Abstract
In the absence of a fully effective human immunodeficiency virus (HIV) vaccine, topical microbicides represent an important strategy for preventing the transmission of HIV through sexual intercourse, the predominant mode of HIV transmission worldwide. Although a comprehensive understanding of HIV transmission has not yet emerged in the microbicide field, it is likely the result of rapid infection of monocyte-derived cells in the vaginal mucosa by CCR5-tropic viruses. Inhibition of HIV transmission requires agents that prevent entry, fusion, reverse transcription, or other preintegrative replication events or agents which directly inactivate HIV or modulate the target cells to render them uninfectible. In vitro assays typically used to evaluate the ability of a microbicide to prevent virus transmission use epithelial or human osteosarcoma-derived cells or immune cells more relevant to the development of anti-HIV therapeutic agents and quantify virus production at short time intervals following infection. We have developed a microbicide transmission and sterilization assay (MTSA) to more sensitively and quantitatively evaluate virus transmission in cell culture in the presence of microbicidal compounds. Results obtained with the MTSA demonstrate that the inhibitory capacity of microbicides is often overestimated in short-term transmission inhibition assays, while some compounds yield equivalent inhibitory results, indicating a biological relevance for the MTSA-based evaluations to identify superior potent microbicides. The MTSA defines the concentration of the microbicide required to totally suppress the transmission of virus in cell culture and may thus help define the effective concentration of the microbicide required in a formulated microbicide product.
The number of individuals living with AIDS continues to increase worldwide despite ongoing efforts to combat the etiologic agent human immunodeficiency virus (HIV). Joint United Nations Programme on HIV/AIDS reported that by the end of 2007, approximately 33.2 million people globally were living with HIV and more than 20 million had died from AIDS. In 2007 alone, approximately 2.5 million people became infected with the virus, with approximately 2.1 million infected individuals succumbing to HIV disease (18). There are approximately 12,000 new infections every day (500 infections/h), of which 50% or more occur in women. Of these newly infected women, married and heterosexual South African women are at the greatest risk for infection (18).
The development of an efficacious vaccine to prevent HIV infection is a logical strategy to reduce or eliminate the continuing spread of HIV. However, despite intensive research over 2 decades, an HIV vaccine has not been successfully developed. In the absence of a prophylactic vaccine, antiretroviral therapy (ART) has become the primary mechanism used to prevent disease progression and prolong the survival of infected individuals. A wide variety of antiretroviral agents are currently approved for use by the FDA. With the introduction of combination therapeutic strategies, resulting in the evolution of highly active ART, profound suppression of the plasma viral load for prolonged periods of time has resulted in significant declines in AIDS-related morbidity and mortality in developed countries where the therapies can be afforded (24, 28). Although highly active ART regimens have improved the prognosis for HIV-infected individuals, serious challenges remain for the effective use of these therapeutic strategies, including patient adherence, side effects, drug interactions and toxicities, drug resistance, and issues of persistent viral replication in latent reservoirs. These challenges have ultimately resulted in the development of alternative strategies to combat the spread of HIV, including prevention in the form of topical microbicides.
With the incidence of HIV infection in women on the rise, especially in underdeveloped countries (18), the development of topical microbicides has become a major worldwide initiative. It has been suggested that a microbicide with 60% efficacy introduced into 73 low-income countries could prevent 2.5 million HIV infections over 3 years (40). For these reasons, topical microbicides are an ideal HIV prevention strategy, allowing a woman to take responsibility for her own protection (3), especially in societies where women are unempowered. Minimally, a microbicide should be safe, acceptable by the user, affordable, stable, and effective without compromising existing physical and other natural barriers to sexually transmitted disease transmission (37). Microbicides are currently being developed as gels, creams, films, suppositories, sponges, and vaginal rings providing a physical and chemical barrier to virus infection of target cells in the vagina by targeting critical stages of the virus replication cycle such as virus entry and reverse transcription (www.microbicide.org). To date, the successful clinical development of a microbicide product has not been achieved. The surfactant nonoxynol-9 was the first promising candidate in human trials but was withdrawn due to toxic effects on the vaginal epithelium that resulted in an enhanced risk of infection (11). More recently, trials with Ushercell (cellulose sulfate) and Carraguard were also terminated due to an increased incidence of HIV infection among women using the product compared with placebo controls (25 July 2007; www.popcouncil.org/microbicides). Currently, there are 16 ongoing clinical trials involving eight microbicides, with three products targeting reverse transcriptase (RT) and four inhibiting virus entry or fusion (15, 23, 29, 30, 33, 35, 38, 39, 41, 42).
The FDA points to consider for HIV therapeutic development (16) and recommendations from the International Working Group on Microbicides have provided guidance for the preclinical and clinical development of HIV microbicides (22). As a more comprehensive understanding of the sexual transmission of HIV has evolved, development strategies have become more refined and directed toward prevention rather than therapeutic use. As an example, the National Institute of Allergy and Infectious Diseases (NIAID) microbicide development algorithm was established as a means to identify and prioritize candidate microbicide products (21). Although the assays constituting this testing algorithm provide efficacy and toxicity data under conditions which attempt to mimic sexual transmission, all are short-term assays with quantitative endpoints ranging from 24 h to 6 days postinfection, and thus they are not sensitive enough to identify rare or low-level infection of target cells and do not address the long-term biological effect of the test compound in the event that the virus is able to infect the target cells. Additionally, activity in these assays is often reported as the amount of the compound required to inhibit 50% of virus replication rather than the concentration which is totally suppressive to virus transmission and replication, which is more important in the prevention context.
As reported herein, we have developed an in vitro microbicide transmission and sterilization assay (MTSA) that more appropriately mimics the sexual transmission of HIV and better defines the active or sterilizing concentration of a candidate microbicide. We have used an approach based on a reported method for determining the “knocking-out” concentration of an HIV therapeutic agent (2) to quantify virus transmission in cell culture in the presence of a potential microbicide compound. These assays demonstrate that the transmission inhibition capacity of some microbicides may be overestimated in the standardized short-term assays used in testing algorithms, whereas with other microbicides the results of both assays yield equivalent results. Data obtained by the MTSA define the concentration of the microbicide required to completely suppress the transmission of virus to target cells and may assist with prioritizing microbicides for further development in animal models and human clinical trials.
MATERIALS AND METHODS
Cell lines and virus.
The CEM-SS (27), HeLa-CD4-LTR-β-Galactosidase (MAGI) (20), GHOST X4/R5 (25), and H9 (32) cell lines and HIV type 1IIIB (HIV-1IIIB) (32) and HIV-1RF (34) virus isolates were obtained from the NIAID AIDS Research and Reference Reagent Program (Rockville, MD). ME180 (17, 31) cells were purchased from the American Type Culture Collection (Manassas, VA), and HIV-1SK-1 (10, 13, 17, 31), which was used to develop chronically infected H9 cells (H9/SK-1), was a gift to ImQuest BioSciences from Duke University (Durham, NC). The cell lines were propagated as recommended and stored in liquid nitrogen; stocks of HIV-1IIIB and HIV-1RF with known titers were stored at −80°C prior to being used in the antiviral assays.
Materials.
