval of the model predictions; the dashed line displays the median predicted concentrations.
An open two-compartmental model with first-order absorption and linear elimination from the central compartment best fitted the data. Table 2 shows the final parameter estimates. The data contained insufficient information to estimate interindividual variability for intercompartmental clearance and the volume of the peripheral compartment. The high correlation between the interindividual variability for CL and V2 can probably be attributed to variability in bioavailability or in the unbound drug fraction.
TABLE 2.
Final parameter estimates for pharmacokinetic model for miltefosine
| Parameter | Estimate (relative SE [%]) | % Interindividual variability (relative SE [%])b |
|---|---|---|
| Absorption rate (ka) (h−1) | 0.36 (10.1) | 24.2 (63.3) |
| Clearance (CL/F) (liters/day) | 3.87 (5.3) | 23.2a (15.4) |
| Volume of central compartment (V2/F) (liters) | 39.6 (4.0) | 18.3a (25.0) |
| Intercompartmental clearance (Q/F) (liters/day) | 0.0375 (22.0) | NE |
| Volume of peripheral compartment (V3/F) (liters) | 1.65 (12.4) | NE |
| Residual variability (%) | 31.5 (6.4) | 23.2 (37.8) |
Interindividual variabilities in CL and V1 were correlated, with a correlation coefficient of 0.83 (relative SE, 23.1%).
NE, not estimated.
Interindividual variability was only modest (∼24%), which is also illustrated in Fig. 1. From the final parameter estimates, the elimination half-lives were calculated. The first half-life was estimated to be 7.05 days (range of individual estimates, 5.45 to 9.10 days), while the terminal half-life was estimated to be 30.9 days (range of individual estimates, 30.8 to 31.2 days).
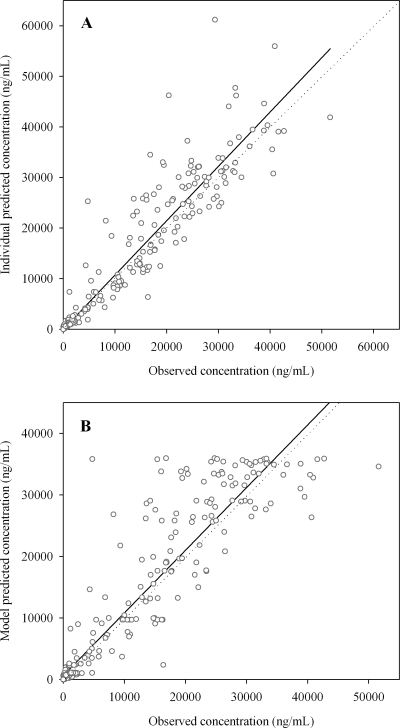
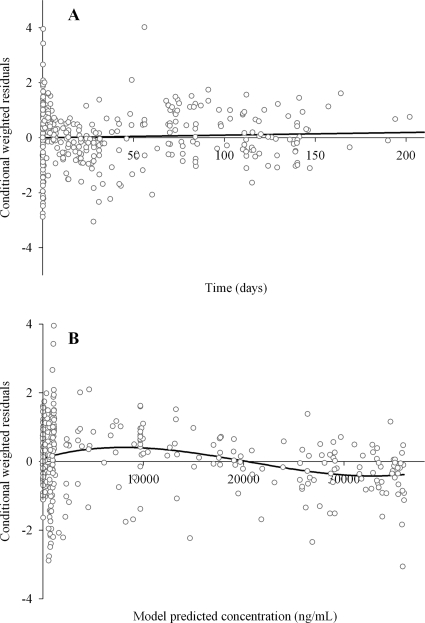
Figure 2 shows the observed concentrations versus the individual and model predicted concentrations. Figure 3 shows the conditional weighted residuals versus time and versus model predicted concentrations. No obvious trends were recognizable from these figures, indicating that the model adequately describes the pharmacokinetic profile of miltefosine.
FIG. 2.
Observed concentrations versus individual (A) and model (B) predicted concentrations. The straight lines represent linear regression lines, while dotted lines represent lines of unity.
FIG. 3.
Conditional weighted residuals versus time (A) and versus model predicted concentrations (B).
