Abstract
The objective of the present study was to test the hypothesis that treatment of schistosomiasis mansoni with praziquantel can alter significantly the immune response of patients and generate a reversal of the level of fibrosis. Peripheral blood mononuclear cell (PBMC) samples were collected from, and abdominal ultrasound examinations conducted on, volunteers infected with Schistosoma mansoni and living in an area where the disease is endemic, both prior to and one year after treatment with praziquantel. Subjects were classified into groups according to the level of pathology (i.e., absent, incipient, moderate, or severe fibrosis). PBMCs were stimulated with schistosome soluble egg antigens (SEA), and the levels of production of the cytokines gamma interferon (IFN-γ), tumor necrosis factor alpha, transforming growth factor β, and interleukin-4 (IL-4), IL-10, and IL-13 were determined. The chemotherapy was effective in reducing morbidity, particularly for individuals presenting with severe fibrosis. When levels of cytokine production in posttreatment PBMC cultures stimulated by SEA were categorized as low or high, significant differences in the distribution of IL-13 levels between groups presenting with or not presenting with fibrosis were established. Comparison of pre- and posttreatment SEA-induced cytokine levels in individuals who had experienced no change in the grade of fibrosis following chemotherapy revealed that the level of IFN-γ decreased in subjects with fibrosis whereas that of IL-10 decreased in individuals with and without fibrosis. The data suggest that chemotherapy is effective in reducing the morbidity of the disease and that the level of IL-13 may be a useful indicator of the persistence of fibrosis following treatment.
Schistosomiasis mansoni has a wide geographical distribution in Africa, South America, and the Caribbean. The severity of the disease results from fibrosis caused by granulomatous reactions around the eggs of Schistosoma mansoni that are trapped in the small vessels of the liver (10, 46). Within areas where the disease is highly endemic, most of the infected subjects control the infection with only minor pathological manifestations. However, in a small percentage of infected individuals, extended periportal fibrosis leads to portal hypertension, varices or ascites, the incidence of which has been found to correlate with the age and gender of the subject and the intensity of infection (2, 5, 7).
The formation of granuloma in the proximity of schistosome eggs is mediated by T lymphocytes (47), and several studies have furnished insights into the cytokine cascade that controls the development of these lesions (7, 10, 45). We have recently evaluated the levels of production of the cytokines gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α) and TGF-β, and interleukin-4 (IL-4), IL-10, and IL-13 following in vitro stimulation with soluble egg antigens (SEA) or soluble adult worm antigens of peripheral blood mononuclear cells (PBMC) from individuals of different ages and sexes and with various intensities of infection and degrees of fibrosis (2). Only IL-13 was strongly associated with fibrosis, while high levels of TGF-β appeared to be related to protection against fibrosis, even though the strength of this association was low.
Chemotherapy with the pyrazinoisoquinoline praziquantel has been demonstrated to be effective in decreasing morbidity in schistosomiasis (19) and is currently the most widely accepted method for the control of the disease. The drug is safe and has a high efficacy against both trematodes and cestodes (9, 11). Furthermore, there is evidence that treatment with praziquantel induces a change in the immune response that provides partial protection against schistosome infection, thus providing benefits that may extend beyond a transient reduction in levels of infection (21, 40, 42). Additionally, although the cumulative fibrosis that occurs during infection is mostly irreversible, periportal fibrosis regresses following eradication of the infection by chemotherapy (16, 44, 48).
The objective of the present study was to test the hypothesis that treatment of schistosomiasis with praziquantel can alter significantly the immune response of patients and generate a reversal of the level of Symmers’ fibrosis. The direct evaluation of the intensity of hepatic fibrosis in large cohorts may be carried out by abdominal ultrasound examination, a technique that is noninvasive, safe, reliable, and relatively inexpensive. Moreover, there are several criteria available for grading the severity of the disease based on the determination of liver and spleen parameters (1, 12, 32, 34, 36). In the present study we evaluated the morbidity (by ultrasound analysis) and the cellular immune response of schistosomiasis patients before and one year after treatment with praziquantel. (Part of the data was presented at the 13th International Congress of Immunology, 21 to 25 August 2007, Rio de Janeiro, Brazil.)
MATERIALS AND METHODS
The protocols involving human subjects were approved by the Ethical Committees of the Centro de Pesquisas René Rachou, Fiocruz, and Universidade Vale do Rio Doce, and the project was conducted in full accordance with all accepted guidelines. Volunteers were enrolled into the study only after informed consent had been obtained from each individual or legal guardian.
Study area and population.
The study was conducted in the village of Virgem das Graças, which is located in the Jequitinhonha Valley in northeastern Minas Gerais, Brazil. The unique characteristics of this region, in which schistosomiasis is highly endemic, have been described previously (2). A total of 91 individuals presenting infection with S. mansoni agreed to undergo clinical and ultrasound examinations and to donate a sample of blood prior to, and one year after, receiving chemotherapy. Following an initial examination, all subjects were treated with praziquantel at the standard dose of 50 mg/kg body weight. Fecal examinations were repeated 2 months after the initial medication, and individuals who remained positive for schistosomiasis received further treatment with praziquantel.
Exposure risk.
Using a combination of direct observations and household surveys, information on the exposure risk of participants was collected in the form of data for three exposure parameters, namely, frequency, duration, and intensity of contact with water in which Biomphalaria glabrata had been found (i.e., streams, canals, ponds, and unprotected springs) (31). The values of these exposure parameters were used to calculate a water exposure index (in total body minutes) as described by Gazzinelli et al. (20).
Parasitological examinations.
Infection with S. mansoni was established from the presence of eggs in fecal samples, obtained from subjects prior to and one year after chemotherapy, as determined by the Kato-Katz stool method (27).
Preparation of antigens.
