Abstract
In vitro susceptibility profiles of 58 Paecilomyces clinical isolates are reported. Amphotericin B, itraconazole, and echinocandins showed poor activity against Paecilomyces lilacinus, while the new triazoles were active against it. Paecilomyces variotii exhibited a different susceptibility pattern, being susceptible to most antifungal agents apart from voriconazole and ravuconazole.
Paecilomyces species are saprophytic filamentous fungi that are found worldwide in soil and as air and water contaminants (9, 13). Among species in this genus, Paecilomyces lilacinus and Paecilomyces variotii are of clinical importance, as they are an increasing cause for opportunistic and usually severe human infections (2, 4, 5, 15) generally associated with the use of immunosuppression therapy, implants, or ocular surgery.
The differentiation between these two species is clinically important, since P. lilacinus and P. variotii seem to present marked differences in their in vitro susceptibilities to the antifungal agents. We report here the in vitro susceptibility profile of a collection of P. variotii and P. lilacinus clinical isolates.
Strains.
This study included 58 clinical isolates of Paecilomyces spp. obtained from a variety of clinical sources.
Morphological identification.
The strains were subcultured at 30°C in malt extract agar (2% malt extract) (Oxoid S.A., Madrid, Spain) and potato dextrose agar (Oxoid) to ascertain their macroscopic and microscopic morphologies.
Molecular identification by sequencing of internal transcribed spacer (ITS) region.
Molds were cultured in GYEP medium (0.3% yeast extract, 1% peptone) (Difco, Soria Melguizo S.A., Madrid, Spain) with 2% glucose (Sigma-Aldrich Quimica, Madrid, Spain) for 24 to 48 h at 30°C. Genomic DNA was isolated using an extraction procedure previously described (10).
DNA segments comprising the ITS1 and ITS2 regions were amplified with primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) in a GeneAmp PCR System 9700 (Applied Biosystems, Madrid, Spain) (19). The reaction products were analyzed in a 0.8% agarose gel. Sequencing reactions were done with 2 μl of a sequencing kit (BigDye Terminator cycle sequencing ready reaction kit; Applied Biosystems), 1 μM of the primers (ITS1 or ITS4), and 3 μl of purified PCR product in a final volume of 10 μl.
Sequence analysis.
Sequences were assembled and edited using the SeqMan II and EditSeq software packages (Lasergene; DNAStar, Inc., Madison, WI). Sequence analysis was performed by comparing the DNA sequences with the ITS sequences of P. lilacinus AY213665 (ATCC 10114) and P. variotii AY753328 (CBS 102.74) and AY373941 (ATCC 22319) obtained from the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/).
Phylogenetic analysis.
All phylogenetic analyses were conducted with InfoQuest FP software v4.50 (Bio-Rad Laboratories, Madrid, Spain) using the maximum parsimony clustering method. Phylogram stability was assessed by parsimony bootstrapping with 2,000 simulations. The ITS sequence of Rhizopus oryzae CNM-CM-4875 (Mold Collection of the Spanish National Center for Microbiology) was used as the outgroup.
Antifungal susceptibility testing.
Microdilution testing was performed by following the EUCAST document (http://www.escmid.org/Files/EUCAST%20moulds%20discussion%bp20document_071019.pdf) (1, 12, 17, 18). Aspergillus fumigatus ATCC 2004305 and Aspergillus flavus ATCC 2004304 were used as quality control strains (14).
The antifungal agents used were amphotericin B (AMB) (Sigma Aldrich Química), itraconazole (ITC) (Janssen S.A., Madrid, Spain), voriconazole (VOR) (Pfizer S.A., Madrid, Spain), ravuconazole (RVC) (Bristol-Myers Squibb, Princeton, NJ), posaconazole (POS) (Schering-Plough Research Institute, Kenilworth, NJ), terbinafine (TRB) (Novartis, Basel, Switzerland), caspofungin (Merck & Co., Inc., Rahway, NJ), micafungin (Astellas Pharma Inc., Tokyo, Japan), and anidulafungin (Pfizer S.A). The endpoint for AMB, ITC, VOR, RVC, POS, and TRB was the antifungal concentration that produced a complete inhibition of visual growth at 48 h. For the echinocandins, the endpoint was the antifungal concentration that produced a visible change in the morphology of the hyphae compared with that of the growth control well (minimum effective concentration [MEC]) (3, 11).
