Abstract
The bactericidal activities and postantibiotic effects (PAEs) of levofloxacin and gatifloxacin at concentrations corresponding to those in antibiotic eye drops against methicillin-resistant Staphylococcus aureus strains were determined. Levofloxacin and gatifloxacin at concentrations simulating those in eye drops showed lower bactericidal activities and shorter PAEs against fluoroquinolone-resistant strains than against fluoroquinolone-sensitive strains.
Ocular infections caused by methicillin-resistant Staphylococcus aureus (MRSA) strains, which are clinically serious and difficult to treat, have been reported (18, 27-30, 34). The prevalence of levofloxacin (LVX) resistance in MRSA strains isolated from patients with ocular infections is 80 to 90% in Japan and the United States (12, 14, 17). Topical treatments may have selected for strains with high-level fluoroquinolone resistance (1, 8, 10, 14, 17), which is associated with multiple mutations in the quinolone resistance-determining regions (QRDRs) of MRSA strains isolated at ophthalmologic clinics (10). Although topical fluoroquinolone eye drops contain much higher concentrations (0.3 to 1.5%; equivalent to 3,000 to 15,000 μg/ml) than their MICs even against strains with high-level fluoroquinolone resistance, the antibiotic concentrations in tear films decline rapidly (by about 1/100 at 30 min) (31), and treatment failures have been noted (6, 33). We performed time-kill and postantibiotic effect (PAE) studies with LVX and gatifloxacin (GAT) at concentrations simulating those of topical ophthalmic administration against fluoroquinolone-resistant and -sensitive MRSA strains to examine the efficacies of these drugs. We examined nine typical Japanese health care facility-associated MRSA strains: staphylococcal cassette chromosome mec type IIa (3, 13, 22, 24) strains with high-level LVX resistance (LHR; MICs, ≥64 μg/ml), low-level LVX resistance (LLR; MICs, 4 to 32 μg/ml), or LVX sensitivity (LS; MICs, ≤1 μg/ml). Cation-adjusted Muller-Hinton II broth (CAMHB; BD, Sparks, MD) and Mueller-Hinton agar (BD) were used for determination of the MICs, time-kill profile analyses, PAE assays, and viable count assays. MICs were measured by broth microdilution methods and were interpreted according to the recommendations of the Clinical and Laboratory Standards Institute (4). To elucidate the contribution of efflux mediated by NorA to fluoroquinolone resistance, the MICs of LVX and GAT were determined in the presence or absence of 20 μg/ml reserpine, which is a multidrug transport inhibitor (21). The QRDRs and norA promoter regions were sequenced as reported previously (10, 20, 21, 35). Time-kill assays were performed according to previously published guidelines (19). LVX and GAT were used at 4×, 8×, and 16× the MICs and at 2,048 μg/ml, which is similar to the concentrations of these two drugs used in eye drops. The initial inoculum (time zero) was adjusted to 1 × 106 CFU/ml. Samples were taken at 0 min, 5 min, and 30 min and at 1, 2, 4, 6, and 24 h. Serial 10-fold dilutions in sterile saline were plated, and the colonies were counted after 48 h of incubation at 37°C (19). Bactericidal activity was defined as a >3-log10 decrease in the numbers of CFU/ml (19). The PAEs of LVX and GAT were determined by the filter method (5). Strains grown in culture to 1 × 106 CFU/ml in the exponential phase were exposed to 3,000 μg/ml LVX or 2,000 μg/ml GAT for 5 min; these antibiotic concentrations were derived from the areas under the concentration-time curves of LVX and GAT in tears after the administration of a single eye drop (25, 31). After removal of the drugs and six washes with saline, the membrane-trapped bacteria were resuspended in prewarmed CAMHB and reincubated (5). Control bacteria not exposed to drug were washed and resuspended in a similar manner. Viable counts were determined every 1 h until the cultures in the test tubes were turbid. The PAE was defined as described previously (5).