Eggs of S mansoni were isolated from the livers of mice that had been exposed to cercaria 8 weeks previously and were ground in cold phosphate-buffered saline (PBS). The resulting homogenate was ultracentrifuged at 100,000 × g for 1 h at 4°C, and the clear supernatant fluid containing the SEA was stored at −70°C until required for use (8). Adult S. mansoni worms (males and females) were suspended in ice-cold PBS and sonicated over ice twice for 10 min each time.
Clinical examinations.
Clinical and ultrasound examinations of the 91 volunteers prior to and one year after the administration of chemotherapy with praziquantel were performed by different physicians. In each case, the physician who performed the ultrasound examination was not aware of the infection status of the subject or of the results of the clinical examination. It is important to mention that all measurements performed using ultrasound, both before and after treatment, were made by the same expert to avoid the potential for the introduction of inaccuracies from the use of different standards of assessment by different persons in performing the examinations. Liver size, portal vein diameter, thickness of the walls of the central portal branches, spleen size, and splenic and mesenteric vein diameters were assessed using a conventional portable diagnostic ultrasound instrument (model EUB-200; Hitachi, Tokyo, Japan). Hepatic fibrosis was evaluated by assessing the central and peripheral echogenic periportal thickness of the liver portal system according to the classification system of Abdel-Wahab et al. (1) and Magalhães et al. (34) with some modifications. Individuals assessed as belonging to group 0 did not present with any clinical or ultrasound evidence of hepatic fibrosis or other alterations in the liver or spleen (periportal thickness < 3 mm [grade 0]). Group 1 individuals presented with incipient periportal fibrosis (periportal thickness of ≥3 to ≤5 mm [grade 1]). Group 2 individuals presented with moderate fibrosis (periportal thickness of >5 to ≤7 mm [grade 2]). Group 3 individuals presented with severe fibrosis (periportal thickness > 7 mm [grade 3]).
Preparation and stimulation of cultures of PBMC.
Human PBMC were purified from heparinized venous blood samples by sedimentation at 400 × g for 45 min at 18°C in a density gradient using Ficoll-Hypaque separation (LMS Litton Biometries, Kensington, MD). After separation, the cells were washed in RPMI 1640 and cultivated in the same medium supplemented with 5% AB Rh-positive heat-inactivated human serum (Sigma, St. Louis, MO) containing 3% antibiotic-antimycotic solution (Gibco-BRL, Grand Island, NY) (the stock mix contained 10,000 U of penicillin, 10 mg of streptomycin, and 25 μg of amphotericin B [Fungizone] per ml) in the presence of soluble antigen at a final concentration of 25 μg/ml. PBMC were incubated for 144 h at 37°C in an atmosphere containing 5% carbon dioxide, after which the supernatant was collected, immediately frozen, and stored at −70°C until required for cytokine assay.
Determination of cytokine concentrations.
The concentrations of human cytokines were determined with the aid of enzyme-linked immunosorbent assay-based kits (Pharmingen, San Diego, CA) used according to the manufacturer's instructions. Briefly, for each cytokine, flat-bottomed plates (Immunolon 4; Dynateeh, Chantilly, VA) were coated overnight at 4°C with the appropriate antibody diluted in carbonate-bicarbonate coating buffer (pH 9.5). Coated plates were washed with PBS (pH 7.2) containing 0.05% Tween 20 (Sigma) (the same solution was used for rinsing in all subsequent washes) and blocked with 10% fetal calf serum-PBS for 1 h at room temperature. Samples or standards were then added to the wells, and the plates were incubated overnight at 4°C, washed, and incubated for 1 h in the presence of horseradish peroxidase-conjugated anti-human cytokine antibodies. Finally, 50 μl of a substrate-o-phenylenediamine (Sigma) solution containing 0.03% hydrogen peroxidase was added to each well and the optical density of the reaction mixture determined at 450 nm using an automated enzyme-linked immunosorbent assay reader (Molecular Devices, Sunnyvale, CA). Concentrations of cytokines (in picograms per milliliter) in the samples were determined by interpolation from a standard calibration curve set up for each plate using appropriate amounts of recombinant human cytokines.
Data analyses.
The data set was organized using EpiInfo (Centers for Disease Control and Prevention, Atlanta, GA) software version 6.04d, and statistical analyses were performed with the aid of SPSS Inc. (Chicago, IL) software version 11.5. Chi-square (χ2) and nonparametric (Kruskal-Wallis and Mann-Whitney) tests with univariate analyses were employed for categorical and continuous variables, respectively. The differences in cytokine levels in groups of patients presenting and not presenting with fibrosis, prior to and one year after treatment, were determined using the Wilcoxon signed-rank test.
RESULTS
Study population.
The 91 individuals included in the study comprised 48 (52.8%) females and 43 (47.2%) males, with ages in the range of 14 to 85 years (mean, 50.2 ± 17.4 years; median, 53 years).
Pre- and posttreatment ultrasound analyses.
One year after treatment, a statistically significant reduction of the mean values for longitudinal and anteroposterior measurements of liver (left and right lobes) as well as of diameters of portal and splenic veins was observed. In contrast, the spleen measurements were augmented significantly (Table 1). In addition, a total reversion of severe morbidity and a considerable reduction in moderate fibrosis were observed one year after treatment; hence, the numbers of individuals with nondetectable fibrosis and those with incipient fibrosis increased (Fig. 1). The changes in the levels of fibrosis may be summarized as follows: of the 7 patients with grade 3 fibrosis, 1 reverted to grade 0, 1 to grade 2, and 5 to grade 1; of the 15 individuals with grade 2 fibrosis, 7 reverted to grade 0, 6 reverted to grade 1, and 2 did not show any change; of the 22 volunteers with grade 1 fibrosis, 6 reverted to group 0, 15 did not show any change, and 1 moved to grade 2; and of the 47 subjects with grade 0 fibrosis, 44 did not show any change and 3 moved to grade 1. Thus, one year after treatment with praziquantel, 26 (29%) individuals reverted to a lower degree of fibrosis, 4 (4%) experienced an increase in fibrosis, and 61 (67%) did not experience any change. Interestingly, the proportion of individuals with pathology (grade 2 or 3) decreased from 24% prior to treatment to 4% after treatment (P < 0.001).
