Abstract
Voriconazole, posaconazole, caspofungin, micafungin, and anidulafungin demonstrated potent in vitro activities against 286 invasive Candida isolates. Analysis of the fluconazole susceptibilities of 204 bloodstream Candida glabrata isolates revealed a rapid shift from susceptible (64% in 1999 to 2001 to 19% in 2007) to susceptible-dose dependent (27% in 1999 to 2001 and 75% in 2007).
The incidence of invasive candidiasis has increased worldwide in recent decades (5, 6, 17, 25). Antifungal resistance is a great concern in the management of patients with invasive candidiasis, particularly as prompt susceptibility testing is not always available.
A total of 286 nonduplicate and consecutive isolates of seven Candida species were obtained from 286 patients with invasive candidiasis treated in a tertiary-care medical center in Taiwan between January 2005 and December 2007. The majority (85%) of the Candida strains were isolated from blood. Only 15% of the isolates originated from cerebrospinal fluid, pleural effusions, or other normally sterile body sites. The collection included 50 isolates of Candida albicans, 51 of C. glabrata, 52 of C. tropicalis, 50 of C. parapsilosis, 40 of C. krusei, 32 of C. guilliermondii, and 11 of C. lusitaniae.
A total of 204 isolates of C. glabrata recovered from patients with candidemia at the National Taiwan University Hospital from 1999 to 2007 were also collected to evaluate the shift in fluconazole susceptibility over time. All of these isolates were identified by conventional methods, and the results were further confirmed with the API ID32C system (bioMerieux Vitek, St. Louis, MO) (7).
The MICs of the isolates were determined and interpreted by following the guidelines established in Clinical and Laboratory Standards Institute (CLSI; formerly the NCCLS) document M27-A2 (11). C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as control strains. Following incubation at 35°C for 48 h, the MICs of the antifungal agents were determined. The MIC interpretive criteria for the susceptibilities of Candida species to fluconazole and itraconazole were as published by the CLSI (11). A provisional susceptibility breakpoint of ≤1 μg/ml was applied for both voriconazole and amphotericin B, and a provisional susceptibility breakpoint of ≤2 μg/ml was applied for the echinocandins (12, 17, 20, 21).
All isolates were inhibited by amphotericin B at 2 μg/ml. An amphotericin B MIC90 of 2 μg/ml was found for C. glabrata (MIC50, 2 μg/ml) and C. krusei (Table 1). All three echinocandins were highly active against all Candida species tested except C. parapsilosis and C. guilliermondii, which were less susceptible to the echinocandins (MIC90s, 1 to 2 μg/ml). All isolates of Candida species were inhibited by posaconazole at 0.5 μg/ml.
TABLE 1.
In vitro susceptibilities of 286 invasive isolates of Candida species to seven antifungal agents determined by the broth dilution method
| Organism (no. of isolates) and agent | MIC (μg/ml)
|