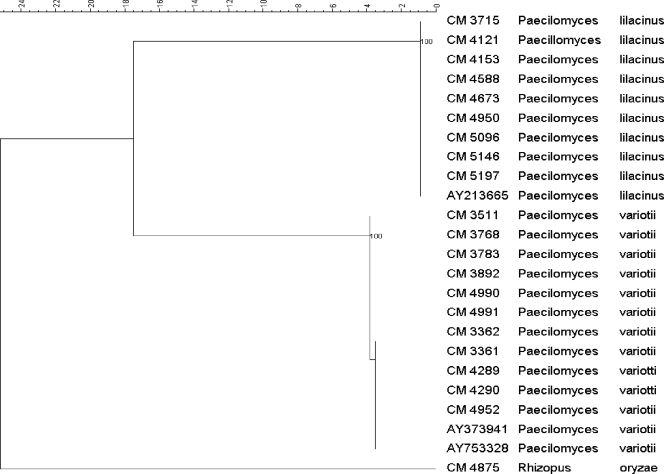
Twenty-seven strains were identified as P. lilacinus and 31 as P. variotii by means of studying the morphology (7). In 20 cases, species were identified also by molecular methods as described above and invariably matched morphological identifications (Fig. 1).
FIG. 1.
Phylogenetic tree of the subset of isolates included in the study obtained by using maximum parsimony phylogenetic analyses and 2,000 bootstrap simulations based on ITS sequences. Rhizopus oryzae CNM-CM 4875 was used as the outgroup to root the tree.
Susceptibility data and MIC distribution are displayed in Table 1. P. lilacinus showed high MICs of AMB, ITC, and echinocandins with geometric means (GM) of MICs/MECs of >8 mg/liter. In contrast, VOR, POS, and TRB were active against this species (GM of MICs, <2 mg/liter), with POS being the drug with the best in vitro activity (GM, 0.28 mg/liter).
TABLE 1.
Susceptibility results of Paecilomyces sp. clinical strains, including GM and MICs distribution in mg/liter by species and antifungal agent
| Antifungal agent | Species | GM | No. of times the indicated MIC was reported
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |||
| AMB | P. variotii | 0.04 | 11 | 8 | 4 | 4 | 2 | 2 | ||||||
| P. lilacinus | 29.6 | 3 | 24 | |||||||||||
| ITC | P. variotii | 0.06 | 4 | 8 | 9 | 3 | 6 | 1 | ||||||
| P. lilacinus | 13.4 | 1 | 2 | 24 | ||||||||||
| VOR | P. variotii | 4.18 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 13 | 6 | |||
| P. lilacinus | 0.45 | 2 | 10 | 9 | 4 | 1 | 1 | |||||||
| RVC | P. variotii | 4.89 | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 4 | 16 | |||
| P. lilacinus | 0.73 | 1 | 2 | 7 | 14 | 3 | ||||||||
| POS | P. variotii | 0.04 | 6 | 12 | 6 | 5 | 1 | 1 | ||||||
| P. lilacinus | 0.28 | 1 | 3 | 14 | 8 | 1 | ||||||||
| TRB | P. variotii | 1.53 | 1 | 1 | 6 | 5 | 10 | 6 | 1 | 1 | ||||
| P. lilacinus | 0.51 | 8 | 11 | 7 | 1 | |||||||||
| Caspofungin | P. variotii | 0.44 | 1 | 3 | 1 | 5 | 13 | 2 | 3 | 3 | ||||
| P. lilacinus | 27.9 | 1 | 26 | |||||||||||
| Micafungin | P. variotii | 0.018 | 25 | 6 | ||||||||||
| P. lilacinus | 25.6 | 1 | 26 | |||||||||||
| Anidulafungin | P. variotii | 0.016 | 29 | 2 | ||||||||||
| P. lilacinus | 27.1 | 1 | 26 | |||||||||||
P. variotii showed a different susceptibility pattern; the GM of MICs/MECs were <2 mg/liter for AMB, ITC, POS, TRB, and echinocandins. Although the GM of MICs for TRB were <2 mg/liter, 18 out of 27 strains showed MICs of ≥2 mg/liter. In addition, 22 out of 27 (81.5%) strains showed MICs of ≥2 mg/liter for VOR. A similar pattern was observed for RVC, for which 21 out of 27 (77.8%) strains had MICs of ≥2 mg/liter.
Limited information about the in vitro antifungal activities for these species is available in the literature, and an optimal treatment has not been established. In addition, some reports show susceptibility results per genus but not species, as well as MICs determined by different methods, which make results incomparable (15).
We have reviewed here the antifungal susceptibility data of 58 Paecilomyces clinical isolates. P. lilacinus and P. variotii showed different susceptibility profiles to the antifungal drugs tested. Our results are in agreement with previous findings for P. lilacinus, except for echinocandins for which contradictory data have been reported (8, 15, 20). Regarding cross-resistance to azole drugs, our data indicate that it is not predictable and all available azoles should be tested in order to set the susceptibility profile of each isolate.
Due to very little data concerning the antifungal susceptibility profiles for these species, this work will contribute toward establishing an optimal antifungal therapy for these fungi. VOR alone or in combination with TRB has been successfully used for the treatment of P. lilacinus oculomycosis and cutaneous or subcutaneous infection (16). AMB seems to be the treatment of choice for P. variotii infections, and VOR resistance has been described previously (6). According our data, new triazoles and TRB constitute the most promising alternatives for the treatment of P. lilacinus infections. In contrast, with the exception of VOR and RVC, all drugs tested against P. variotii showed good in vitro activity.