Three highly active pyrimidinedione compounds (6) were used in these evaluations. These three compounds were defined as potential microbicides in a previously reported structure-activity relationship (SAR) evaluation; they are designated SJ-3991 (previously reported as SAR compound 19) (6), SJ-3366 (previously reported as SAR compound 62) (6), and SJ-3339 (previously reported as SAR compound 63) (6) and were obtained from Samjin Pharmaceutical Company, Ltd., as dry white powders. The compounds were solubilized at 1 or 40 mM in 100% dimethyl sulfoxide and stored at 4°C. Zidovudine (AZT) (26), dextran sulfate (molecular weight, 500,000) (1), and Chicago Sky Blue (12) were used as experimental control compounds and were obtained from Sigma-Aldrich Corporation (St. Louis, MO). Thiocarboxanilide UC781 (7, 9) and cyanovirin-N (CV-N) (4) were a gift to ImQuest BioSciences Inc. from Biosyn Inc. (now a part of Cellegy Pharmaceuticals). Tenofovir (36), efavirenz (42), and lamivudine (3TC) (14) were obtained from the NIAID AIDS Research and Reference Reagent Program.
Virus entry inhibition assays.
Twenty-four hours prior to compound and virus addition, MAGI cells diluted in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS) and antibiotics were plated in 96-well flat-bottom microtiter plates at 1 × 104 per well in a volume of 100 μl and incubated overnight at 37°C in 5% CO2. Following the overnight incubation, test compound was serially diluted and added to the cells in triplicate in a volume of 50 μl per well. HIV-1IIIB was diluted to a predetermined titer in assay medium and added to the microtiter plate. The cultures were incubated for 2 h at 37°C in 5% CO2. Following the incubation, the monolayers were washed three times with RPMI 1640 medium (without additives) to remove residual extracellular compound and unbound virus and incubated at 37°C in 5% CO2 for an additional 48 h. Following the incubation, toxicity plates were evaluated by 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) staining as previously described (8) and efficacy plates were evaluated by chemiluminescence detection with Gal-Screen (Applied Biosystems) according to the manufacturer's instructions.
CD4-independent and cell-associated virus transmission inhibition assays.
ME180 cells resuspended in RPMI 1640 medium with 10% FBS and antibiotics were plated at a density of 5 × 103 per well in a 96-well flat-bottom microtiter plate and incubated overnight at 37°C in 5% CO2. Following the incubation, test compound was serially diluted in assay medium and added in triplicate to the cells. Thoroughly washed, mitomycin C-treated, chronically HIV-infected H9 (H9/SK-1) cells were added at 2 × 104 per well, and the plates were incubated at 37°C in 5% CO2 for 4 h. The mitomycin C concentration used was that which resulted in complete killing of the chronically infected cells within 24 h to prevent virus production from the H9/SK-1 inoculum from contributing to the endpoint of the assay. Immediately following the infection period and again at 24 and 48 h postinfection, the cell monolayers were washed with RPMI 1640 medium (without additives) to remove residual compound and virus-infected cells. Following a 6-day incubation at 37°C in 5% CO2, cell-free supernatant samples from the cell cultures were evaluated for virus content with the HIV-1 p24 Antigen ELISA kit (Beckman Coulter) according to the manufacturer's instructions. Duplicate assay plates were evaluated for cellular toxicity by XTT staining.
Cell-free virus transmission inhibition assay.
GHOST X4/R5 cells diluted in Dulbecco modified Eagle medium supplemented with 10% FBS and antibiotics were incubated in a 96-well flat-bottom microtiter plate (5 × 103 per well) overnight prior to assay initiation. Following the incubation, the cultures were washed to remove nonadherent cells. Serially diluted test compound was added in triplicate wells, and the cells were infected with a predetermined titer of HIV-1IIIB for 4 h at 37°C. Following the incubation, residual virus and test material were removed by washing with RPMI 1640 medium (without additives). The cultures were incubated for 6 days, at which time antiviral activity was assessed by evaluating cell-free supernatants for virus content by RT assay as described previously (8). Cytotoxicity was evaluated in parallel by XTT dye reduction.
Cell-to-cell virus transmission inhibition assay.
CEM-SS cells were added to a 96-well flat-bottom microtiter plate at a concentration of 1 × 105 per well. Serially diluted test compounds were then added to each well with the uninfected cells. Chronically HIV-1RF-infected CEM-SS cells were washed and resuspended in complete RPMI 1640 medium and then added to appropriate wells of the microtiter plate in serial logarithmic dilutions (105 per well through 100 per well). The cultures were incubated at 37°C in 5% CO2 for 48 h. Following the incubation, the number of syncytia per well was quantified microscopically and cell-free supernatant samples were analyzed for virus content by the RT assay (8).
Cytopathic effect (CPE) inhibition assay.
The CPE assay was performed as previously described (8). Briefly, serially diluted compound was added to a 96-well round-bottom microtiter plate in triplicate. CEM-SS cells at a concentration of 2.5 × 103 per well and HIV-1IIIB at the appropriate predetermined titer were sequentially added to the microtiter plate. The cultures were incubated in 5% CO2 at 37°C for 6 days. Following the incubation, the microtiter plates were stained with XTT tetrazolium dye to evaluate the efficacy and toxicity of the test compound(s).
MTSA.
CEM-SS cells at a concentration of 5 × 105 per well and the appropriate predetermined titer of HIV-1IIIB were cocultured for 1 h at 37°C in a 96-well round-bottom microtiter plate. Following the initial infection, the cells and virus suspension were transferred to a T25 tissue culture flask containing 5 ml of complete tissue culture medium (RPMI 1640 medium supplemented with 10% FBS, 1% penicillin-streptomycin, and l-glutamine). Five or six concentrations of test or control compound were added to the appropriate individual flasks. The compound concentrations used in the MTSA were based on both the defined 50% effective concentration (EC50) and the overall therapeutic index (TI; defined as the toxic concentration [TC50] divided by the EC50) of the test compound as determined in the CEM-SS-based CPE inhibition assay. The lowest test concentration in the MTSA was chosen as 10 times the defined EC50 (10 × EC50). Successive test concentrations were increased in fivefold increments. This test concentration scheme was varied as required, depending on the overall TI of the test compound (based on the defined EC50 and the TC50 of the compound). Three days following the initial infection, cell-free supernatant samples were evaluated for virus content by the RT assay (8). Twenty percent of the existing culture (1 ml of resuspended cells and supernatant) was added to 4 ml of fresh cells (at a cell density of 1 × 105/ml) in fresh tissue culture medium containing the test compound at the identical fixed concentration. Each subculturing performed at 3-day intervals was considered a new passage. Subculturing of the infected cultures was performed every third day, for a total of 15 passages. Cells in passages 11 through 15 were cultured in the absence of the test compound to confirm complete sterilization of the culture.
RT assay.
Cell-free supernatants were collected from infected cultures, and virion-associated RT was quantified. The RT assay uses poly(rA) as the assay template and oligo(dT) as the primer (10). The quantitative RT assay was performed exactly as previously described (8).
RESULTS
Efficacy of select pyrimidinediones in standard HIV transmission assays.
Three highly active and structurally related compounds from the pyrimidinedione SAR series (5, 6) were evaluated for efficacy and toxicity in five transmission inhibition assays routinely used to quantify the activity of potential microbicide products. Each of these assays is performed over a short duration, ranging from 48 h to 6 days, uses either cell-free or cell-associated virus as the inoculum and target cells with or without cell surface CD4, and uses diverse quantitative virus replication endpoints as described in Materials and Methods. All three pyrimidinediones were found to be nontoxic to each of the target cell lines used in these transmission inhibition assays up to the highest concentration evaluated (10 to 100 μM, depending on the assay) (data not presented). In each assay, an appropriate positive control compound (the sulfonated dye Chicago Sky Blue or the sulfated polysaccharide dextran sulfate) was used to control for the relative multiplicity of infection in the assay and to confirm that the assay performed as expected.