TABLE 1.
Ultrasound measurements for 91 patients before and one year after specific chemotherapy, Virgem das Graças, Brazil
| Ultrasound parametera | Value before treatment | Value 1 yr after treatment | P valueb |
|---|---|---|---|
| No. of subjects | 91 | 91 | |
| Liver right lobe | |||
| Lon (cm) | 10.35 ± 2.06 | 9.65 ± 1.23 | 0.0023 |
| AP (cm) | 9.51 ± 1.90 | 6.82 ± 0.99 | <0.0001 |
| Liver left lobe | |||
| Lon (cm) | 8.09 ± 1.96 | 7.28 ± 1.08 | <0.0001 |
| AP (cm) | 6.29 ± 1.63 | 5.52 ± 0.93 | 0.0002 |
| Portal vein (diam in cm) | 1.08 ± 0.21 | 0.98 ± 0.16 | 0.0027 |
| Splenic vein (diam in cm) | 0.72 ± 0.16 | 0.67 ± 0.10 | 0.0259 |
| Spleen | |||
| Lon (cm) | 8.09 ± 1.63 | 8.66 ± 1.57 | 0.0055 |
| AP (cm) | 4.71 ± 1.71 | 5.34 ± 0.81 | 0.0054 |
Lon, longitudinal measurement; AP, anteroposterior measurement.
Significance levels based on Mann-Whitney test results.
FIG. 1.
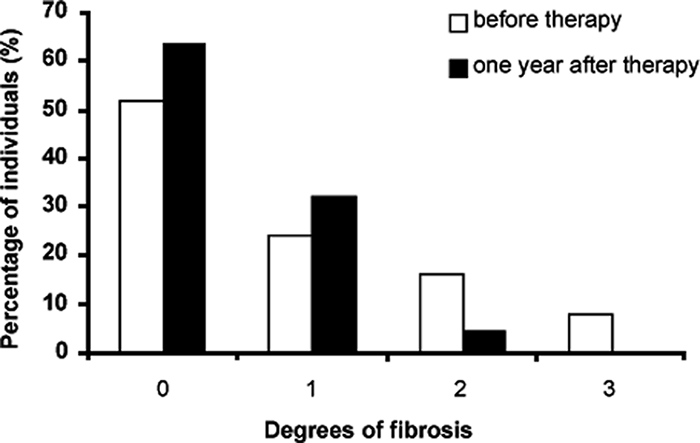
Percentages of the 91 individuals with Schistosoma mansoni infection distributed according to the degree of periportal fibrosis prior to and one year after chemotherapy.
Table 2 shows the pretreatment profiles of individuals who reverted to a lower degree of periportal fibrosis one year after treatment and of those who did not so revert. Most (15/17) of the individuals in the nonreverting group initially exhibited grade 1 fibrosis, while those in the reverting group (20/26) presented fibroses classified as grade 2 or 3 (P < 0.001). The mean age of individuals who had reverted was higher than that of individuals who had remained at the same grade of fibrosis after treatment (P = 0.010). Additionally, the group of individuals who had not reverted presented with a higher intensity of infection than the reverting group (P = 0.037). Although the prevalences of infection in both groups did not differ in relation to gender, the intensity of infection in females in the reverting group was 13 times lower than in males, while in the nonreverting group, females presented with an intensity of infection 4 times higher than that seen with males.
TABLE 2.
Profiles of individuals not reverting to periportal fibrosis and of those reverting to a nondetectable or lower level of periportal fibrosis
| Parameter | Reverting subjects | Nonreverting subjects | P valuea |
|---|---|---|---|
| Total no. of subjects | 26 | 17 | |
| Mean age ± SD | 57 ± 13 | 43 ± 17 | 0.010 |
| Mean eggb count ± SD | 40 ± 89 | 213 ± 345 | 0.037 |
| Prevalence of infection | 42% | 65% | 0.151 |
| No. (%) of females | 10 (38) | 9 (53) | 0.350 |
| No. (%) of males | 16 (62) | 8 (47) | |
| Mean eggb count (females) ± SD | 5 ± 9 | 322 ± 450 | 0.057 |
| Mean eggb count (males) ± SD | 63 ± 109 | 90 ± 88 | 0.452 |
Significance levels based on χ2 test (categorical variables) or Mann-Whitney tests (continuous variables). The minimum accepted significance level was P < 0.05.
S. mansoni eggs.
Posttreatment demographic and infection data.
Information concerning patient characteristics such as age, gender, water contact time (in total body minutes), level of infection, and the presence or absence of other parasitic diseases distributed according to the degree of hepatic fibrosis (classified into three groups determined by posttreatment ultrasound measurements) that was collected one year after treatment is provided in Table 3. There were no statistically significant differences between the three groups with respect to any of the measured parameters. A total of 28 individuals (31%) exhibited coinfection by various parasites, including Ascaris lumbricoides, Ancylostoma duodenale, Trichuris trichiura, Enterobius vermicularis, and species of Taenia. Nine (9.9%) participants remained positive for the presence of eggs of S. mansoni, and their infection levels ranged from 4 to 184 eggs/g of feces.
TABLE 3.
