Abstract
Polymyxin B, minocycline, and tigecycline were the most potent of 10 antibiotics against 170 isolates of multidrug-resistant Acinetobacter baumannii. In time-kill studies, the exposure of a highly tigecycline-resistant isolate to tigecycline resulted in enhanced susceptibility to amikacin and synergistic bactericidal activities of the two drugs.
Determining optimal therapy for Acinetobacter baumannii infections is a challenge because of emerging multidrug resistance (1-4, 8, 12-14, 16). Tigecycline has potentially useful activity (5, 9) but may not always be effective as monotherapy (10, 11). Various antibiotic combinations have been suggested as potential therapies (12), but little is known about the activity of tigecycline-based combinations. Therefore, a study was designed to investigate the in vitro activities of tigecycline and comparison agents against multidrug-resistant A. baumannii, followed by investigations of the activities of combinations of tigecycline and either polymyxin B or amikacin against isolates with various levels of tigecycline susceptibility.
The isolates were obtained from Walter Reed Medical Center, Washington, DC, from military personnel wounded in Iraq (n = 150) and from the Creighton University culture collection (n = 20). The latter set included producers of IMP-1, VIM-2, and OXA-23 carbapenemases. MICs of tigecycline, minocycline, imipenem, ampicillin-sulbactam, levofloxacin, ceftazidime, gentamicin, amikacin, polymyxin B, and piperacillin-tazobactam were determined by CLSI microdilution methodology with frozen panels made from freshly prepared Mueller-Hinton broth (TREK Diagnostic Systems, Cleveland, OH). Because of trailing end points, MICs of piperacillin-tazobactam were determined with conservative and liberal interpretations based on CDC recommendations (15).
The activities of tigecycline, amikacin, and polymyxin B and combinations of tigecycline and either amikacin or polymyxin B were further evaluated in time-kill studies using concentrations that did not cause significant killing by any drug alone after 24 h. Mueller-Hinton broth cultures were inoculated with ≥5 × 105 CFU of each isolate in log phase/ml, and killing was assessed at 0, 2, 4, 6, and 24 h. The emergence of resistance was investigated if regrowth followed killing. After 24 h of incubation, bactericidal activity was defined by a ≥3-log10 decrease in the number of CFU per milliliter, and synergy was defined by a ≥2-log10 decrease in the number of CFU per milliliter in a comparison of the results for the combination and its most active constituent. The tigecycline, polymyxin B, and amikacin microdilution MICs for the three isolates used in the time-kill studies are given in Table 1.
TABLE 1.
MICs for the three isolates used in the time-kill study
| Isolate | MIC (μg/ml) of:
|
Susceptibility to other drugs | ||
|---|---|---|---|---|
| Tigecycline | Polymyxin B | Amikacin | ||
| 853 | 0.12 | 1 | ≤0.5 | Relatively susceptible |
| 1198 | 4 | 1 | >128 | Resistant to all except minocycline |
| 1826 | 64 | 2 | 2 | Resistant to imipenem, levofloxacin, gentamicin, and piperacillin-tazobactam |
Based on MIC90s, polymyxin B (MIC90 = 2 μg/ml) was the most potent agent, followed by tigecycline and minocycline (8 μg/ml), imipenem and levofloxacin (16 μg/ml), and ampicillin-sulbactam (128 μg/ml) (Table 2). Amikacin, ceftazidime, gentamicin, and piperacillin-tazobactam were less active, exhibiting high, out-of-range MIC90s. Although tigecycline and minocycline had similar overall activities, they differed distinctly against some isolates. Minocycline was significantly more potent than tigecycline against 13 isolates (minocycline MICs, 0.5 to 4 μg/ml; tigecycline MICs, 8 to 64 μg/ml), and tigecycline was significantly more potent against 27 isolates (tigecycline MICs, 2 to 4 μg/ml; minocycline MICs, 8 to 32 μg/ml). Examples include isolates GM186 (minocycline MIC, 4 μg/ml; tigecycline MIC, 64 μg/ml), 841 (minocycline MIC, 0.5 μg/ml; tigecycline MIC, 8 μg/ml), and GM248 (tigecycline MIC, 4 μg/ml; minocycline MIC, 32 μg/ml). This phenomenon is worth further investigation to understand its mechanistic basis and also to utilize pharmacokinetic-pharmacodynamic studies to explore potential clinical implications.
TABLE 2.