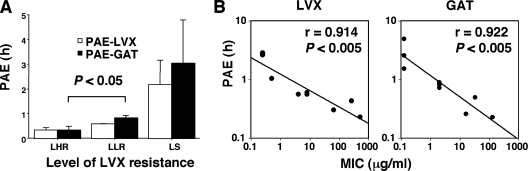
The MIC profiles and the mechanisms of fluoroquinolone resistance in the MRSA strains used are shown in Table 1. The level of fluoroquinolone resistance was dependent upon multiplication of the mutation sites in the QRDRs. The correlation between changes in the norA promoter region and the effects of reserpine on the MICs of LVX and GAT suggested that the NorA efflux system did not confer fluoroquinolone resistance on the strains used, as reported previously (9, 10, 21). The results of the time-kill assays for LVX and GAT are presented in Fig. 1 and 2. At up to 60 min of exposure, LVX and GAT showed no detectable bactericidal activities at 4× to 16× the MICs. Only GAT was bactericidal against the LLR strains at 120 min of exposure. At a high concentration (2,048 μg/ml), close to that present in eye drops, LVX completely killed (>99.9%) only one of three LHR strains, zero of three LLR strains, and two of three LS strains, whereas GAT completely killed two of three LHR strains, two of three LLR strains, and three of three LS strains at 60 min. All the strains were killed completely by LVX and GAT at 4×, 8×, and 16× the MICs and at 2,048 μg/ml after 24 h of exposure (data not shown). Overall, GAT was more bactericidal than LVX for the MRSA strains. Similar findings have been obtained in previous studies, and it has been suggested that the differences observed are attributable to the C-8 methoxy moiety (2, 11). The PAEs of LVX and GAT are presented in Fig. 3. The PAEs of both drugs against the LHR and LLR strains were shorter in duration than those against the LS strains (Fig. 3A). There was a linear correlation between the PAEs of both drugs and their MICs (Fig. 3B). Since LVX and GAT at sub-MICs show PAEs against S. aureus strains (15, 23), the infrequent ophthalmic administration of both drugs may be effective against ocular infections caused by LS strains. The shorter PAEs and lower bactericidal activities against LHR and LLR strains compared to those against LS strains may affect clinical outcomes. The maintenance of adequate concentrations of fluoroquinolones (preferably GAT) by frequent administration may be more clinically efficacious against fluoroquinolone-resistant strains. Indeed, the frequent administration of eye drops containing GAT has successfully been used to treat moderately or highly resistant S. aureus strains (MICs, 12 μg/ml and >64 μg/ml, respectively) in a rabbit keratitis model (26). However, high concentrations of fluoroquinolones can cause corneal perforations in humans (7, 16, 32). We did not investigate how the growth-inhibitory effects of long-duration supra-MIC and sub-MIC dosages of antibiotics following brief exposure to a high concentration of the antibiotic affected the antibacterial responses. Further studies are required to determine the usefulness of these two antibiotics in topical formulations (eye drops) in different in vitro simulation models that reproduce the pharmacokinetic-pharmacodynamic properties of the antibiotics in the human eye when they are administered on traditional dosing schedules.
TABLE 1.
Fluoroquinolone susceptibilities and mutation profiles of MRSA strains
| Strain | MIC (μg/ml)
|
Type of LVX resistance | Amino acid or nucleotide change ina:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OXA | LVX | LVX + reserpine | GAT | GAT + reserpine | gyrA | gyrB | grlA | norAb | ||
| JCSC 6763 | 512 | 512 | 512 | 128 | 128 | LHR | Ser84Leu + Ser85Pro | Ser80Phe + Glu84Lys | In362-IS256 | |
| JCSC 6764 | 512 | 256 | 256 | 32 | 32 | LHR | Ser84Leu | Asp437Glu | Ser80Tyr + Glu84Lys | T389C |
| JCSC 6765 | 256 | 64 | 64 | 16 | 16 | LHR | Ser84Leu | Ser80Tyr + Glu84Lys | T389C | |
| JCSC 6766 | 512 | 8 | 8 | 2 | 2 | LLR | Glu88Lys | Ser80Phe | ||
| JCSC 6767 | 256 | 8 | 8 | 2 | 2 | LLR | Glu88Lys | Ser80Phe | ||
| JCSC 6768 | 256 | 4 | 2 | 2 | 1 | LLR | Glu88Lys | Ser80Phe | ||
| JCSC 6769 | 512 | 0.5 | 0.5 | 0.125 | 0.125 | LS | Glu84Gly | |||
| JCSC 6770 | 256 | 0.25 | 0.25 | 0.125 | 0.125 | LS | ||||
| JCSC 6822 | 128 | 0.25 | 0.25 | 0.125 | 0.125 | LS | In346-A | |||
No changes were detected in grlB.
Portion of the norA gene from nucleotides 305 to 476 encompassing the −35 and −10 sequences in the promoter region representing the transcriptional start site described by Yoshida et al. (35)
FIG. 1.
Killing activities (log10 CFU/ml) of LVX and GAT against LS, LLR, and LHR MRSA strains at exposure times of 5 to 120 min. Each test group contained three different strains. The data are presented as means with 1 standard deviation (bars). P values for comparisons of LVX and GAT treatments are as follows: *, P < 0.002; **, P < 0.02; ***, P < 0.03; ****, P < 0.05. The dashed lines designate nearly complete bactericidal activity (>99.9% killing) for each drug.