% of isolates in each susceptibility categorya
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | S-DD | R | |
| C. albicans (50) | ||||||
| Amphotericin B | 1 | 1 | 1 | 100 | ||
| Fluconazole | 0.12-4 | 0.12 | 0.25 | 100 | 0 | 0 |
| Itraconazole | 0.015-0.25 | 0.03 | 0.03 | 98 | 2 | 0 |
| Voriconazole | 0.015-0.06 | 0.015 | 0.03 | 100 | 0 | 0 |
| Posaconazole | 0.015-0.03 | 0.015 | 0.03 | |||
| Caspofungin | 0.06-0.5 | 0.25 | 0.25 | |||
| Micafungin | 0.007-0.015 | 0.007 | 0.015 | |||
| Anidulafungin | 0.015-0.03 | 0.015 | 0.015 | |||
| C. glabrata (51) | ||||||
| Amphotericin B | 1-2 | 2 | 2 | 47 | ||
| Fluconazole | 4->64 | 16 | 32 | 22 | 73 | 6 |
| Itraconazole | 0.25->16 | 1 | 1 | 0 | 37 | 63 |
| Voriconazole | 0.12-8 | 0.5 | 1 | 94 | 2 | 4 |
| Posaconazole | 0.25-0.5 | 0.5 | 0.5 | |||
| Caspofungin | 0.12-0.5 | 0.25 | 0.25 | |||
| Micafungin | 0.03-0.06 | 0.06 | 0.06 | |||
| Anidulafungin | 0.015-0.03 | 0.03 | 0.03 | |||
| C. tropicalis (52) | ||||||
| Amphotericin B | 0.5-2 | 0.5 | 1 | 98 | ||
| Fluconazole | 0.5-8 | 1 | 2 | 100 | 0 | 0 |
| Itraconazole | 0.03-0.5 | 0.12 | 0.25 | 65 | 35 | 0 |
| Voriconazole | 0.015-0.25 | 0.12 | 0.12 | 100 | 0 | 0 |
| Posaconazole | 0.03-0.25 | 0.06 | 0.25 | |||
| Caspofungin | 0.12-0.5 | 0.25 | 0.5 | |||
| Micafungin | 0.06-0.12 | 0.06 | 0.12 | |||
| Anidulafungin | 0.015-0.12 | 0.03 | 0.06 | |||
| C. parapsilosis (50) | ||||||
| Amphotericin B | 0.5-2 | 1 | 1 | 98 | ||
| Fluconazole | 0.25-32 | 1 | 2 | 98 | 2 | 0 |
| Itraconazole | 0.03-0.25 | 0.06 | 0.25 | 78 | 22 | 0 |
| Voriconazole | 0.015-0.25 | 0.03 | 0.06 | 100 | 0 | 0 |
| Posaconazole | 0.03-0.12 | 0.03 | 0.06 | |||
| Caspofungin | 0.5-2 | 1 | 2 | |||
| Micafungin | 0.25-4 | 2 | 2 | |||
| Anidulafungin | 0.03-2 | 1 | 2 | |||
| C. guilliermondii (32) | ||||||
| Amphotericin B | 0.5-2 | 1 | 1 | 97 | ||
| Fluconazole | 2-64 | 4 | 8 | 88 | 9 | 3 |
| Itraconazole | 0.12-1 | 0.5 | 0.5 | 6 | 88 | 6 |
| Voriconazole | 0.03-2 | 0.06 | 0.12 | 97 | 3 | 0 |
| Posaconazole | 0.06-0.5 | 0.12 | 0.25 | |||
| Caspofungin | 1-4 | 2 | 2 | |||
| Micafungin | 0.5-2 | 1 | 1 | |||
| Anidulafungin | 0.5-2 | 1 | 2 | |||
| C. krusei (40) | ||||||
| Amphotericin B | 1-2 | 2 | 2 | 5 | ||
| Fluconazole | 32->64 | 32 | 64 | 0 | 63 | 38 |
| Itraconazole | 0.25-1 | 0.5 | 0.5 | 0 | 90 | 10 |
| Voriconazole | 0.25-8 | 0.5 | 0.5 | 95 | 3 | 3 |
| Posaconazole | 0.12-0.25 | 0.25 | 0.25 | |||
| Caspofungin | 0.5-2 | 1 | 1 | |||
| Micafungin | 0.25-0.5 | 0.25 | 0.5 | |||
| Anidulafungin | 0.06-0.5 | 0.12 | 0.25 | |||
| C. lusitaniae (11) | ||||||
| Amphotericin B | 0.5-1 | 1 | 1 | 100 | ||
| Fluconazole | 0.12-1 | 0.25 | 0.5 | 100 | 0 | 0 |
| Itraconazole | 0.03-0.12 | 0.06 | 0.06 | 100 | 0 | 0 |
| Voriconazole | 0.015 | 0.015 | 0.015 | 100 | 0 | 0 |
| Posaconazole | 0.015-0.03 | 0.03 | 0.03 | |||
| Caspofungin | 1-2 | 2 | 2 | |||
| Micafungin | 0.12-0.25 | 0.25 | 0.25 | |||
| Anidulafungin | 0.03-0.12 | 0.06 | 0.12 | |||
S, susceptible; S-DD, susceptible-dose dependent; R, resistant.