Demographic data, parasite infection data, and durations of water contact time of S. mansoni-infected patients with different degrees of hepatic fibrosis determined one year after treatment with praziquantel
| Parameter | Severity of fibrosis
|
P valuea | ||
|---|---|---|---|---|
| Group 0 (absent) | Group 1 (incipient) | Group 2 (moderate) | ||
| No. (%) of subjects | 58 (63.7%) | 29 (31.9%) | 4 (4.4%) | |
| Age (yr) | ||||
| Range | 15-85 | 14-85 | 54-63 | |
| Mean ± SD | 50.4 ± 18.1 | 48.5 ± 17.2 | 59.0 ± 4.2 | |
| Median | 52.5 | 51 | 59.5 | 0.405 |
| Gender | ||||
| No. (%) of females | 34 (59%) | 12 (41%) | 2 (50%) | 0.314 |
| No. (%) of males | 24 (41%) | 17 (59%) | 2 (50%) | |
| Eggb counts (no. of eggs/g of feces) | ||||
| Range | 0-184 | 0-12 | 0 | |
| Mean ± SD | 7.7 ± 30.9 | 0.4 ± 2.2 | 0 ± 0 | |
| Median | 0 | 0 | 0 | 0.241 |
| Eggb distribution | ||||
| No. (%) of negative results | 50 (86%) | 28 (97%) | 4 (100%) | 0.249 |
| No. (%) of positive results | 8 (14%) | 1 (3%) | 0 (0%) | |
| Other parasitic infectionsc | ||||
| No. (%) of negative results | 38 (65%) | 22 (76%) | 3 (75) | 0.596 |
| No. (%) of positive results | 20 (35%) | 7 (24%) | 1 (25) | |
| Water contact TBMd in no. of total body min (no. of subjects) | 153 (57) | 154 (29) | 138 (4) | 0.917 |
Significance levels based on χ2 test (categorical variables) or Kruskal-Wallis tests (continuous variables). The minimum accepted significance level was P < 0.05.
S. mansoni eggs.
Infections of Ascaris lumbricoides, Ancylostoma duodenale, Trichuris trichiura, Enterobius vermicularis, and species of Taenia.
TBM, total body minutes.
Levels of cytokines produced following stimulation of PBMC with SEA.
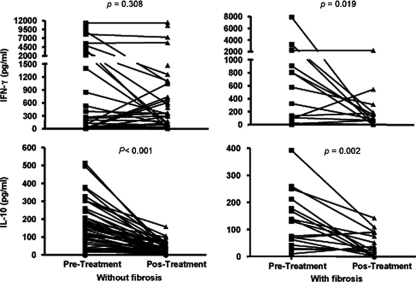
The levels of the cytokines IFN-γ, IL-10, IL-4, TGF-β, TNF-α, and IL-13 were determined in supernatants collected from SEA-stimulated cultures of posttreatment PBMC samples obtained from all members of the study population. When distributed according to the degree of hepatic fibrosis (classified into three groups as determined by posttreatment ultrasound measurements), no statistically significant differences in levels of cytokines could be detected. However, when the levels of these cytokines were categorized as low or high (on the basis of the median value of each cytokine titer for 91 patients) for individuals not presenting (group 0) or presenting (groups 1 and 2) with fibrosis, the proportion of subjects with a high level of IL-13 was significantly larger in the latter two groups (Table 4). Further analysis was performed by comparing the cytokine levels induced by SEA prior to and one year after treatment of the 61 individuals that had experienced no change in the grade of fibrosis following chemotherapy. Of this set, one group of 44 individuals remained without fibrosis, while a second group of 17 subjects continued to exhibit fibrosis (15 with incipient fibrosis and 2 with moderate fibrosis). Figure 2 displays the results of cytokine assays obtained before and one year after treatment for these two groups of patients. It may be observed that IL-10 levels decreased in both groups following treatment, with a significance level of P = 0.002 for the group with fibrosis and of P < 0.001 for the group without fibrosis. In contrast, IFN-γ levels decreased only in the group with fibrosis (P = 0.019).
TABLE 4.
Percentages of individuals with low or high cytokine levels produced by SEA-stimulated cultures of PBMC derived from subjects not presenting (group 0) and presenting (groups 1 and 2) with fibrosis as determined one year after treatment with praziquantela
| Cytokine | Level | No. or % of subjects with indicated severity of fibrosis
|
P valueb | |||
|---|---|---|---|---|---|---|
| Group 0
|
Groups 1 and 2
|
|||||
| n | % | n | % | |||
| IFN-γ | Low | 26 | 44.8 | 20 | 60.6 | 0.148 |
| High | 32 | 55.2 | 13 | 39.4 | ||
| IL-10 | Low | 31 | 53.4 | 15 | 45.5 | 0.463 |
| High | 27 | 46.6 | 18 | 54.5 | ||
| IL-4 | Low | 33 | 56.9 | 13 | 39.4 | 0.108 |
| High | 25 | 43.1 | 20 | 60.6 | ||
| TGF-β | Low | 22 | 56.4 | 8 | 40.0 | 0.233 |
| High | 17 | 43.6 | 12 | 60.0 | ||
| TNF-α | Low | 31 | 53.4 | 15 | 45.5 | 0.463 |
| High | 27 | 46.6 | 18 | 54.5 | ||
| IL-13 | Low | 29 | 59.2 | 10 | 34.5 | 0.035 |
| High | 20 | 40.8 | 19 | 65.5 | ||
Cytokine levels were categorized as low or high according to the median value (experimental value − control value) of the cytokine titer (IFN-γ = 0.133, TNF-α = 0.106, IL-4 = 0.016, IL-13 = 0.122, IL-10 = 0.024, and TGF-β = 0.013) and compared between groups of subjects presenting without (group 0) and with (groups 1 and 2) fibrosis one year after treatment.
Significance levels based on χ2 test (categorical variables). The minimum accepted significance level was P < 0.05.
FIG. 2.
Levels of IFN-γ and IL-10 in SEA-stimulated PBMC supernatants from groups of individuals not presenting with or presenting with fibrosis, as determined before and one year after treatment with praziquantel. Since these data were not normally distributed, the analyses of significant differences were performed using the Wilcoxon signed-rank test (the minimum accepted significance level was P < 0.05).