The activity of the three pyrimidinedione compounds was quantified in each of the standardized virus transmission assays and yielded the following results. (i) In the cell-based entry inhibition assay (MAGI assay), SJ-3991, SJ-3366, and SJ-3339 exhibited EC50s against HIV-1IIIB of 17, 59, and 53 nM, respectively (Table 1). (ii) Each of the pyrimidinediones was inactive (>100 μM) in the CD4-independent virus transmission inhibition assay, consistent with the requirement for CD4 in the mechanism of entry inhibition by the pyrimidinediones (Robert W. Buckheit, Jr., unpublished data). (iii) SJ-3991, SJ-3366, and SJ-3339 inhibited CD4-dependent virus transmission to MAGI cells at concentrations of 67, 61, and 87 nM, respectively (Table 1). (iv) Each of the pyrimidinediones was determined to be an active inhibitor of HIV-1 transmission in both cell-free and cell-associated virus transmission inhibition assays, with EC50s ranging from 7 to 30 nM (Table 1). In the cell-free virus transmission inhibition assay, the EC50s of SJ-3991, SJ-3366, and SJ-3339 were determined to be 7, 30, and 10 nM, respectively, and in the cell-associated virus transmission assay, the defined EC50s were 19, 18, and 30 nM, respectively.
TABLE 1.
Efficacies of select pyrimidinediones in standard HIV transmission assaysa
| Transmission inhibition assay | Target cells | Viral inoculum | Time | Endpoint | EC50 of:
|
||||
|---|---|---|---|---|---|---|---|---|---|
| SJ-3991 (μM) | SJ-3366 EC50 (μM) | SJ-3339 EC50 (μM) | Chicago Sky Blue (μg/ml) | Dextran sulfate (μg/ml) | |||||
| Entry | MAGI | HIV-1IIIB | 48 h | β-Galactosidase | 0.017 | 0.059 | 0.053 | 0.22 | NDb |
| CD4 independent | ME180 | H9/SK-1 | 6 days | p24 | >100 | >100 | >100 | ND | 1.2 |
| CD4 dependent | GHOST X4/R5 | HIV-1IIIB | 6 days | RT | 0.067 | 0.061 | 0.087 | 0.53 | 0.42 |
| Cell free | MAGI | HIV-1IIIB | 48 h | β-Galactosidase | 0.007 | 0.03 | 0.01 | ND | 0.93 |
| Cell associated | CEM-SS | CEM-SS/RF | 48 h | RT/SFU | 0.019 | 0.018 | 0.03 | ND | 0.07 |
The results presented were obtained from a single representative antiviral assay with appropriate control compounds evaluated in parallel selected from a minimum of three antiviral assays. We have demonstrated that the standard error among multiple antiviral assays averaged less than 10% of the respective mean EC50 and TC50. In each individual assay, mean efficacy and toxicity values are derived from a minimum of three replicate wells. Toxic concentrations were greater than the highest concentration evaluated in each assay as follows: entry, 10 μM and 10 μg/ml; CD4 independent, 100 μM; CD4 dependent, 10 μM, 10 μg/ml, and 25 μg/ml; cell free, 10 μM and 25 μg/ml; cell associated, 10 μM and 25 μg/ml.
ND, not done. This compound is not used as a standard control in the specified assay and therefore was not evaluated.
Efficacy of select pyrimidinediones in the MTSA.
In an effort to further quantify the abilities of these compounds to prevent virus transmission in cell culture and to prioritize the compounds for further preclinical development, we evaluated virus transmission and the potential for the maintenance of low-level HIV infection in treated cultures by using the MTSA. The intent of these biological transmission inhibition assays was to quantify the concentration of the potential microbicide which would totally suppress virus transmission and yield sterilization of the cell culture and then to compare this sterilizing concentration of the compound to the inhibitory values obtained in the standardized but much shorter term and potentially less sensitive standard assay methods. For each test compound, a broad dose-response range of five or six concentrations spanning the TI of each compound (selected based on the defined EC50 and TC50 of the compound in the CPE inhibition assay) were evaluated for efficacy in the MTSA. The sterilizing concentration obtained in the MTSA is presented as the difference relative to the EC50 defined in the microtiter anti-HIV assay following 10 passages in the presence of fixed concentrations of the test compound and after an additional 5 passages (for a total of 15 passages) of culture in the absence of the test compound. The sterilizing concentrations of each compound at passages 10 and 15 are presented for comparison.
(i) Determination of the sterilizing concentration of SJ-3991.
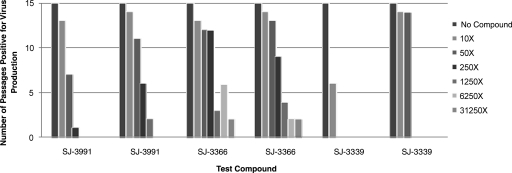
SJ-3991 had an EC50 of 0.2 nM against HIV-1 in the CEM-SS-based CPE inhibition assay and was evaluated in the MTSA at concentrations of 2, 10, 50, 250, 1,250, and 6,250 nM (or 10, 50, 250, 1,250, 6,250, and 31,250 times the EC50, respectively). In the first replicate experiment (Fig. 1), virus was initially detected at passage 3 at 2 nM (10 times the EC50). Virus replication was suppressed at all of the other concentrations until passage 9, where virus breakthrough was observed at 10 nM (50 times the EC50) and at passage 15, where breakthrough was observed at 50 nM (250 times the EC50). There was no virus replication observed visually or by the RT assay through passage 15 at 250, 1,250, or 6,250 nM, resulting in a defined sterilizing concentration of 250 nM, 1,250 times the EC50 of SJ-3991. In a second experiment (Fig. 1), virus replication was observed at 2 nM (10 times the EC50) by passage 2 and at 10 nM (50 times the EC50) by passage 5. Virus replication was detected by passage 10 at 50 nM and by passage 14 at 250 nM, yielding a sterilizing concentration that was 6,250 times the EC50 (1,250 nM). Virus breakthrough at concentrations of 2 and 10 nM was detected two and four passages earlier, respectively, in the second experiment, and there was a fivefold difference between the sterilizing concentrations defined in the two replicate experiments. These differences suggest a relatively small range of error in the MTSA. Sterilizing concentration data obtained from the MTSAs with SJ-3991 are summarized in Table 2.
FIG. 1.
Determination of sterilizing concentrations of pyrimidinedione inhibitors. SJ-3991, SJ-3366, and SJ-3339 were evaluated in the MTSA, and the results are presented as the number of passages which were positive for virus replication at each compound concentration. The results of two replicate assays for each compound are presented. Each pyrimidinedione was evaluated at concentrations that were 10, 50, 250, 1,250, 6,250, and 31,250 times the EC50 that was defined in the CPE inhibition assay. All of the concentrations tested were significantly below the defined TC50 for CEM-SS cells. Passages which were positive for virus production were defined by detection of virus in the cell-free supernatant by RT assay. Cells were passaged for 10 passages and in the absence of the compound for an additional 5 passages.
TABLE 2.