For the C. glabrata isolates, the rate of susceptibility to fluconazole was 22%. Voriconazole retained high potency against C. glabrata (94% of the isolates had MICs ≤1 μg/ml). The triazole susceptibility pattern for C. parapsilosis was similar to that for C. tropicalis. C. parapsilosis showed a high degree of susceptibility to voriconazole (MIC90, 0.06 μg/ml).
None of the C. krusei isolates were susceptible to fluconazole (MICs, >8 μg/ml) or itraconazole (MICs, >0.125 μg/ml). Thirty-eight percent of the C. krusei isolates were resistant to fluconazole. C. lusitaniae was highly susceptible to the three triazoles tested. Micafungin and anidulafungin were highly active against C. lusitaniae (MIC90s, 0.25 μg/ml and 0.12 μg/ml, respectively), but the caspofungin MIC90 for this species was 2 μg/ml.
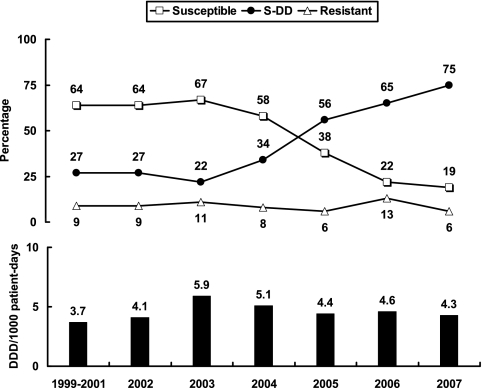
The change of fluconazole susceptibility and annual fluconazole consumption (expressed as defined daily doses [DDDs]/1,000 patient-days) are shown in Fig. 1. Pearson's correlation coefficient and linear regression analysis were used to determine statistical significance. A remarkable increase in the rates of susceptible-dose dependent (S-DD) isolates (P = 0.003) and a decrease in the rates of susceptible isolates (P = 0.003) were found. There was no significant change in the rate of fluconazole resistance (P = 0.696) during the study period.
FIG. 1.
Intravenous and oral fluconazole consumption (black bars) and trends of susceptibility to fluconazole (broken lines) among 204 Candida glabrata isolates recovered from the blood of patients with candidemia over a 9-year period.
Generally, the pattern of distribution of the MICs of the antifungal agents for invasive Candida strains in this study was consistent with that described in previous reports (3-5, 16, 17, 22, 23, 26). C. glabrata has emerged as an important Candida species that causes invasive candidiasis and has raised great clinical concern due to high rates of fluconazole resistance (1, 17, 18, 24). In this survey, 6% of the C. glabrata isolates tested were resistant to fluconazole. This rate is lower than that described in previous reports (10 to 20%) (18, 19, 26) but is comparable to the findings of our previous study (7). The prevalence of fluconazole resistance among C. glabrata isolates varies by country and region (18). Although the rate of resistance to fluconazole in this survey was relatively low (6%) compared to that found in previous studies, the rate of susceptibility was also low (22%). By contrast, the percentage of S-DD isolates was notably high (73%). Indeed, analysis of the data on the rates of fluconazole resistance among bloodstream isolates of C. glabrata revealed an increase in the numbers of S-DD isolates, which occurred together with a rapid decline in the rate of fluconazole susceptibility.