DISCUSSION
The data presented in this study provide the first extensive pharmacokinetic data on miltefosine in (cutaneous) leishmaniasis patients and show that the elimination of miltefosine from the body is best described by a two-compartmental disposition model. This is a contribution to the few, anecdotal published data that are available on the clinical pharmacokinetics of miltefosine. The already available data report a single elimination half-life of 150 to 200 h (∼6 to 8 days) for adults (2) and an expected similar value for children (26), although in a previous case report from our group, a much longer value of 14.8 days was calculated for a one-compartmental disposition model (8). Daily administration of 100 mg/day (∼2.5 mg/kg of body weight/day for 28 days) resulted in a mean maximum concentration of drug in serum at day 23 of treatment of 70,000 ng/ml (2). The median minimum concentration of drug in serum determined on days 26 to 28 of treatment for children who received 2.5 mg/kg of body weight/day for 28 days was 26,000 ng/ml (26).
The results of this study show that miltefosine keeps accumulating during a 4-week treatment course and that concentrations observed in the last week of treatment are comparable to the values cited above, although our patients received a lower dose, with a median of 1.76 mg/kg of body weight/day for 28 days. Consistent with the data provided by the manufacturer and as published in a review by Berman (2), we estimated a first elimination half-life of 7.05 days. However, plasma concentrations of miltefosine measured in this study were best described by a two-compartmental model. Besides this first elimination half-life, an even longer terminal elimination half-life of 30.9 days was found. We were able to observe and estimate this terminal elimination half-life because of the long follow-up and sampling of our patients. This also explains why we previously calculated an elimination half-life (14.8 days) between these two values with a one-compartmental disposition model in a single case study (8). Due to this extremely long terminal elimination half-life, concentrations of miltefosine were still above the lower limit of quantification (4 ng/ml) in samples taken 5 to 6 months after the end of treatment. It cannot be excluded that the current terminal elimination half-life estimate is actually an underestimate or that there is another, even slower, terminal elimination. This is corroborated by Fig. 1, as the 90% confidence interval and the median predicted concentration of the model do not completely follow the observed concentrations at the end of the period of follow-up, probably because of the more limited sampling in this period.
The findings of our study have implications for both the efficacy and toxicity of miltefosine. Resistance of Leishmania parasites to other chemotherapeutics has been reported; for example, resistance to pentavalent antimony has been reported in India, where Bihar, a region of endemicity, is especially highly affected, with treatment failure rates as high as 65% (25, 28). As a result of these reported therapy failures, there is also much concern about the induction of resistance to miltefosine or any other new drug to treat leishmaniasis. In vitro studies showed that this concern is not unfounded: Leishmania promastigotes resistant to miltefosine were easily generated when parasites were cultured in medium containing miltefosine at concentrations of up to 40 μM (∼16,300 ng/ml), and these resistant promastigotes showed up to 15-fold higher 50% effective dose values for miltefosine than those for wild-type organisms (19).
The previously established long elimination half-life of 7 days invariably results in subtherapeutic levels of miltefosine in the blood for some weeks after ending the therapy, a property that was thought to contribute to the emergence of resistance (3, 6, 18, 19). This study shows that miltefosine has a much longer terminal elimination half-life than was expected. Subtherapeutic concentrations remain in the blood for up to 5 months posttreatment, and possibly even longer. This corroborates the concern for selection of miltefosine-resistant parasites.
Other factors contributing to the emergence of resistance are the increase of human immunodeficiency virus coinfections, which prevent complete eradication of Leishmania parasites from the body so that more viable or relapsing parasites will be exposed to subtherapeutic drug levels (3), and also the deregulation of the sale of miltefosine by the Indian government may enhance inappropriate and suboptimal use of miltefosine (18, 29). Miltefosine is now used as a monotherapy and is widely available over the counter without prescription or restriction on the quantity dispensed, which highly increases the risk of selection of miltefosine-resistant parasites.