Sterilizing concentrations of test compounds as determined by the MTSA
| Compound | HIV-1/CEM-SS CPE assaya EC50 (μM) | HIV-1/CEM-SS CPE assaya TC50 (μM) | TI | Sterilizing concn (n-fold increase over EC50)
|
|||
|---|---|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
||||||
| Passage 10 | Passage 15 | Passage 10 | Passage 15 | ||||
| SJ-3991 | 0.0002 | >20 | >100,000 | 250 | 1,250 | 1,250 | 6250 |
| SJ-3366 | 0.0004 | >100 | >250,000 | 1,250 | >31,250 | 1,250 | >31,250 |
| SJ-3339 | 0.0004 | >50 | >125,000 | 50 | 50 | 250 | 250 |
| AZT | 0.0015 | >31.3 | >20,867 | 31,250 | >31,250 | 31,250 | >31,250 |
| 3TC | 0.012 | >62.5 | >5,208 | >6,250 | >6,250 | >6,250 | >6,250 |
| Efavirenz | 0.0009 | >10 | >11,111 | 10 | 50 | 10 | 50 |
| UC781 | 0.0015 | >50 | >33,333 | 50 | 250 | 50 | 1,250 |
| CV-N | 0.016b | >62.5b | >3,906 | 250 | 1,250 | 250 | 1,250 |
| Tenofovir | 1.3 | >100 | >77 | 39 | >97.7 | 97.7 | >97.7 |
The results presented were obtained from representative antiviral assays with appropriate control compounds evaluated in parallel selected from a minimum of three antiviral assays. We have demonstrated that the standard error among multiple antiviral assays averaged less than 10% of the respective mean EC50 and TC50. In each individual assay, efficacy values are derived from a minimum of three replicate wells while toxicity is defined from two replicate wells. Appropriate compound colorimetric controls and controls for cell growth and virus-induced CPEs were evaluated in sextuplet wells.
Value is in micrograms per milliliter.
(ii) Determination of the sterilizing concentration of SJ-3366.
SJ-3366 had an EC50 of 0.4 nM against HIV-1IIIB in CEM-SS cells. The compound was evaluated in the MTSA at concentrations of 4, 20, 100, 500, 2,500, and 12,500 nM. In the initial experiment (Fig. 1), virus replication was first detected at passage 3 at the lowest concentration (4 nM or 10 times the EC50). Virus breakthrough was observed at 20 nM (50 times the EC50) and 100 nM (250 times the EC50) by passage 4. SJ-3366 was able to suppress virus replication at 500 nM through passage 12 and at 2,500 nM through passage 14. By passage 14, virus replication was detected in the presence of 12,500 nM, resulting in a sterilizing concentration that was greater than 31,250 times the defined EC50. In a second experiment (Fig. 1), virus replication was first observed at passage 2 at the lowest concentration (4 nM or 10 times the EC50) and then quickly progressed in the presence of 20 nM (50 times the EC50) and 100 nM (250 times the EC50) at passages 3 and 7, respectively. At 500 nM, virus replication was observed at passage 12 and at 2,500 and 12,500 nM by passage 14, yielding again a sterilizing concentration of greater than 31,250 times the EC50 (>12,500 nM). The patterns of virus replication were similar in the two experiments, with a noticeable delay in virus breakthrough at the 500 nM concentration but ultimately achieving the same sterilizing concentration. Sterilizing concentration data obtained in the MTSA with SJ-3366 are summarized in Table 2.
(iii) Determination of the sterilizing concentration of SJ-3339.
SJ-3339 had a defined EC50 of 0.4 nM against HIV-1IIIB in the CPE assay and was evaluated in the MTSA at concentrations of 4, 20, 100, 500, 2,500, and 12,500 nM (10, 50, 250, 1,250, 6,250, and 31,250 times the EC50). In the first replicate experiment (Fig. 1), SJ-3339 suppressed virus replication for nine passages at all of the concentrations before virus breakthrough was observed at 4 nM (10 times the EC50). Following removal of the compound at passage 10, SJ-3339 was able to suppress virus replication for the remaining five passages at all of the remaining concentrations, yielding a sterilizing concentration of 50 times the EC50 (20 nM). In a second experiment, virus breakthrough was observed at the two lowest concentrations (4 and 20 nM or 10 and 50 times the EC50) by passage 2; however, virus replication was suppressed at all of the remaining test concentrations through passage 15, resulting in a sterilizing concentration of 250 times the EC50 (100 nM). Data obtained in the MTSA for SJ-3339 are summarized in Table 2.
Biological effects of compound pretreatment of cells on defined sterilizing concentrations of the pyrimidinediones in the MTSA.
As a prevention strategy, the use of a microbicide will involve vaginal application of the test compound prior to sexual intercourse and the introduction of infectious virus or virus-infected cells. Thus, the microbicide will be present prior to the introduction of virus and will be required to prevent the initial transmission of virus to the vaginal target cells. The standard MTSA mimics this sequence of events, with the initial virus infection of cells prior to microbicide application yielding a small pool of infected cells or cell-free virions, which then represent the viral inoculum from the semen. Quantitative evaluation of the number of cells initially infected in the MTSA indicates that 0.001% of the cells in the culture are infected (Robert W. Buckheit, Jr., unpublished data). However, it was also important to evaluate the results of the MTSA with compound treatment occurring prior to the introduction of the cell-free virus inoculum to mirror the presence of the microbicide and preloading of target cells with compound prior to the introduction of any infectious virus. Thus, we evaluated the performance of the pyrimidinedione compounds in the MTSA following pretreatment of the target CEM-SS cells with comparative evaluation of compound addition prior to or after infection with virus. In the pretreatment assay, cells were treated with SJ-3339 for 2 h prior to virus infection. In the assay performed without pretreatment of the target cells, SJ-3339 had a sterilizing concentration that was 1,875 times the EC50 (750 nM) (data not shown). Pretreatment of the target cells with SJ-3339 at the same concentrations used in the assay, followed by virus infection, also resulted in a sterilizing concentration of 750 nM, or 1,875 times the EC50 (data not shown), indicating that compound pretreatment did not have any additional biological effect on virus transmission or the defined sterilizing concentration of this compound. The replication of virus in these comparative cultures was similar, with detectable virus replication appearing at the lowest concentration of SJ-3339 at passage 1 (20 nM) and at the 150 nM concentration by passage 6 in both experiments. Similar pretreatment MTSA results were also obtained with SJ-3991, SJ-3366, efavirenz, and AZT (data not shown).
Efficacy of known inhibitors in the MTSA.
In order to compare the results obtained with the pyrimidinedione compounds to those obtained with other known HIV inhibitors, a variety of control compounds were evaluated, including the entry inhibitor CV-N, the nucleoside RT inhibitors (NRTIs) AZT and 3TC, the nucleotide RT inhibitor (NtRTI) tenofovir, and the non-NRTIs (NNRTIs) UC781 and efavirenz. These compounds were chosen for evaluation in the MTSA in order to comparatively evaluate data obtained with representative approved HIV drugs with differing antiviral mechanisms of action (AZT, 3TC, efavirenz, and tenofovir), as well as potential microbicide candidates (CV-N, UC781, and tenofovir).
(i) Determination of the sterilizing concentration of AZT.