The increasing incidence of fluconazole S-DD isolates of C. glabrata suggests that a higher dose of fluconazole is needed when this agent is chosen as empirical treatment for invasive C. glabrata infections. The Infectious Diseases Society of America also recommended that disseminated C. glabrata infections be treated with amphotericin B (≥0.7 mg/kg of body weight per day) or 12 mg/kg/day fluconazole (800 mg/day for a 70-kg patient) (15). In the present study, the cause of the decrease in the rate of fluconazole susceptibility was not easy to determine. The decrease in the rate of fluconazole susceptibility among Candida species has been attributed to increasing fluconazole exposure (2, 8, 14). However, the total level of fluconazole consumption in our hospital did not change significantly during this period of decreasing susceptibility (P = 0.766). The correlation between each of the susceptibility categories and annual fluconazole consumption was also not significant (P = 0.668, 0.614, and 0.477 for susceptible, S-DD, and resistant isolates, respectively).
C. lusitaniae is a species which is often considered less susceptible to amphotericin B, and clinically apparent amphotericin B resistance has been reported (9, 10). In the present study, however, amphotericin B retained high potency against C. lusitaniae with an MIC90 of ≤1 μg/ml. A similar result was reported by Ostrosky-Zeichner et al. in a study of bloodstream Candida isolates in the United States in 2003 (13).
In conclusion, this survey from a university hospital in Taiwan found that all invasive isolates of Candida species other than C. glabrata and C. krusei were susceptible to fluconazole. Voriconazole and posaconazole were the most potent triazoles against invasive isolates of Candida species. All three echinocandins were highly active against all invasive Candida isolates except C. parapsilosis. In addition, a shift from susceptible to S-DD bloodstream C. glabrata isolates was noted.
Footnotes
Published ahead of print on 5 May 2008.
REFERENCES
- 1.AbiSaid, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. E., K. Izumikawa, and K. A. Marr. 2004. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 48:1773-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuenca-Estrella, M., L. Rodero, G. Garcia-Effron, and J. L. Rodriguez-Tudela. 2002. Antifungal susceptibilities of Candida spp. isolated from blood in Spain and Argentina, 1996-1999. J. Antimicrob. Chemother. 49:981-987. [DOI] [PubMed] [Google Scholar]
- 5.Eggimann, P., J. Garbino, and D. Pittet. 2003. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 3:685-702. [DOI] [PubMed] [Google Scholar]
- 6.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsueh, P. R., Y. J. Lau, Y. C. Chuang, J. H. Wan, W. K. Huang, J. M. Shyr, J. J. Yan, K. W. Yu, J. J. Wu, W. C. Ko, Y. C. Yang, Y. C. Liu, L. J. Teng, C. Y. Liu, and K. T. Luh. 2005. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob. Agents Chemother. 49:512-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masia Canuto, M., and F. Gutierrez Rodero. 2002. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2:550-563. [DOI] [PubMed] [Google Scholar]
- 9.Miller, N. S., J. D. Dick, and W. G. Merz. 2006. Phenotypic switching in Candida lusitaniae on copper sulfate indicator agar: association with amphotericin B resistance and filamentation. J. Clin. Microbiol. 44:1536-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minari, A., R. Hachem, and I. Raad. 2001. Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin. Infect. Dis. 32:186-190. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth microdilution antifungal susceptibility testing of yeasts. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 12.Nguyen, M. H., C. J. Clancy, V. L. Yu, Y. C. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi. 1998. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425-430. [DOI] [PubMed] [Google Scholar]
- 13.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panackal, A. A., J. L. Gribskov, J. F. Staab, K. A. Kirby, M. Rinaldi, and K. A. Marr. 2006. Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J. Clin. Microbiol. 44:1740-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, J. F. Meis, I. M. Gould, W. Fu, A. L. Colombo, and E. Rodriguez-Noriega. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 45:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spreghini, E., C. M. Maida, M. E. Milici, G. Scalise, and F. Barchiesi. 2008. Posaconazole activity against Candida glabrata after exposure to caspofungin or amphotericin B. Antimicrob. Agents Chemother. 52:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 25.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 26.Yang, Y. L., S. Y. Li, H. H. Cheng, and H. J. Lo. 2005. Susceptibilities to amphotericin B and fluconazole of Candida species in TSARY 2002. Diagn. Microbiol. Infect. Dis. 51:179-183. [DOI] [PubMed] [Google Scholar]