Since they have different susceptibility profiles, correct characterization of these species is compulsory. Our study shows that both morphological and molecular identification methods are useful for distinguishing these species as the ITSs are good targets for molecularly characterizing these organisms.
Acknowledgments
María Victoria Castelli has a research contract from Agencia Española de Cooperación Internacional. Ana Alastruey-Izquierdo has a predoctoral fellowship from Fondo de Investigaciones Sanitarias (grant FI05/00856). Isabel Cuesta has a research contract from Red Española de Investigación de Patología Infecciosa (REIPI). This work was supported in part by the research project PI05/32 from the Instituto de Salud Carlos III and from REIPI.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Aberkane, A., M. Cuenca-Estrella, A. Gomez-Lopez, E. Petrikkou, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and the Eurofung Network. 2002. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 50:719-722. [DOI] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., T. J. Walsh, and E. Roilides. 2007. Fungal infections in primary immunodeficiencies. Eur. J. Pediatr. 166:1099-1117. [DOI] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey, J., R. D'Amico, D. A. Sutton, and M. G. Rinaldi. 2003. Paecilomyces lilacinus vaginitis in an immunocompetent patient. Emerg. Infect. Dis. 9:1155-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro, L. G., A. Salebian, and M. N. Sotto. 1990. Hyalohyphomycosis by Paecilomyces lilacinus in a renal transplant patient and a review of human Paecilomyces species infections. J. Med. Vet. Mycol. 28:15-26. [PubMed] [Google Scholar]
- 6.Chamilos, G., and D. P. Kontoyiannis. 2005. Voriconazole-resistant disseminated Paecilomyces variotii infection in a neutropenic patient with leukaemia on voriconazole prophylaxis. J. Infect. 51:e225-e228. [DOI] [PubMed] [Google Scholar]
- 7.de Hoog, G., J. Guarro, C. S. Tan, R. G. F. Wintermans, and J. Gene. 1995. Hyphomycetes, p. 380-1007. In G. S. de Hoog and J. Guarro (ed.), Atlas of clinical fungi. Universitat Rovira i Virgili and Centraalbureau voor Schimmelcultures, Reus, España and Utrecht, The Netherlands.
- 8.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez, F., M. Masia, J. Ramos, M. Elia, E. Mellado, and M. Cuenca-Estrella. 2005. Pulmonary mycetoma caused by an atypical isolate of Paecilomyces species in an immunocompetent individual: case report and literature review of Paecilomyces lung infections. Eur. J. Clin. Microbiol. Infect. Dis. 24:607-611. [DOI] [PubMed] [Google Scholar]
- 10.Holden, D. W. 1994. DNA mini prep method for Aspergillus fumigatus (and other filamentous fungi), p. 3-4. In B. Maresca and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungi, a laboratory manual. Telos Press, New York, NY.
- 11.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lass-Florl, C., M. Cuenca-Estrella, D. W. Denning, and J. L. Rodriguez-Tudela. 2006. Antifungal susceptibility testing in Aspergillus spp. according to EUCAST methodology. Med. Mycol. 44(Suppl.):319-325. [DOI] [PubMed] [Google Scholar]
- 13.Madsen, A. M., V. M. Hansen, N. V. Meyling, and J. Eilenberg. 2007. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann. Agric. Environ. Med. 14:5-24. [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute/NCCLS. 2005. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 15.Pastor, F. J., and J. Guarro. 2006. Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clin. Microbiol. Infect. 12:948-960. [DOI] [PubMed] [Google Scholar]
- 16.Pastor, F. J., and J. Guarro. 2007. The role of voriconazole in the treatment of emerging mycoses. Rev. Iberoam. Micol. 24:228-232. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 17.Petrikkou, E., J. L. Rodriguez-Tudela, M. Cuenca-Estrella, A. Gomez, A. Molleja, and E. Mellado. 2001. Inoculum standardization for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J. Clin. Microbiol. 39:1345-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Tudela, J. L., E. Chryssanthou, E. Petrikkou, J. Mosquera, D. W. Denning, and M. Cuenca-Estrella. 2003. Interlaboratory evaluation of hematocytometer method of inoculum preparation for testing antifungal susceptibilities of filamentous fungi. J. Clin. Microbiol. 41:5236-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Tudela, J. L., T. M. Diaz-Guerra, E. Mellado, V. Cano, C. Tapia, A. Perkins, A. Gomez-Lopez, L. Rodero, and M. Cuenca-Estrella. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 49:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida, K., Y. Nishiyama, N. Yokota, and H. Yamaguchi. 2000. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J. Antibiot. (Tokyo) 53:1175-1181. [DOI] [PubMed] [Google Scholar]