DISCUSSION
The objective of the study was to test the hypothesis that chemotherapy for treatment of schistosomiasis decreases the level of fibrosis and alters the cytokine pattern of patients living in areas in which the disease is endemic. Chemotherapy with praziquantel was highly efficient in eliminating the infection in the study population, as has been previously reported (2), and only 9 of the 91 patients remained positive for the presence of eggs one year after treatment, with infection levels ranging from 4 to 184 eggs/g of feces. Although the patients had also been treated with albendazol (400 mg), 28 (31%) were still infected with one or more additional parasites. This variation in treatment efficacy has also been previously reported (25, 26, 29).
Measurements of several ultrasound parameters, such as liver size and vein size, were found to have been reduced one year after treatment. However, this span of time was not sufficient to reduce the spleen size. These results tend to confirm that the chemotherapy favors involution of liver fibrosis (12, 34). In addition, our study confirmed that a decrease in worm burden favors the reversal of liver fibrosis. A total of 26 subjects reverted to a lower or nondetectable level of periportal fibrosis, while 17 individuals showed no regression of fibrosis and remained with the same grade of fibrosis. Most (20/26) of the individuals who reverted had presented with moderate to severe fibrosis, while 15 out of the 17 patients who had grade 1 (incipient) fibrosis did not revert. Although the reasons for nonreversion are not yet clear, several possibilities may be suggested, including (i) genetic differences in the individuals, (ii) inability of an individual to develop adequate posttreatment immune mechanisms in order to control fibrosis, and (iii) the difficulty in distinguishing, by ultrasound examination, healthy patients and patients presenting a small amount of echogenic thickening of the portal veins (group 0) from those in group 1. However, it is important to mention that in our study there was a clear distinction that distinguished grades 0 and 1 from grades 2 and 3. In addition, the lack of a characteristic pattern of fibrosis reversal emphasizes the need for further in-depth analyses of the factors involved. The lack of complete reversion following chemotherapy has already been described in reports of human studies (12, 23, 33) and mouse studies (3, 4). Comparison of old and young patients with respect to the time factor related to fibrosis regression among the treated cases was not considered, because in our study only 4.4% of subjects were less than 20 years of age.
It is interesting that treatment with praziquantel showed greater efficacy for individuals presenting with forms of fibrosis of greater severity. Thus, all seven patients with grade 3 fibrosis experienced reversion, and only a few (4/15 [27%]) subjects with grade 2 fibrosis remained with the same grade. On the other hand, liver morbidity increased in some (4%) individuals presenting with low grades of fibrosis in spite of praziquantel administration, a result that had been observed previously (6, 16). Although there is no obvious explanation for the observed increase in fibrosis, there is no reason to believe that the treatment itself could have been responsible. Clearly, further studies are necessary in order to identify individuals who are at particular risk of hepatoesplenic complications even when receiving praziquantel therapy.
There were no clear differences in the levels of production of the cytokines IFN-γ, IL-10, IL-4, IL-13, TGF-β, and TNF-α determined one year after chemotherapy among subjects categorized according to posttreatment degree of fibrosis. However, when patients within each group were separated into groups of low- and high-level cytokine producers, those subjects who presented with fibrosis and those who had not could be clearly classified according to the median value of each set of cytokine titers. Moreover, it was observed that a significantly larger group of individuals with elevated IL-13 levels belonged to the fibrosis group (P = 0.035), suggesting that a high level of this cytokine may be an indicator of persistency of fibrosis following treatment and/or of fibrosis development in cases of human and experimental schistosomiasis (2, 17, 24, 28, 41).
In the present study, comparison of cytokine production levels before and after chemotherapy revealed a significant posttreatment decrease in levels of IFN-γ in the group with fibrosis. The association between low IFN-γ production and the development of fibrosis must be analyzed in the light of a large body of evidence indicating that IFN-γ is the most active antifibrogenic cytokine in experimental granulomas induced by schistosome eggs and in many injury-induced hepatic fibroses (13, 14, 22). In addition, the observation that IFN-γ levels are inversely related to the intensity of infection prior to treatment (r = −0.387; P < 0.001) suggests that high infection levels may contribute to periportal fibrosis by downmodulating IFN-γ. The key role of IFN-γ in periportal fibrosis is also implied by the existence of a major susceptibility locus for periportal fibrosis that is closely linked to IFNGR1, the gene that encodes the γ-chain of the IFN-γ receptor (15), and the demonstration that cases of severe portal fibrosis are associated with low levels of IFN-γ (22). It is likely, therefore, that proinflammatory activity involving IFN-γ secretion is an important factor in human schistosomiasis. The fact that we observed a decrease in posttreatment IFN-γ secretion in groups with fibrosis indicates either that the mechanism regulating this cytokine is active or that the effectiveness of the treatment in eliminating the parasite and, consequently, egg deposition is sufficient to alter the immune response.
Among the cytokines involved in the regulation of T-cell responses in cases of human schistosomiasis, IL-10 undoubtedly plays a key role (30, 35, 38). In the present study, we observed a negative correlation between the presence of IL-10 and that of fibrosis prior to treatment (r = −0.249; P = 0.017) and a posttreatment decrease in IL-10 production in groups with and without fibrosis. Previous research involving subjects with chronic schistosomiasis had suggested that the levels of IL-10 secretion are dependent on the intensity of infection, as determined by the number of eggs in the stool (43). The positive correlation between the level of IL-10 and the intensity of infection before treatment (r = 0.317; P = 0.002) reported here supports this hypothesis.
Previous studies of patients with acute and chronic schistosomiasis have suggested that IFN-γ and IL-10 cross-regulate each other (38, 43) and that the presence of IL-10 in patients with acute infections or with hepatosplenomegaly is beneficial. We observed an inverse correlation between these two cytokines (r = −0.249; P = 0.017) that changed after treatment (r = 0.451; P < 0.001). This finding suggests that chemotherapy affected the influence of IL-10 on IFN-γ either as a consequence of the elimination of parasite eggs or through the induction of a burst of formation of new antigens by dead and/or damaged parasites.