Summary of MIC data for 170 strains of A. baumanniia
| Antibiotic | MIC range | Susceptibility breakpoint | MIC50 | MIC90 | % of susceptible strains |
|---|---|---|---|---|---|
| Tigecycline | ≤0.06-64 | NDb | 2 | 8 | NDb |
| Minocycline | ≤0.06-32 | ≤4 | 2 | 8 | 78 |
| Imipenem | ≤0.12-128 | ≤4 | 1 | 16 | 86 |
| Ampicillin-sulbactam | 0.5->128 | ≤8 | 16 | 128 | 31 |
| Polymyxin B | 0.5-4 | ≤2 | 1 | 2 | 99 |
| Ceftazidime | 2->64 | ≤8 | 64 | >64 | 17 |
| Levofloxacin | ≤0.06->16 | ≤2 | 8 | 16 | 35 |
| Amikacin | ≤0.5->128 | ≤16 | 8 | >128 | 53 |
| Piperacillin-tazobactam (conservative interpretation)c | ≤4->128 | ≤16 | >128 | >128 | 9 |
| Piperacillin-tazobactam (liberal interpretation)d | ≤4->128 | ≤16 | >128 | >128 | 22 |
| Gentamicin | 0.5->32 | ≤4 | >32 | >32 | 16 |
MICs are expressed in micrograms per milliliter.
ND, not determined. No breakpoint criteria have been established for tigecycline against Acinetobacter spp. Therefore, the percentage of susceptible strains was not calculated.
Conservative piperacillin-tazobactam MIC readings were made at the lowest concentration at which no growth occurred.
Liberal piperacillin-tazobactam MIC readings were made at a concentration that ignored any subtle growth above an obvious end point.
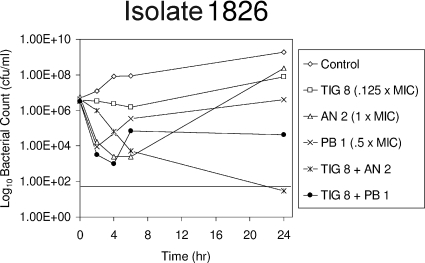
An 8-μg/ml concentration of tigecycline (0.12× MIC) in combination with a 2-μg/ml concentration of amikacin (1.0× MIC) was bactericidal, and the two drugs were synergistic against highly tigecycline-resistant isolate 1826 (Fig. 1). Alone, both agents suppressed growth for 4 h, and the suppression was followed by regrowth. Tigecycline alone selected a less susceptible mutant (for which the tigecycline MIC was >128 μg/ml) with enhanced susceptibility to amikacin (the MIC decreased from 2 to ≤0.5 μg/ml). The synergy that appeared to be due to the enhanced amikacin susceptibility was interesting because a similar phenomenon with Pseudomonas aeruginosa, in which imipenem-levofloxacin combinations prevented the emergence of resistance in strains lacking susceptibility to one or both drugs, was reported previously (6, 7).
FIG. 1.
Effects of tigecycline (TIG), amikacin (AN), polymyxin B (PB), and combinations of tigecycline and amikacin and tigecycline and polymyxin B on the viability of highly tigecycline-resistant A. baumannii 1826.
An 8-μg/ml concentration of tigecycline (0.12× MIC) in combination with a 1-μg/ml concentration of polymyxin B (0.5× MIC) produced a >3-log10 reduction in the CFU of isolate 1826 at 4 h, followed by regrowth with no susceptibility changes. The combination of tigecycline and polymyxin B was more active than either agent alone.
The combination of 0.06 μg of tigecycline/ml (0.5× MIC) and 0.5 μg of polymyxin B/ml (0.5× MIC) was more active against isolate 853 than either drug alone but was neither bactericidal nor synergistic, producing a >3-log10 decrease in CFU at 6 h, followed by regrowth without susceptibility changes. A 0.06-μg/ml concentration of tigecycline in combination with a 0.5-μg/ml concentration of amikacin produced a >3-log10 reduction in CFU at 4 to 6 h, followed by regrowth due to the emergence of reduced susceptibilities to tigecycline and amikacin (the tigecycline MIC increased from 0.12 to 1 μg/ml; the amikacin MIC increased from ≤0.5 to 8 μg/ml). Exposure to tigecycline alone resulted in reduced susceptibilities to tigecycline (the MIC increased from ≤0.12 to 0.25 μg/ml) and piperacillin-tazobactam (the MICs increased from ≤4/4 μg/ml to 32/4 μg/ml, respectively).