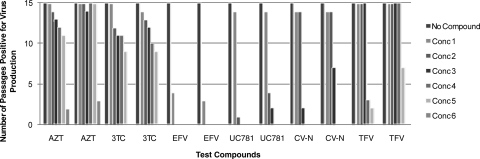
AZT, an approved NRTI, had an EC50 of 1.5 nM in the CEM-SS-based CPE inhibition assay against HIV-1 and was evaluated in the MTSA at concentrations of 10, 50, 250, 1,250, 6,250, and 31,250 nM (10, 50, 250, 1,250, 6,250, and 31,250 times the EC50, respectively). Virus replication was immediately observed at the two lowest concentrations (10 and 50 nM) of AZT by passages 1 and 2, respectively, and at the intermediate concentrations (250 and 1,250 nM) by passages 3 and 4, respectively. By passage 5, virus replication was observed at 6,250 times the EC50 (6,250 nM). At the highest concentration evaluated, 31,250 nM, AZT was able to suppress virus replication through 10 passages in the presence of the drug and for 3 passages without reapplication of the drug. In a replicate experiment, virus replication was observed at all of the concentrations tested except the highest concentration (31.25 nM) by passage 2 and at 31.25 nM by passage 12. The sterilizing concentration defined in two replicate experiments was the same (>31,250 times the EC50). Data obtained for AZT in the MTSA are presented in Fig. 2 and summarized in Table 2.
FIG. 2.
Determination of sterilizing concentrations of control compounds. The entry inhibitor CV-N, the NRTIs AZT and 3TC, the NtRTI tenofovir, and the NNRTIs UC781 and efavirenz were evaluated in the MTSA, and the results are presented as the number of passages which were positive for virus replication at each compound concentration (Conc) tested. The results of two replicate assays for each compound are presented. The concentrations used for each compound in the series are as follows: AZT, 10 to 31,250 nM (10 through 31,250 times the EC50); 3TC, 100 to 62,500 nM (10 through 6,250 times the EC50); efavirenz, 10 to 6,250 nM (10 through 6,250 times the EC50); UC781, 15 to 46,875 nM (10 through 31,250 times the EC50); CV-N, 0.1 to 62.5 μg/ml (10 through 6,250 times the EC50); tenofovir, 2.5 to 97.7 μM (2.5 through 97.7 times the EC50). All concentrations were in 5-fold increments, with the exception of those of tenofovir, which were in 2.5-fold increments. Passages which were positive for virus production were defined by detection of virus in the cell-free supernatant by RT assay. Cells were passaged for 10 passages and in the absence of the compound for an additional 5 passages.
(ii) Determination of the sterilizing concentration of 3TC.
3TC, an FDA-approved NRTI, had an EC50 of 12 nM in the CPE assay and was evaluated at 100, 500, 2500, 12,500, and 62,500 nM (10, 50, 250, 1,250, and 6,250 times the EC50, respectively) in the MTSA. Through passage 3, virus replication was only observed at 100 nM. At passage 4, virus replication was observed at 500 nM, followed by virus replication at 2,500 and 12,500 nM at passage 5. By passage 7, virus replication was observed in the samples cultured in the presence of 62,500 nM 3TC. Since virus replication was observed at all of the concentrations in the presence of 3TC, continued evaluation in the absence of 3TC was not necessary and the sterilizing concentration was defined as greater than 6,250 times the EC50. A replicate assay performed with 3TC yielded an identical result, with virus breakthrough occurring at all of the concentrations of the compound tested during the first 10 passages. Data obtained with 3TC in the MTSA are presented in Fig. 2 and summarized in Table 2.
(iii) Determination of the sterilizing concentration of efavirenz.
The NNRTI efavirenz had an EC50 of 0.9 nM in the HIV-1 CPE assay and was evaluated at 10, 50, 250, 1,250, 6,250, and 31,250 nM (10, 50, 250, 1,250, 6,250, and 31,250 times the EC50, respectively). At the highest concentration evaluated (31,250 nM), drug toxicity to the CEM-SS cells was noted microscopically and this culture could not be maintained. Virus replication was completely suppressed compared to the virus control when cells were cultured in the presence of all of the concentrations of efavirenz tested. This suppressive effect was observed through the first passage in the absence of efavirenz. By passage 2 in the absence of reapplied compound, virus was detected in the culture that had been passaged in the presence of 10 nM efavirenz (10 times the EC50). Virus replication remained suppressed in those cultures treated with all other concentrations of efavirenz through five passages in the absence of the compound (passage 15), resulting in a sterilizing concentration of 50 nM (50 times the EC50). In a replicate experiment, virus replication was again suppressed for 10 passages in the presence of the drug and for 2 passages without drug readdition at 10 times the EC50; thus, efavirenz was determined to exhibit a sterilizing concentration of 50 times the EC50 (50 nM) in the replicate assay. Data obtained with efavirenz in the MTSA are presented in Fig. 2 and summarized in Table 2.
(iv) Determination of the sterilizing concentration of UC781.
The NNRTI UC781 had an EC50 of 1.5 nM in the HIV-1 CPE assay and was evaluated at 15, 75, 375, 1,875, 9,375, and 46,875 nM (10, 50, 250, 1,250, 6,250, and 31,250 times the EC50, respectively). In two separate experiments with 10 passages in the presence of UC781, virus replication was suppressed at all of the concentrations when cells were cultured in the presence of the compound, except at the 15 nM concentration (10 times the EC50). At this concentration, virus replication was first detected at passage 2 and remained constant through all of the remaining passages. Following removal of the compound, virus replication was observed at 75 nM (50 times the EC50) at passage 15 in the first experiment and at 75 and 375 nM (50 and 250 times the EC50, respectively) at passages 12 and 14, respectively, in the second experiment, yielding sterilizing concentrations of 250 (375 nM) and 1,250 (1,875 nM) times the EC50, respectively. Data obtained with UC781 in the MTSA are presented in Fig. 2 and summarized in Table 2.
(v) Determination of the sterilizing concentration of CV-N.
The entry inhibitor CV-N had an EC50 of 0.016 μg/ml in the HIV-1 CPE assay and was evaluated at 0.1, 0.5, 2.5, 12.5, and 62.5 μg/ml (10, 50, 250, 1,250, and 6,250 times the EC50, respectively). In the first experiment, virus was first detected at passage 2 at the two lowest concentrations of CV-N (0.1 and 0.5 μg/ml). The compound was able to suppress virus replication at the three highest concentrations through passage 10 (all in the presence of the compound). In the absence of the compound, virus replication was observed at a concentration of 2.5 μg/ml (250 times the EC50) by passage 14. Virus replication remained suppressed at concentrations of 12.5 μg/ml (1,250 times the EC50) and 62.5 μg/ml (6,250 times the EC50) for five passages in the absence of compound, yielding a sterilizing concentration of 1,250 times the EC50 of CV-N (12.5 μg/ml). Identical sterilization results were obtained in a replicate assay. Data obtained with CV-N in the MTSA are presented in Fig. 2 and summarized in Table 2.
(vi) Determination of the sterilizing concentration of tenofovir.
The NtRTI tenofovir had an EC50 of 1.3 μM in the HIV-1 CPE assay and was evaluated at 2.5, 6.25 15.6, 39.0, 97.7, and 244.0 μM (2.5, 6.25, 15.6, 39, 97.7, and 244 times the EC50, respectively). With tenofovir, the range of concentrations evaluated in the MTSA had to be modified significantly to compensate for the toxicity of the compound to the target cells, resulting in a TI that was much narrower than those observed with most of the other compounds evaluated. Additionally, at the highest concentration evaluated (244 μM), drug toxicity to the CEM-SS cells was noted microscopically and this culture could not be maintained. In the first experiment, virus replication was detected at passage 1 at all of the concentrations tested except the two highest ones. Suppression of virus replication at these concentrations was sustained for the remaining passages in the presence of the compound, as well as through two passages in the absence of the compound. By passage 13, virus replication was detected in those cultures maintained in the presence of 39.9 μM (39 times the EC50) and by passage 14 in the cultures at 97.7 μM (97.7 times the EC50), yielding a sterilizing concentration of >97.7 times the EC50. In a second experiment, virus replication was observed at passage 1 at all of the concentrations tested except the highest one (97.7 μM). Virus replication was observed at this concentration at passage 9, also yielding a sterilizing concentration of >97.7 μM. Data obtained with tenofovir in the MTSA are presented in Fig. 2 and summarized in Table 2.