The fact that no association between the cytokines TGF-β, IL-4, and TNF-α and periportal fibrosis was detected does not mean that such an association could not be found under other conditions. The data presented here could be a reflection of the analytical power, limited by sample size, employed to detect these interactions. Other groups, for example, have found high levels of TNF-α associated with an elevated risk of periportal fibrosis (22), and Moukoko et al. (39) suggested that TNF-α polymorphism might play an important role in the progression from mild to severe periportal fibrosis. Moreover, in cases of experimental schistosomiasis, TGF-β levels have been shown to be associated with the progression of fibrosis (18) and in the regulation of granuloma after treatment and reinfection (37). Additionally, TGF-β levels have been found to be associated with protection against fibrosis in cases of human schistosomiasis (2).
In conclusion, the data presented here suggest that chemotherapy is effective in reducing morbidity in schistosomiasis patients with fibrosis of greater severity and that the level of IL-13 may be a useful indicator of the persistence of fibrosis following treatment with praziquantel.
Acknowledgments
This work was supported by grants from FIOCRUZ/CNPq (PAPES IV) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil. P.M.-L. is a Fellow of FAPEMIG.
We thank Maria de Fátima da Silva, Marlucy Rodrigues Lima, Lília Cardoso Moreira, and Ivanete dos Santos Nascimento (Universidade Vale do Rio Doce, Governador Valadares, Minas Gerais, Brazil) for their technical assistance in various aspects of the field and laboratory work. We also thank Anna Carolina Lustosa Lima for help with the statistical analysis.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Abdel-Wahab, M. F., G. Esmat, A. Farrag, Y. A. el-Boraey, and G. T. Strickland. 1992. Grading of hepatic schistosomiasis by the use of ultrasonography. Am. J. Trop. Med. Hyg. 46:403-408. [DOI] [PubMed] [Google Scholar]
- 2.Alves-Oliveira, L. F., E. C. Moreno, G. Gazzinelli, O. A. Martins-Filho, A. M. S. Silveira, A. Gazzinelli, L. C. C. Malaquias, P. LoVerde, P. Martins Leite, and R. Correa-Oliveira. 2006. Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infect. Immun. 74:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade, Z. A., A. Baptista, and T. S. Santana. 2006. Remodeling of hepatic vascular changes after specific chemotherapy of schistosomal periportal fibrosis. Mem. Inst. Oswaldo Cruz 101(Suppl. I):267-272. [DOI] [PubMed] [Google Scholar]
- 4.Andrade, Z. A., and J. A. Grimaud. 1986. Evolution of the schistosomal hepatic lesions in mice after curative chemotherapy. AJP 124:59-65. [PMC free article] [PubMed] [Google Scholar]
- 5.Arap Siongok, T. K., A. A. Mahmoud, J. H. Ouma, K. S. Warren, A. S. Muller, A. K. Handa, and H. B. Houser. 1976. Morbidity in schistosomiasis mansoni in relation to intensity of infection: study of a community in machacos, Kenya. Am. J. Trop. Med. Hyg. 25:273-284. [DOI] [PubMed] [Google Scholar]
- 6.Boisier, P., C.-E. Ramarokoto, V. E. Ravaoalimalala, L. Rabarijaona, J. Serieye, J. Roux, and P. Esterre. 1998. Reversibility of Schistosoma mansoni-associated morbidity after yearly mass praziquantel therapy: ultrasonographic assessment. Trans. Royal Soc. Trop. Med. Hyg. 92:451-453. [DOI] [PubMed] [Google Scholar]
- 7.Booth, M., J. K. Mwatha, S. Joseph, F. M. Jones, H. Kadso, E. Ireri, F. Kazibwe, J. Kemijumbi, C. Kariuki, G. Kimani, J. H. Ouma, N. B. Kabatereine, B. J. Vennervald, and D. W. Dunne. 2004. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-y, high TNF-a, or low RANTES, depending on age and gender. J. Immunol. 172:1295-1303. [DOI] [PubMed] [Google Scholar]
- 8.Boros, D. L., and K. S. Warren. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolates from Schistosoma mansoni eggs. J. Exp. Med. 132:488-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botros, S., H. Sayed, H. El-Dusoki, H. Sabry, I. Rabie, M. El-Ghannam, M. Hassanein, Y. A. El-Wahab, and D. Engels. 2005. Efficacy of Mirazid in comparison with praziquantel in Egyptian Schistosoma mansoni-infected school children and households. Am. J. Trop. Med. Hyg. 72:119-123. [PubMed] [Google Scholar]
- 10.Chiaramonte, M. G., A. W. Cheever, J. D. Malley, D. D. Donaldson, and T. A. Wynn. 2001. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 34:273-282. [DOI] [PubMed] [Google Scholar]
- 11.Cioli, D. 1998. Chemotherapy of schistosomiasis: an update. Parasitol. Today 14:418-422. [DOI] [PubMed] [Google Scholar]
- 12.Cota, G. F., R. A. Pinto-Silva, C. M. F. Antunes, and J. R. Lambertucci. 2006. Ultrasound and clinical investigation of hepatosplenic schistosomiasis: evaluation of splenomegaly and liver fibrosis four years after mass chemotherapy with oxamniquine. Am. J. Trop. Hyg. 74:103-107. [PubMed] [Google Scholar]
- 13.Czaja, M. J., F. R. Weiner, S. Takahashi, M. A. Giambrone, P. H. van der Meide, H. Schellekens, L. Biempica, and M. A. Zern. 1989. γ-Interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology 10:795-800. [DOI] [PubMed] [Google Scholar]