The clinical pharmacodynamic properties of miltefosine are ill defined, and the lower limit of the therapeutic range of miltefosine is unknown. The relative ease with which resistant parasites were generated in vitro and the long duration of treatment that is required to achieve complete cure indicate that the therapeutic range of miltefosine is quite narrow. From a pharmacokinetic viewpoint, the thrice-daily dosing of miltefosine (50 mg three times daily) which was used in our patients does not seem sensible because of the extremely slow elimination and strong accumulation of miltefosine, as shown in this study. However, the upper limit of dosing is determined mainly by gastrointestinal side effects directly after oral administration, and thus, toxicity determines the maximum dosage regimen.
In reproductive studies with rats and rabbits, embryotoxic, fetotoxic, and teratogenic effects were seen following multiple doses of miltefosine at a dose level of 1.2 mg/kg, which is still below the therapeutic dose in these animals (2, 20). Nevertheless, more extensive published data on the teratogenicity of miltefosine are lacking. The levels for teratogenicity of miltefosine in humans remain undetermined, since pregnant women were not included in controlled studies with miltefosine. Use of miltefosine is therefore strictly contraindicated during pregnancy, and the use of contraception is compulsory in women of child-bearing age. Based on the previously reported terminal elimination half-life of 7 days, a period of 2 months after the end of treatment was calculated to be required for plasma concentrations to drop below the undefined no-effect level for teratogenicity (20). To account for worst-case scenarios, recommendations were that reproductive contraception should be continued in females with child-bearing potential for about eight half-lives (2 to 3 months) after the treatment period (18). In the present study, we observed that all blood samples collected 5 months after treatment still contained miltefosine concentrations well above our lower limit of quantification, and taking into account that the maximum nonteratogenic concentration is uncertain, the previous recommendations should be reconsidered. Based on the results presented here, the mandatory recommendation for women of child-bearing age to use reproductive contraception should be extended to at least 5 months or even longer after the end of the miltefosine treatment.
In conclusion, we present the first extensive pharmacokinetic data on miltefosine in a group of cutaneous leishmaniasis patients. We observed an extremely long second and terminal elimination half-life of 30.9 days, which was estimated from our two-compartmental population pharmacokinetic model. We were still able to detect miltefosine in samples taken at 5 months posttreatment. This may have consequences for the selection of resistant parasites and is a plea to extend current recommendations for contraception to at least 5 months after discontinuation of miltefosine treatment.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Beal, S. L., and L. B. Sheiner. 1999. NONMEM user's guides. University of California, San Francisco.
- 2.Berman, J. 2005. Miltefosine to treat leishmaniasis. Expert Opin. Pharmacother. 6:1381-1388. [DOI] [PubMed] [Google Scholar]
- 3.Berman, J., A. D. M. Bryceson, S. Croft, J. Engel, W. Gutteridge, J. Karbwang, H. Sindermann, J. Soto, S. Sundar, and J. A. Urbina. 2006. Miltefosine: issues to be addressed in the future. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S41-S44. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya, S. K., T. K. Jha, S. Sundar, C. P. Thakur, J. Engel, H. Sindermann, K. Junge, J. Karbwang, A. D. Bryceson, and J. D. Berman. 2004. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin. Infect. Dis. 38:217-221. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya, S. K., P. K. Sinha, S. Sundar, C. P. Thakur, T. K. Jha, K. Pandey, V. R. Das, N. Kumar, C. Lal, N. Verma, V. P. Singh, A. Ranjan, R. B. Verma, G. Anders, H. Sindermann, and N. K. Ganguly. 2007. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J. Infect. Dis. 196:591-598. [DOI] [PubMed] [Google Scholar]
- 6.Bryceson, A. 2001. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop. Med. Int. Health 6:928-934. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). 2004. Update: cutaneous leishmaniasis in U.S. military personnel—Southwest/Central Asia, 2002-2004. Morb. Mortal. Wkly. Rep. 53:264-265. [PubMed] [Google Scholar]
- 8.de Vries, P. J., W. F. van der Meide, M. H. Godfried, H. D. F. H. Schallig, H. J. Dinant, and W. R. Faber. 2006. Quantification of the response to miltefosine treatment for visceral leishmaniasis by QT-NASBA. Trans. R. Soc. Trop. Med. Hyg. 100:1183-1186. [DOI] [PubMed] [Google Scholar]
- 9.Dorlo, T. P. C., M. J. X. Hillebrand, H. Rosing, T. A. Eggelte, P. J. de Vries, and J. H. Beijnen. 2008. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 865:55-62. [DOI] [PubMed] [Google Scholar]
- 10.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 11.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 12.Hooker, A. C., C. E. Staatz, and M. O. Karlsson. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm. Res. 24:2187-2197. [DOI] [PubMed] [Google Scholar]
- 13.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 15.Keizer, R. J. 2008. Piraña 1.0 RC1. http://sourceforge.net/projects/pirana/.