Comparison of sterilizing concentrations defined in the MTSA to EC50s and EC99s defined in standardized transmission inhibition assays.
Since the MTSA results are a measurement of the concentration of the test compound which affords complete suppression of virus replication, it is important to assess the sterilizing concentration determined in the MTSA compared to not only the EC50s in standard transmission assays but more relevantly to the EC99s defined in these assays. In Table 3, the relative EC50s and EC99s which were determined in the MAGI transmission inhibition assay are presented in comparison to the sterilizing concentrations determined in the MTSA. As expected, in all cases, the EC99 is higher than the EC50, with the exception of the compounds that were inactive in the standard MAGI transmission inhibition assay (AZT and tenofovir). Direct comparison of these inhibitory values to the MTSA-defined sterilizing concentration suggests that the sterilizing concentrations of the pyrimidinediones ranged from 1- to >212-fold compared to the EC50 and from 0.06- to >12.5-fold compared to the EC99. With the control compounds AZT, UC781, CV-N, efavirenz, and tenofovir, the range of concentration changes varied from 5- to 12,500-fold compared to the EC50 and 0.3- to 125-fold compared to the EC99. Although these results indicate that the sterilizing concentration determined in the MTSA does more closely relate to the defined EC99 rather than the EC50 determined in standard transmission inhibition assays, the MTSA does predict that higher concentrations of some test compounds are necessary to totally suppress virus transmission in cell culture. Direct comparison of the MTSA results to the calculated EC99s in the entry inhibition assay suggests that SJ-3339, UC781, efavirenz, and SJ-3991 act more effectively in the MTSA. Only SJ-3339 worked equivalently in the MTSA compared with its EC50 in the entry inhibition assay.
TABLE 3.
Comparison of EC50s and EC99s determined by a standard transmission assay to sterilizing concentrations determined by MTSA
| Compound | Entry transmission assay
|
Sterilizing concn determined by MTSA
|
||
|---|---|---|---|---|
| EC50a | EC99 | Expt 1 | Expt 2 | |
| SJ-3991 | 0.017b | 1.0 | 0.25 | 1.25 |
| SJ-3366 | 0.059 | 1.0 | >12.5 | >12.5 |
| SJ-3339 | 0.053 | 1.0 | 0.02 | 0.1 |
| AZT | >0.5 | >0.5 | >31.25 | >31.25 |
| UC781 | 0.009 | 2.98 | 0.37 | 1.9 |
| CV-N | 0.001 | 0.1 | 12.5 | 12.5 |
| Efavirenz | 0.03 | 0.5 | 0.05 | 0.05 |
| Tenofovir | >10 | >10 | >97.7 | >97.7 |
The results presented were obtained from a single representative antiviral assay with appropriate control compounds evaluated in parallel selected from a minimum of three antiviral assays. We have demonstrated that the standard error among multiple antiviral assays averaged less than 10% of the respective mean EC50 and TC50. In each individual assay, mean efficacy and toxicity values were derived from a minimum of three replicate wells.
All values are micromolar concentrations, except those for CV-N, which are in micrograms per milliliter.
DISCUSSION
Although significant discovery resources have been expended and a wide variety of compounds are being developed as potential female-controlled topical microbicides, a microbicide candidate has yet to be approved for use by the FDA (www.microbicide.org). With the increased incidence of HIV infection in women, especially in developing parts of the world, and with social stigmas surrounding the use of condoms and the lack of empowerment of women in many places around the world, the development of a female-controlled means to prevent sexual HIV transmission is urgently needed (18).
Due to the preventative nature of a microbicide, inhibitors targeting the early steps in virus replication prior to integration of the viral DNA are the primary focus of development efforts (37). A variety of in vitro mechanistic assays are currently being used in the development of topical microbicides to quantify the ability of a product to prevent HIV transmission (virus attachment, fusion, and entry inhibition assays). These assays are performed to measure the inhibitory activity of potential microbicides under the conditions that would be expected to be encountered in the vaginal environment, taking into account the cell population that would be infected (CD4+/CD4−), the virus-containing inoculum being presented to the cells (cell free or cell associated), and the short time frame that this transmission event would require (minutes to hours). To be effective, the microbicide product must prevent the infection of target cells in the vagina (or rectum) and thereby prevent HIV from establishing a life-long persistent infection. Thus, it is critical that the concentration of a microbicide to be used clinically be able to completely inhibit virus transmission to target cells in these environments (22, 37). Currently accepted and widely used transmission inhibition assays quantify the ability of a test compound to suppress cell-free and cell-associated virus transmission to the expected target cells, but the assays are often of short duration, with inhibitory activity being measured at 48 h to, at most, 6 days postinfection (21, 22). This short duration for the antiviral assay only allows for substantial transmission events to be detected and quantified, resulting in assays which may not define the true effects of a potential microbicide on rare virus transmission events. Another critical issue in microbicide development is that the activity of the compound in these assay systems is routinely reported as an EC50. While this EC50 quantification is the standard for the comparative evaluation of compounds being developed as both therapeutics and microbicides, the concentration required to totally suppress virus transmission is more important for microbicide development. Thus, in the development of preventative strategies, it is critical to define the ability of a microbicide candidate to reduce virus transmission below detectable limits. In addition, these transmission inhibition concentrations should more closely relate to the clinical concentration of a microbicide that would be needed in the vaginal environment at the time and place that the virus-containing semen inoculum is deposited.
In an effort to determine a lead microbicide candidate among the structurally similar pyrimidinediones, we modified a method previously described by Balzarini et al. (2), which we have termed the MTSA. Although this assay was originally designed for quantifying the knocking-out concentration of a systemic drug which would result in virus clearance from the body upon continued therapeutic treatment of an HIV-infected individual, the assay also has the potential to evaluate the virus transmission suppression characteristics of potential topical microbicides. After the initial infection in the system (which mimics the deposition of the viral inoculum in a cell-free or cell-associated form in the semen), which results in approximately 0.001% of the cells becoming infected, virus can be transmitted in the cultures through three independent mechanisms. First, infectious, cell-free virus produced from the infected cells or present in the inoculum may be transmitted directly in the presence of the microbicide. Second, transmission may also occur from cell to cell in the presence of the microbicide (19). Finally, it is also possible that the presence of virus in the culture results from the continued growth and expansion of the infected cells, with virus production from daughter cells contributing to the overall levels of virus detected in the culture. It should also be kept in mind that the culture is maintained in the presence of high but fixed concentrations of the microbicide product and thus the outgrowth of drug-resistant virus also may occur in a cell-free or cell-associated form. In this assay, the sterilizing concentration of the compound is defined as the concentration that completely suppresses virus replication. Balzarini et al. determined that several classes of NNRTIs, including 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine (HEPT), tetrahydroimidazobenzodiazepinones (TIBO), nevirapine, pyridinone, bis(heteroaryl)piperazines, and 2′-5′-bis- O-(tert-butyldimethylsilyl)-3′-spiro-5‴-(4‴-amino-1‴,2‴-oxathiole- 2‴,2‴-dioxide)pyrimidine (TSAO), were able to effectively sterilize the cell cultures and were able to do so at concentrations that were 100 to 250 times the defined EC50s of the compounds (2). The tested compounds prevented virus outgrowth even with subsequent passage in the absence of the test compound. This was in contrast to the nucleoside AZT, which, even at a concentration 1,000 times the EC50, failed to prevent virus breakthrough in as few as two passages. Our MTSA results match and expand those of Balzarini et al. by showing how compounds with minimal structural differences from a series of highly related pyrimidinedione congeners yield much different sterilizing concentrations. These results confirm that the MTSA measures real and relevant biological properties of the test compounds and is not simply the result of tissue culture artifacts due to the mechanical loss of infected cells as a result of the 80% dilution of the test culture every 3 days.