- 14.Dessein, A., B. Kouriba, C. Eboumbou, H. Dessein, L. Argiro, S. Marquet, N. E. M. A. Elwali, V. Rodrigues, Y. Li, O. Doumbo, and C. Chevillard. 2004. Interleukin-13 in the skin and interferon-γ in the liver are key players in immune protection in human schistosomiasis. Immunol. Rev. 201:180-190. [DOI] [PubMed] [Google Scholar]
- 15.Dessein, A. J., D. Hillaire, N. E. M. A. Elwali, S. Marquet, Q. Mahamed-Ali, A. Mirghani, S. Henri, A. A. Abdelhameed, O. K. Saeed, M. M. A. Magzoub, and L. Abel. 1999. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am. J. Hum. Genet. 65:699-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doehring-Schwerdtfeger, E., I. M. Abdel-Rahim, R. Kardoff, C. Kaiser, D. Franke, J. Schlake, J. Richter, M. Elsheikh, Q. Mohamed-Ali, and J. H. H. Ehrich. 1992. Ultrasonographical investigation of periportal fibrosis in children with Schistosoma mansoni infection: reversibility of morbidity twenty-three months after treatment with praziquantel. Am. J. Trop. Med. Hyg. 46:409-415. [DOI] [PubMed] [Google Scholar]
- 17.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. J. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164:2585-2591. [DOI] [PubMed] [Google Scholar]
- 18.Farah, I. O., P. W. Mola, T. M. Kariuki, M. Nyindo, R. E. Blanton, and C. L. King. 2000. Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-β and IL-4. J. Immunol. 164:5337-5343. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari, M. L. A., P. M. Z. Coelho, C. M. F. Antunes, C. A. P. Tavares, and A. S. da Cunha. 2003. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bull. World Health Org. 81:190-196. [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzinelli, A., J. Bethony, L. A. Fraga, P. LoVerde, R. Correa-Oliveira, and H. Kloos. 2001. Exposure to Schistosoma mansoni infection in a rural area of Brazil: water contact. Trop. Med. Int. Health 6:126-135. [DOI] [PubMed] [Google Scholar]
- 21.Grogan, J. L., P. G. Kremsner, G. J. van Dam, W. Metzger, B. Mordmüller, A. M. Deelder, and M. Yazdanbakhsh. 1996. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J. Infect. Dis. 173:1242-1247. [DOI] [PubMed] [Google Scholar]
- 22.Henri, S., C. Chevillard, A. Mergani, P. Paris, J. Gaudart, C. Camilla, H. Dessein, F. Montero, N. E. M. A. Elwali, O. K. Saeed, M. Magzoub, and A. J. Dessein. 2002. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-γ is associated with protection against fibrosis and TNF-α with aggravation of disease. J. Immunol. 169:929-936. [DOI] [PubMed] [Google Scholar]
- 23.Homeida, M. A., I. E. Tom, T. Nash, and J. L. Bennett. 1991. Association of the therapeutic activity of praziquantel with the reversal of Symmers’ fibrosis induced by Schistosoma mansoni. Am. J. Trop. Med. Hyg. 45:360-365. [DOI] [PubMed] [Google Scholar]
- 24.Jesus, A. R., A. Magalhães, D. G. Miranda, R. G. Miranda, M. I. Araújo, A. A. De Jesus, A. Silva, L. B. Santana, E. Pearce, and E. M. Carvalho. 2004. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect. Immun. 72:3391-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabatereine, N. B., J. Kemijumbi, J. H. Ouma, R. F. Sturrock, A. E. Butterworth, H. Madsen, N. Ornbjerg, D. W. Dunne, and B. J. Vennnervald. 2003. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Trans. Royal Soc. Trop. Med. Hyg. 97:599-603. [DOI] [PubMed] [Google Scholar]
- 26.Kabatereine, N. B., S. Brooker, A. Koukounari, F. Kazibwe, E. M. Tukahebwa, F. M. Fleming, Y. Zhang, J. P. Webster, J. R. Stothard, and A. Fenwick. 2007. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull. World Health Org. 85:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique in Schistosoma mansoni. Rev. Inst. Med. Trop. São Paulo 14:397-400. [PubMed] [Google Scholar]
- 28.Kaviratne, M., M. Hesse, M. Leusink, A. W. Cheever, S. J. Davies, J. H. McKerrow, L. M. Wakefield, J. J. Letterio, and T. A. Wynn. 2004. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J. Immunol. 173:4020-4029. [DOI] [PubMed] [Google Scholar]
- 29.Kihara, J. H., N. Muhoho, D. Njomo, I. K. Mwobobia, K. Josyline, Y. Mitsui, T. Awazawa, T. Amano, and C. Mwandawiro. 2007. Drug efficacy of praziquantel and albendazole in school children in Mwea Division, Central Province, Kenya. Acta Trop. 102:165-171. [DOI] [PubMed] [Google Scholar]
- 30.King, C. L., A. Medhat, I. Malhotra, M. Nafeh, A. Helmy, J. Khaudary, S. Ibrahim, M. El-Sherbiny, S. Zaky, R. J. Stupi, K. Brustoski, M. Shehata, and M. T. Shata. 1996. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J. Immunol. 156:4715-4721. [PubMed] [Google Scholar]
- 31.Kloos, H., J. K. L. Passos, P. LoVerde, R. Correa-Oliveira, and A. Gazzinelli. 2004. Distribution and Schistosoma mansoni infection of Biomphalaria glabrata in different habitats in a rural area in the Jequitinhonha Valley, Minas Gerais, Brazil: environmental and epidemiological aspects. Mem. Inst. Oswaldo Cruz 99:673-681. [DOI] [PubMed] [Google Scholar]