FIG. 2.
Time-kill assays for 2,048 μg/ml LVX and GAT against LS, LLR, and LHR MRSA strains. The dashed lines designate nearly complete bactericidal activity (>99.9% killing) for each drug.
FIG. 3.
PAEs (A) and relationship between PAEs and MICs (B) of LVX (3,000 μg/ml) and GAT (2,000 μg/ml) against LS, LLR, and LHR MRSA strains after 5 min of exposure to the antibiotics. Each test group comprised three different strains. The data are presented as means with 1 standard deviation (bars).
Acknowledgments
This work was supported by a Grant-in-Aid for 21st Century COE Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Alexandrakis, G., E. C. Alfonso, and D. Miller. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497-1502. [DOI] [PubMed] [Google Scholar]
- 2.Ba, B. B., C. Arpin, C. Vidaillac, A. Chausse, M.-C. Saux, and C. Quentin. 2006. Activity of gatifloxacin in an in vitro pharmacokinetic-pharmacodynamic model against Staphylococcus aureus strains either susceptible to ciprofloxacin or exhibiting various levels and mechanisms of ciprofloxacin resistance. Antimicrob. Agents Chemother. 50:1931-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J.-H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 6.Dajcs, J. J., B. A. Thibodeaux, M. E. Marquart, D. O. Girgis, M. Traidej, and R. J. O'Callaghan. 2004. Effectiveness of ciprofloxacin, levofloxacin, or moxifloxacin for treatment of experimental Staphylococcus aureus keratitis. Antimicrob. Agents Chemother. 48:1948-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangopadhyay, N., M. Daniell, L. Weih, and H. R. Taylor. 2000. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br. J. Ophthalmol. 84:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein, M. H., R. P. Kowalski, and Y. J. Gordon. 1999. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 106:1313-1318. [PubMed] [Google Scholar]
- 9.Horii, T., Y. Suzuki, A. Takeshita, and M. Maekawa. 2007. Molecular characterization of 8-methoxyfluoroquinolone resistance in a clinical isolate of methicillin-resistant Staphylococcus aureus. Chemotherapy (Basel) 53:104-109. [DOI] [PubMed] [Google Scholar]
- 10.Iihara, H., T. Suzuki, Y. Kawamura, K. Ohkusu, Y. Inoue, W. Zhang, M. M. Shah, Y. Katagiri, Y. Ohashi, and T. Ezaki. 2006. Emerging multiple mutations and high-level fluoroquinolone resistance in methicillin-resistant Staphylococcus aureus isolated from ocular infections. Diagn. Microbiol. Infect. Dis. 56:297-303. [DOI] [PubMed] [Google Scholar]
- 11.Ince, D., and D. C. Hooper. 2001. Mechanisms and frequency of resistance to gatifloxacin in comparison to AM-1121 and ciprofloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamo, J., H. Yamamoto, S. Muramatsu, and A. Unno. 2006. Kinds of bacteria from conjunctivitis discharge at both ward and clinic and their susceptibility to drugs 2001-2005. Atarashii Ganka 23:219-224. (In Japanese.) [Google Scholar]
- 13.Kikuchi, K., N. Takahashi, C. Piao, K. Totsuka, H. Nishida, and T. Uchiyama. 2003. Molecular epidemiology of methicillin-resistant Staphylococcus aureus strains causing neonatal toxic shock syndrome-like exanthematous disease in neonatal and perinatal wards. J. Clin. Microbiol. 41:3001-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotulus, B. S., R. A. Wymbs, E. M. Vellozzi, and I. J. Udell. 2006. In vitro activity of fluoroquinolones, vancomycin, and gentamicin against methicillin-resistant Staphylococcus aureus ocular isolates. Am. J. Ophthalmol. 142:726-729. [DOI] [PubMed] [Google Scholar]
- 15.Licata, L., C. E. Smith, R. M. Goldschmidt, J. F. Barrett, and M. Frosco. 1997. Comparison of the postantibiotic and postantibiotic sub-MIC effects of levofloxacin and ciprofloxacin on Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:950-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd, P., T. Mallari, D. J. McCarty, M. Daniell, and H. Taylor. 2001. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am. J. Ophthalmol. 131:131-133. [DOI] [PubMed] [Google Scholar]
- 17.Marangon, F. B., D. Miller, M. S. Muallem, A. C. Romano, and E. C. Alfonso. 2004. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am. J. Ophthalmol. 137:453-458. [DOI] [PubMed] [Google Scholar]