A combination of 4 μg of tigecycline/ml (1.0× MIC) and 0.5 μg of polymyxin B/ml (0.5× MIC) was bactericidal and synergistic against isolate 1198, whereas the addition of 16 μg of amikacin/ml (≤0.06× MIC) to tigecycline did not enhance activity (data not shown). Growth in 16 μg of amikacin/ml was comparable to that in the antibiotic-free control. Exposure to sub-MIC tigecycline concentrations of 1 and 2 μg/ml (0.25× and 0.5× MIC, respectively) led to the emergence of reduced susceptibility to tigecycline (the MIC increased from 4 to 32 μg/ml).
Overall, tigecycline alone was confirmed to be active against many multiple-antibiotic-resistant isolates of A. baumannii (5, 9, 10). In this study, tigecycline was shown to be more active in vitro when combined with an appropriate codrug. The differences in tigecycline and minocycline activities against some isolates indicated that neither drug should be used as a surrogate test agent for the other and that laboratories should test and report the results for only the compound intended for therapy. The phenomenon of tigecycline's selecting enhanced susceptibility to amikacin was particularly interesting from a scientific perspective, as it may indicate a basis for designing more effective antibiotic combinations for treating A. baumannii infections caused by other strains. Further studies are needed to elucidate the mechanism responsible for this effect and to explore its therapeutic potential.
Acknowledgments
This study was supported by Wyeth, Pearl River, NY.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Arroyo, L. A., A. Garcia-Curiel, M. E. Pachon-Ibanez, A. C. Llanos, M. Ruiz, J. Pachon, and J. Aznar. 2005. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 43:903-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomo, R. A., and D. Szabo. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 43(Suppl. 2):S49-S56. [DOI] [PubMed] [Google Scholar]
- 3.Brown, S., and S. Amyes. 2006. OXA (beta)-lactamases in Acinetobacter: the story so far. J. Antimicrob. Chemother. 57:1-3. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 5.Henwood, C. J., T. Gatward, M. Warner, D. James, M. W. Stockdale, R. P. Spence, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936). J. Antimicrob. Chemother. 49:479-487. [DOI] [PubMed] [Google Scholar]
- 6.Lister, P. D., and D. J. Wolter. 2005. Levofloxacin-imipenem combination prevents the emergence of resistance among clinical isolates of Pseudomonas aeruginosa. Clin. Infect. Dis. 40(Suppl. 2):S105-S114. [DOI] [PubMed] [Google Scholar]
- 7.Lister, P. D., D. J. Wolter, P. A. Wickman, and M. D. Reisbig. 2006. Levofloxacin/imipenem prevents the emergence of high-level resistance among Pseudomonas aeruginosa strains already lacking susceptibility to one or both drugs. J. Antimicrob. Chemother. 57:999-1003. [DOI] [PubMed] [Google Scholar]
- 8.Manikal, V. M., D. Landman, G. Saurina, E. Oydna, H. Lal, and J. Quale. 2000. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin. Infect. Dis. 31:101-106. [DOI] [PubMed] [Google Scholar]
- 9.Milatovic, D., F. J. Schmitz, J. Verhoef, and A. C. Fluit. 2003. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrob. Agents Chemother. 47:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navon-Venezia, S., A. Leavitt, and Y. Carmeli. 2007. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 59:772-774. [DOI] [PubMed] [Google Scholar]
- 11.Peleg, A. Y., B. A. Potoski, R. Rea, J. Adams, J. Sethi, B. Capitano, S. Husain, E. J. Kwak, S. V. Bhat, and D. L. Paterson. 2007. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J. Antimicrob. Chemother. 59:128-131. [DOI] [PubMed] [Google Scholar]
- 12.Perez, F., A. M. Hujer, K. M. Hujer, B. K. Decker, P. N. Rather, and R. A. Bonomo. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quale, J., S. Bratu, D. Landman, and R. Heddurshetti. 2003. Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin. Infect. Dis. 37:214-220. [DOI] [PubMed] [Google Scholar]
- 14.Sader, H. S., and R. N. Jones. 2005. Comprehensive in vitro evaluation of cefepime combined with aztreonam or ampicillin/sulbactam against multi-drug resistant Pseudomonas aeruginosa and Acinetobacter spp. Int. J. Antimicrob Agents 25:380-384. [DOI] [PubMed] [Google Scholar]
- 15.Swenson, J. M., G. E. Killgore, and F. C. Tenover. 2004. Antimicrobial susceptibility testing of Acinetobacter spp. by NCCLS broth microdilution and disk diffusion methods. J. Clin. Microbiol. 42:5102-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon, J., C. Urban, C. Terzian, N. Mariano, and J. J. Rahal. 2004. In vitro double and triple synergistic activities of polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 48:753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]