- 32.Lambertucci, J. R., J. Carlos Serufo, R. Gerspacher-Lara, A. A. M. Rayes, R. Teixeira, V. Nobre, and C. M. F. Antunes. 2000. Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop. 77:101-109. [DOI] [PubMed] [Google Scholar]
- 33.Li, Y. S., A. C. Sleigh, Y. Li, M. Tanner, A. Dessein, G. M. Williams, and D. P. Mcmanus. 2002. Five-year impact of repeated praziquantel treatment on subclinical morbidity due to Schistosoma japonicum in China. Trans. Royal Soc. Trop. Med. Hyg. 96:438-443. [DOI] [PubMed] [Google Scholar]
- 34.Magalhães, T. V., G. Gazzinelli, M. C. Alvarez, F. C. Lima e Silva, L. A. Fraga, A. M. Silveira, A. Gazzinelli, J. Bethony, P. Loverde, I. R. Caldas, R. Correa-Oliveira, and A. Prata. 2005. Comparative clinical and ultrasound study of egg-negative and egg-positive individuals from Schistosoma mansoni low morbidity endemic areas, and hospitalized patients with hepatosplenic disease. Rev. Soc. Bras. Med. Trop. 38:33-37. [DOI] [PubMed] [Google Scholar]
- 35.Malaquias, L. C. C., P. L. Falcão, A. M. S. Silveira, G. Gazzinelli, A. Prata, R. L. Coffman, V. Pizziolo, C. P. Souza, D. G. Colley, and R. Correa-Oliveira. 1997. Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand. J. Immunol. 46:393-398. [DOI] [PubMed] [Google Scholar]
- 36.Mohamed-Ali, Q., N. E. M. A. Elwali, A. A. Abdelhameed, A. Mergani, S. Rahoud, K. E. Elagib, O. K. Saeed, L. Abel, M. M. A. Magzoub, and A. J. Dessein. 1999. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J. Infect. Dis. 180:1298-1306. [DOI] [PubMed] [Google Scholar]
- 37.Mola, P. W., I. O. Farah, T. M. Kariuki, M. Nyindo, R. E. Blanton, and C. L. King. 1999. Cytokine control of the granulomatous response in Schistosoma mansoni-infected baboons: role of exposure and treatment. Infect. Immun. 67:6565-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montenegro, S. M., P. Miranda, S. Mahanty, F. G. Abath, K. M. Teixeira, E. M. Coutinho, J. Brinkman, I. Gonçalves, L. A. Domingues, A. Sher, and T. A. Wynn. 1999. Cytokine production in acute versus chronic human schistosomiasis mansoni: the cross-regulatory role of interferon-gamma and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J. Infect. Dis. 179:1502-1514. [DOI] [PubMed] [Google Scholar]
- 39.Moukoko, C. E., N. El-Wali, O. K. Saeed, Q. M. Ali, J. Gaudart, A. Dessein, and C. Chevillard. 2003. No evidence for a major effect of tumor necrosis factor alpha gene polymorphism in periportal fibrosis caused by S. mansoni infection. Infect. Immun. 71:5456-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutapi, F., P. D. Ndhlovu, P. Hagan, J. T. Spicer, T. Mduluza, C. M. R. Turner, S. K. Chandiwana, and M. E. J. Woolhouse. 1998. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J. Infect. Dis. 178:289-293. [DOI] [PubMed] [Google Scholar]
- 41.Reiman, R. M., R. W. Thompson, C. G. Feng, D. Hari, R. Knight, A. W. Cheever, H. F. Rosenberg, and T. A. Wynn. 2006. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 74:1471-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimert, C. L., C. M. Fitzsimmons, S. Joseph, J. K. Mwatha, F. M. Jones, G. Kimani, K. F. Hoffmann, M. Booth, N. B. Kabatereine, D. W. Dunne, and B. J. Vennervald. 2006. Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre-and post-treatment with praziquantel. Clin. Vaccine Immunol. 13:584-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silveira, A. M. S., G. Gazzinelli, L. F. Alves-Oliveira, J. Bethony, A. Gazzinelli, C. Carvalho-Queiroz, M. C. B. Alvarez, F. C. Lima-Silva, A. Prata, P. T. LoVerde, and R. Correa-Oliveira. 2004. Human schistosomiasis mansoni: intensity of infection differentially affects the production of interleukin-10, interferon-γ and interleukin-13 by soluble egg antigen or adult worm antigen stimulated cultures. Trans. Royal Soc. Trop. Med. Hyg. 98:514-519. [DOI] [PubMed] [Google Scholar]
- 44.Singh, K. P., H. C. Gerard, A. P. Hudson, and D. L. Boros. 2004. Expression of matrix metalloproteinases and their inhibitors during the resorption of schistosome egg-induced fibrosis in praziquantel-treated mice. Immunology 111:343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talaat, M. R., A. I. El-Bassiouny, A. M. Osman, M. Yossif, R. Charmy, and M. M. Al-Sherbiny. 2007. Cytokine secretion profile associated with periportal fibrosis in S. mansoni-infected Egyptian patients. Parasitol. Res. 101:289-299. [DOI] [PubMed] [Google Scholar]
- 46.Warren, K. S. 1968. Pathophysiology and pathogenesis of hepatoesplenic schistosomiasis mansoni. Bull. N. Y. Acad. Med. 44:280-294. [PMC free article] [PubMed] [Google Scholar]
- 47.Warren, K. S. 1979. The pathogenesis of hepatoesplenic schistosomiasis: from man to monkey to mouse to molecule, p. 439-455. In H. Popper and F. Scaffner (ed.), Progress in liver diseases. Grune and Stratton, New York, NY. [PubMed]
- 48.Zwingenberger, K., G. Harma, H. Feldmeier, O. Muller, A. Steiner, and U. Bienzle. 1988. Liver involvement in human schistosomiasis mansoni. Regression of immunological and biochemical disease markers after specific treatment. Acta Trop. 45:263-275. [PubMed] [Google Scholar]