When evaluated in the MTSA, the pyrimidinediones had various effects on virus transmission, ranging from a highly active molecule (SJ-3339) to one that was completely inactive (SJ-3366), despite the fact that they all had very similar EC50s and EC99s in standard transmission assays. In a representative standard transmission inhibition assay, the defined EC99 was 17- to 60-fold higher than the EC50 among the pyrimidinediones. The sterilizing concentration determined in the MTSA compared to the EC99 in the standard assay was different for each compound, ranging from 16-fold less than the EC99 to greater than 125-fold higher than the defined EC99. These results suggest that the ability of a test compound to effectively prevent virus transmission is an inherent biological property of each individual compound and cannot be predicted, even among molecules which are as closely related as the pyrimidinediones. Our results suggest that superior microbicides would most likely be those in which the MTSA-defined sterilizing concentration is equal to or less than the observed EC99 in standardized assays (SJ-3339 > efavirenz > UC781 > SJ-3991). Although standard assays provide a great deal of information regarding the mechanism of inhibitory activity of potential microbicides, assays such as the MTSA may offer a more useful and informative means to assess inhibitory activity on rare transmission events, as well as provide inhibitory concentrations which may correlate more closely to the EC50s required in a formulated clinical product. Additionally, we believe that the results from the pyrimidinedione comparison justify using the MTSA as a standard transmission inhibition assay and may be used to prioritize active compounds for further development (SJ-3339 > SJ-3991 ≫ SJ-3366).
Using the MTSA, we also evaluated a series of compounds and approved drugs representing different HIV therapeutic drug classes, as well as compounds currently being developed as topical microbicides. These studies indicate that the NRTIs have significant problems with complete transmission suppression and virus sterilization, whereas the NNRTIs were among the most potent in the MTSA. In our experiments, AZT and 3TC were unable to suppress virus replication at up to 31,250 times their EC50s, even with constant drug presence. Tenofovir (NtRTI) was not able to suppress virus replication, even at the highest concentration tested (97 μM). The NNRTIs efavirenz and UC781 were two of the most potent compounds evaluated. Based on the data generated from the evaluation of efavirenz, it is apparent that the drug has significant potential as a microbicide. The entry inhibitor CV-N was also able to sterilize the culture and prevent virus transmission. These results further justify using the MTSA as a means to discriminate between active and inactive inhibitors of virus transmission, and further evaluations of these, as well as other, potential microbicides is in progress.
We have shown that the MTSA transmission assay is robust enough to segregate compounds that have highly similar chemical structures and biological anti-HIV activities and mechanisms of action. Although the MTSA may be a highly informative addition to the microbicide development algorithm, it should be understood that the assay measures the transmission inhibition potential of the active pharmaceutical ingredient (the microbicide) and not the final formulated product, since a gel or film formulation product is incompatible with the growth of cells in culture (i.e., viscosity issues). We have evaluated many biological variables of the MTSA, including variations in viral multiplicity of infection and time of microbicide addition and pretreatment versus delayed treatment conditions, in order to standardize the MTSA (unpublished data). Additional assay parameters which are currently being addressed include the effects of pretreatment without readdition of compound for the duration of the assay, evaluation of compound efficacy in the presence of seminal plasma, vaginal fluid, and mucin; with a pH shift; and in the presence of other normal and pathogenic organisms that may be present in the vaginal environment. Additionally, evaluation of the MTSA with fresh human peripheral blood mononuclear cells and clinical virus strains is being done. The MTSA can also be used to evaluate the transmission of virus with a cell-associated virus inoculum in comparison to cell-free virus transmission, as well as the transmission of drug-resistant virus. We are also aware of the possibility that culture in the presence of high fixed concentrations of a compound might result in the rapid selection of drug-resistant viruses which preexist within the heterogeneous virus inoculum, and we are currently evaluating the presence of resistant strains at the concentrations of each compound where virus breakthrough occurs. The results of assays reported herein suggest that the sterilizing concentration defined by the MTSA may provide important information which will allow the prioritization of compounds for development and for establishing the dose ranges for studies to be performed with nonhuman primates and humans. Our results thus suggest that SJ-3339, SJ-3991, efavirenz, and UC781 represent attractive microbicide candidates with the potential to be used at formulated concentrations which would totally suppress virus transmission in the vagina.
Acknowledgments
The studies presented herein were made possible by support obtained from SBIR grant R44 AI067047-02 and Microbicide Innovation Program grant R21 AI076967 from the NIAID, NIH.
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Baba, M., R. Pauwels, J. Balzarini, J. Arnout, J. Desmyter, and E. De Clercq. 1988. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 85:6132-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Karlsson, M. J. Perez-Perez, M. J. Camarasa, and E. De Clercq. 1993. Knocking-out concentrations of HIV-1-specific inhibitors completely suppress HIV-1 infection and prevent the emergence of drug-resistant virus. Virology 196:576-585. [DOI] [PubMed] [Google Scholar]
- 3.Blocker, M. E., and M. S. Cohen. 2000. Biologic approaches to the prevention of sexual transmission of human immunodeficiency virus. Infect. Dis. Clin. N. Am. 14:983-999. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurencot, M. J. Currens, J. H. Cardellina, 2nd, R. W. Buckheit, Jr., P. L. Nara, L. K. Pannell, R. C. Sowder, 2nd, and L. E. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckheit, R. W., Jr., T. L. Hartman, K. M. Watson, S. G. Chung, and E. H. Cho. 2008. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob. Agents Chemother. 52:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckheit, R. W., Jr., T. L. Hartman, K. M. Watson, H. S. Kwon, S. H. Lee, J. W. Lee, D. W. Kang, S. G. Chung, and E. H. Cho. 2007. The structure-activity relationships of 2,4(1H,3H)-pyrimidinedione derivatives as potent HIV type 1 and type 2 inhibitors. Antivir. Chem. Chemother. 18:259-275. [DOI] [PubMed] [Google Scholar]
- 7.Buckheit, R. W., Jr., M. Hollingshead, S. Stinson, V. Fliakas-Boltz, L. A. Pallansch, J. Roberson, W. Decker, C. Elder, S. Borgel, C. Bonomi, R. Shores, T. Siford, L. Malspeis, and J. P. Bader. 1997. Efficacy, pharmacokinetics, and in vivo antiviral activity of UC781, a highly potent, orally bioavailable nonnucleoside reverse transcriptase inhibitor of HIV type 1. AIDS Res. Hum. Retrovir. 13:789-796. [DOI] [PubMed] [Google Scholar]
- 8.Buckheit, R. W., Jr., T. L. Kinjerski, V. Fliakas-Boltz, J. D. Russell, T. L. Stup, L. A. Pallansch, W. G. Brouwer, D. C. Dao, W. A. Harrison, R. J. Schultz, et al. 1995. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type-1-specific compounds related to oxathiin carboxanilide. Antimicrob. Agents Chemother. 39:2718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckheit, R. W., Jr., M. J. Snow, V. Fliakas-Boltz, T. L. Kinjerski, J. D. Russell, L. A. Pallansch, W. G. Brouwer, and S. S. Yang. 1997. Highly potent oxathiin carboxanilide derivatives with efficacy against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus isolates. Antimicrob. Agents Chemother. 41:831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckheit, R. W., Jr., and R. Swanstrom. 1991. Characterization of an HIV-1 isolate displaying an apparent absence of virion-associated reverse transcriptase activity. AIDS Res. Hum. Retrovir. 7:295-302. [DOI] [PubMed] [Google Scholar]
- 11.Centers of Disease Control and Prevention. 2000. CDC statement on study results of product containing nonoxynol-9. JAMA 284:1376. [DOI] [PubMed] [Google Scholar]
- 12.Clanton, D. J., R. A. Moran, J. B. McMahon, O. S. Weislow, R. W. Buckheit, Jr., M. G. Hollingshead, V. Ciminale, B. K. Felber, G. N. Pavlakis, and J. P. Bader. 1992. Sulfonic acid dyes: inhibition of the human immunodeficiency virus and mechanism of action. J. Acquir. Immune. Defic. Syndr. 5:771-781. [PubMed] [Google Scholar]
- 13.Cloyd, M. W., and B. E. Moore. 1990. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology 174:103-116. [DOI] [PubMed] [Google Scholar]
- 14.Coates, J. A., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (-)-2′-deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315-336. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. 1997. Guidance for industry: points to consider in the preclinical development of antiviral drugs. Food and Drug Administration, Washington, DC.