- 18.Moshirfar, M., V. Feiz, A. T. Vitale, J. A. Wegelin, S. Basavanthappa, and D. H. Wolsey. 2007. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones. Ophthalmology 114:686-691. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Noguchi, N., H. Okada, K. Narui, and M. Sasatsu. 2004. Comparison of the nucleoside sequence and expression of norA genes and microbial susceptibility in 21 strains of Staphylococcus aureus. Microb. Drug Resist. 10:197-203. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi, N., T. Okihara, Y. Namiki, Y. Kumaki, Y. Yamanaka, M. Koyama, K. Wakasugi, and M. Sasatsu. 2005. Susceptibility and resistance gene to fluoroquinolones in methicillin-resistant Staphylococcus aureus isolated in 2002. Int. J. Antimicrob. Agents 25:374-379. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira, D. C., A. Tomasz, and H. de Lencastre. 2002. Secrets of success of a human pathogen: molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 2:180-189. [DOI] [PubMed] [Google Scholar]
- 23.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1999. Postantibiotic effects of gatifloxacin against gram-positive and -negative organisms. Antimicrob. Agents Chemother. 43:2574-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piao, C., T. Karasawa, K. Totsuka, T. Uchiyama, and K. Kikuchi. 2005. Prospective surveillance of community-onset and healthcare-associated methicillin-resistant Staphylococcus aureus isolated from a university-affiliated hospital in Japan. Microbiol. Immunol. 49:959-970. [DOI] [PubMed] [Google Scholar]
- 25.Raizman, M. B., J. M. Rubin, A. L. Graves, and M. Rinehart. 2002. Tear concentrations of levofloxacin following topical administration of a single dose of 0.5% levofloxacin ophthalmic solution in healthy volunteers. Clin. Ther. 24:1439-1450. [DOI] [PubMed] [Google Scholar]
- 26.Romanowski, E. G., F. S. Mah, K. A. Yates, R. P. Koalski, and J. Gordon. 2005. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with Zymar® (gatifloxacin 0.3%) in a NZW rabbit model. Am. J. Ophthalmol. 139:867-877. [DOI] [PubMed] [Google Scholar]
- 27.Rutar, T., H. F. Chambers, J. B. Crawford, F. Perdreau-Remington, O. M. Zwick, M. Karr, J. J. Diehn, and K. P. Cockerham. 2006. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology 113:1455-1462. [DOI] [PubMed] [Google Scholar]
- 28.Rutar, T., O. M. Zwick, K. P. Cockerham, and J. C. Horton. 2005. Bilateral blindness from orbital cellulitis caused by community-acquired methicillin-resistant Staphylococcus aureus. Am. J. Ophthalmol. 140:740-742. [DOI] [PubMed] [Google Scholar]
- 29.Solomon, R., E. D. Donnenfeld, H. D. Perry, R. S. Rubinfeld, M. Ehrenhaus, J. R. Wittpenn, K. D. Solomon, E. E. Manche, M. Moshirfar, D. C. Matzkin, R. M. Mozayeni, and R. K. Maloney. 2007. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am. J. Ophthalmol. 143:629-634. [DOI] [PubMed] [Google Scholar]
- 30.Sotozono, C., K. Inagaki, A. Fujita, N. Koizumi, Y. Sano, T. Inatomi, and S. Kinoshita. 2002. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infectious in the cornea. Cornea 21(Suppl. 2):S94-S101. [DOI] [PubMed] [Google Scholar]
- 31.Wada, T., T. Tajika, H. Takahashi, H. Sakaki, and Y. Miyagawa. 2004. Postantibiotic effect of gatifloxacin in ophthalmic use. Atarashii Ganka 21:1520-1524. (In Japanese.) [Google Scholar]
- 32.Walter, K., and M. E. Tyler. 2006. Severe corneal toxicity after topical fluoroquinolone therapy. report of two cases. Cornea 25:855-857. [DOI] [PubMed] [Google Scholar]
- 33.Wilhelmus, K. R., R. L. Abshire, and B. A. Schlech. 2003. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch. Ophthalmol. 121:1229-1233. [DOI] [PubMed] [Google Scholar]
- 34.Wroblewski, K. J., J. F. Pasternak, K. S. Bower, S. C. Schallhorn, W. J. Hubickey, C. E. Harrison, M. F. Torres, and S. D. Barnes. 2006. Infectious keratitis after photorefractive keratectomy in the United States army and navy. Ophthalmology 113:520-525. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, H., M. Bogaki, S. Nakamura, K. Ubukata, and M. Konno. 1990. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 172:6942-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]