- 16.Lindbom, L., J. Ribbing, and E. N. Jonsson. 2004. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 75:85-94. [DOI] [PubMed] [Google Scholar]
- 17.Mohebali, M., A. Fotouhi, B. Hooshmand, Z. Zarei, B. Akhoundi, A. Rahnema, A. R. Razaghian, M. J. Kabir, and A. Nadim. 2007. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 103:33-40. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Victoria, F. J., M. P. Sanchez-Canete, K. Seifert, S. L. Croft, S. Sundar, S. Castanys, and F. Gamarro. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Updat. 9:26-39. [DOI] [PubMed] [Google Scholar]
- 19.Seifert, K., S. Matu, F. Javier Perez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 20.Sindermann, H., and J. Engel. 2006. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S17-S20. [DOI] [PubMed] [Google Scholar]
- 21.Soto, J., B. A. Arana, J. Toledo, N. Rizzo, J. C. Vega, A. Diaz, M. Luz, P. Gutierrez, M. Arboleda, J. D. Berman, K. Junge, J. Engel, and H. Sindermann. 2004. Miltefosine for new world cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266-1272. [DOI] [PubMed] [Google Scholar]
- 22.Soto, J., J. Toledo, P. Gutierrez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:E57-E61. [DOI] [PubMed] [Google Scholar]
- 23.Soto, J., J. Toledo, L. Valda, M. Balderrama, I. Rea, R. Parra, J. Ardiles, P. Soto, A. Gomez, F. Molleda, C. Fuentelsaz, G. Anders, H. Sindermann, J. Engel, and J. Berman. 2007. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin. Infect. Dis. 44:350-356. [DOI] [PubMed] [Google Scholar]
- 24.Stojkovic, M., T. Junghanss, E. Krause, and R. N. Davidson. 2007. First case of typical Old World cutaneous leishmaniasis treated with miltefosine. Int. J. Dermatol. 46:385-387. [DOI] [PubMed] [Google Scholar]
- 25.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 26.Sundar, S., T. K. Jha, H. Sindermann, K. Junge, P. Bachmann, and J. Berman. 2003. Oral miltefosine treatment in children with mild to moderate Indian visceral leishmaniasis. Pediatr. Infect. Dis. J. 22:434-438. [DOI] [PubMed] [Google Scholar]
- 27.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 28.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104-1107. [DOI] [PubMed] [Google Scholar]
- 29.Sundar, S., and H. W. Murray. 2005. Availability of miltefosine for the treatment of kala-azar in India. Bull. W.H.O. 83:394-395. [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar, S., F. Rosenkaimer, M. K. Makharia, A. K. Goyal, A. K. Mandal, A. Voss, P. Hilgard, and H. W. Murray. 1998. Trial of oral miltefosine for visceral leishmaniasis. Lancet 352:1821-1823. [DOI] [PubMed] [Google Scholar]
- 31.van der Meide, W. F., G. J. Schoone, W. R. Faber, J. E. Zeegelaar, H. J. C. de Vries, Y. Ozbel, A. F. Lai, L. I. A. R. Coelho, M. Kassi, and H. D. F. H. Schallig. 2005. Quantitative nucleic acid sequence-based assay as a new molecular tool for detection and quantification of Leishmania parasites in skin biopsy samples. J. Clin. Microbiol. 43:5560-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weina, P. J., R. C. Neafie, G. Wortmann, M. Polhemus, and N. E. Aronson. 2004. Old World leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin. Infect. Dis. 39:1674-1680. [DOI] [PubMed] [Google Scholar]