- 17.Halliday, S. M., C. Lackman-Smith, J. P. Bader, W. G. Rice, D. J. Clanton, L. H. Zalkow, and R. W. Buckheit, Jr. 1996. Inhibition of human immunodeficiency virus replication by the sulfonated stilbene dye resobene. Antivir. Res. 33:41-53. [DOI] [PubMed] [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS and World Health Organization. 2007. AIDS epidemic: December 2007. World Health Organization, Geneva, Switzerland.
- 19.Jolly, C., and Q. J. Sattentau. 2007. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J. Virol. 81:7873-7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackman-Smith, C., C. Osterling, K. Luckenbaugh, M. Mankowski, B. Snyder, G. Lewis, J. Paull, A. Profy, R. G. Ptak, R. W. Buckheit, Jr., K. M. Watson, J. E. Cummins, Jr., and B. E. Sanders-Beer. 2008. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob. Agents Chemother. 52:1768-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lard-Whiteford, S. L., D. Matecka, J. J. O'Rear, I. S. Yuen, C. Litterst, and P. Reichelderfer. 2004. Recommendations for the nonclinical development of topical microbicides for prevention of HIV transmission: an update. J. Acquir. Immune. Defic. Syndr. 36:541-552. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy, T. D., P. Karellas, S. A. Henderson, M. Giannis, D. F. O'Keefe, G. Heery, J. R. Paull, B. R. Matthews, and G. Holan. 2005. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharmacol. 2:312-318. [DOI] [PubMed] [Google Scholar]
- 24.Mocroft, A., S. Vella, T. L. Benfield, A. Chiesi, V. Miller, P. Gargalianos, A. d'Arminio Monforte, I. Yust, J. N. Bruun, A. N. Phillips, and J. D. Lundgren. 1998. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 352:1725-1730. [DOI] [PubMed] [Google Scholar]
- 25.Morner, A., A. Bjorndal, J. Albert, V. N. Kewalramani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima, H., T. Matsui, S. Harada, N. Kobayashi, A. Matsuda, T. Ueda, and N. Yamamoto. 1986. Inhibition of replication and cytopathic effect of human T cell lymphotropic virus type III/lymphadenopathy-associated virus by 3′-azido-3′-deoxythymidine in vitro. Antimicrob. Agents Chemother. 30:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nara, P. L., W. C. Hatch, N. M. Dunlop, W. G. Robey, L. O. Arthur, M. A. Gonda, and P. J. Fischinger. 1987. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res. Hum. Retrovir. 3:283-302. [DOI] [PubMed] [Google Scholar]
- 28.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 29.Patton, D. L., Y. C. Sweeney, P. K. Cummings, L. Meyn, L. K. Rabe, and S. L. Hillier. 2004. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex. Transm. Dis. 31:290-296. [DOI] [PubMed] [Google Scholar]
- 30.Patton, D. L., Y. T. Sweeney, J. E. Balkus, L. C. Rohan, B. J. Moncla, M. A. Parniak, and S. L. Hillier. 2007. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob. Agents Chemother. 51:1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips, D. M., X. Tan, R. Pearce-Pratt, and V. R. Zacharopoulos. 1995. An assay for HIV infection of cultured human cervix-derived cells. J. Virol. Methods 52:1-13. [DOI] [PubMed] [Google Scholar]
- 32.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 33.Ramjee, G., N. S. Morar, S. Braunstein, B. Friedland, H. Jones, and J. van de Wijgert. 2007. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res. Ther. 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starcich, B. R., B. H. Hahn, G. M. Shaw, P. D. McNeely, S. Modrow, H. Wolf, E. S. Parks, W. P. Parks, S. F. Josephs, R. C. Gallo, et al. 1986. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45:637-648. [DOI] [PubMed] [Google Scholar]
- 35.Trottier, S., R. F. Omar, A. Desormeaux, J. Drouin, M. T. Gagnon, F. Vezina, E. Guilbert, B. Masse, and M. G. Bergeron. 2007. Safety, tolerance and acceptability of the Invisible Condom and its vaginal applicator in healthy women and their male sexual partners. Contraception 76:117-125. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C. C., K. E. Follis, A. Sabo, T. W. Beck, R. F. Grant, N. Bischofberger, R. E. Benveniste, and R. Black. 1995. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 270:1197-1199. [DOI] [PubMed] [Google Scholar]
- 37.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 38.Van Damme, L., A. Wright, K. Depraetere, I. Rosenstein, V. Vandersmissen, L. Poulter, M. McKinlay, E. Van Dyck, J. Weber, A. Profy, M. Laga, and V. Kitchen. 2000. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex. Transm. Infect. 76:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Herrewege, Y., J. Michiels, J. Van Roey, K. Fransen, L. Kestens, J. Balzarini, P. Lewi, G. Vanham, and P. Janssen. 2004. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob. Agents Chemother. 48:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts, C., and C. Zimmerman. 2002. Violence against women: global scope and magnitude. Lancet 359:1232-1237. [DOI] [PubMed] [Google Scholar]
- 41.Woolfson, A. D., R. K. Malcolm, R. J. Morrow, C. F. Toner, and S. D. McCullagh. 2006. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int. J. Pharm. 325:82-89. [DOI] [PubMed] [Google Scholar]
- 42.Young, S. D., S. F. Britcher, L. O. Tran, L. S. Payne, W. C. Lumma, T. A. Lyle, J. R. Huff, P. S. Anderson, D. B. Olsen, S. S. Carroll, et al. 1995. L-743, 726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 39:2602-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]