Abstract
Phosphorothioated oligonucleotides have a sequence-independent antiviral activity as amphipathic polymers (APs). The activity of these agents against herpesvirus infections in vitro and in vivo was investigated. The previously established sequence-independent, phosphorothioation-dependent antiviral activity of APs was confirmed in vitro by showing that a variety of equivalently sized homo- and heteropolymeric AP sequences were similarly active against herpes simplex virus type 1 (HSV-1) infection in vitro compared to the 40mer degenerate parent compound (REP 9), while the absence of phosphorothioation resulted in the loss of antiviral activity. In addition, REP 9 demonstrated in vitro activity against a broad spectrum of other herpesviruses: HSV-2 (50% effective concentration [EC50], 0.02 to 0.06 μM), human cytomegalovirus (EC50, 0.02 to 0.13 μM), varicella zoster virus (EC50, <0.02 μM), Epstein-Barr virus (EC50, 14.7 μM) and human herpesvirus types 6A/B (EC50, 2.9 to 10.2 μM). The murine microbicide model of genital HSV-2 was then used to evaluate in vivo activity. REP 9 (275 mg/ml) protected 75% of animals from disease and infection when provided 5 or 30 min prior to vaginal challenge. When an acid-stable analog (REP 9C) was used, 75% of mice were protected when treated with 240 mg/ml 5 min prior to infection (P < 0.001), while a lower dose (100 mg/ml) protected 100% of the mice (P < 0.001). The acid stable REP 9C formulation also provided protection at 30 min (83%, P < 0.001) and 60 min (50%, P = 0.07) against disease. These observations suggest that APs may have microbicidal activity and potential as broad-spectrum antiherpetic agents and represent a novel class of agents that should be studied further.
Microbicides are intended to prevent the transmission of sexually transmitted infections (STIs) from one sexual partner to another. The ideal microbicide would be completely safe and broadly effective against a variety of STIs (reviewed in reference 9). Despite recent setbacks, including failed trials of nonoxynol 9, Savvy (C31G), and cellulose sulfate (6), there are more than 30 other microbicides being investigated (9). Because genital herpes infections are not only one of the most common STIs but also have recently been shown to have profound effects on the spread of human immunodeficiency virus (HIV), they have been another major target for microbicides (reviewed in references 11 and 8). Thus, several preclinical microbicide evaluations have used animal models of genital herpes for the initial testing (20).
Recently, the use of a novel class of compounds, phosphorothioate oligonucleotides (PS-ONs), has been suggested as a promising microbicide approach (17). The presence of sequence-independent antiviral activity with PS-ONs in HSV has been previously described (10, 12, 13). Recently, this sequence-independent antiviral activity of PS-ONs was more clearly elucidated in HSV and other viruses (17, 22, 27) and was shown to be derived from the chemical nature of PS-ONs amphipathic polymers, independent of the stability that phosphorothioation provides to oligonucleotides. Furthermore, the antiherpetic activity of amphipathic polymers (APs) in herpes simplex virus (HSV) and HIV was due not only to blocking virus binding but also to virus entry (17, 27).
Here we report that APs possess an even broader spectrum of activity than previously reported including potent in vitro activity against not only HSV type 1 (HSV-1) and HSV-2 and human cytomegalovirus (HCMV) but also varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and human herpesviruses 6A and 6B (HHV-6A and HHV-6B). Furthermore, the in vivo activity of different APs was demonstrated in a murine microbicide model of genital HSV-2 infections.
MATERIALS AND METHODS
Oligonucleotide synthesis.
ONs used in in vitro studies were prepared at the University of Calgary DNA synthesis lab using standard phosphoramidite chemistry with commercially available reagents as previously described (27). Fully degenerate ONs (which we have termed “randomers”) were prepared by mixing equimolar concentrations of all four amidites during coupling. ONs were deprotected and cleaved with NH4OH, desalted on a Sephadex 250 column, and lyophilized prior to quantitation by absorbance at 260 nm. All ONs were then prepared as 1 mM stocks in 10 mM Tris (pH 7.2) and diluted appropriately. For in vivo testing, both the degenerate 40mer and 40mer polycytosine PS-ONs (amphipathic polymers) were prepared as highly purified sodium salts under Good Manufacturing Practice-like conditions by Girindus America, Inc. Randomers were then dissolved in a suitable phosphate-buffered saline (PBS [pH 7.2]) prior to administration.
In vitro antiviral activity. (i) Plaque reduction assays.
Confluent monolayers of VERO cells were infected with HSV-1 (strain KOS) or HSV-2 (strain MS2) or a variety of acyclovir or foscarnet-resistant HSV-1 and HSV-2 strains (14, 25). Viral adsorption proceeded for 90 min, after which the cells were washed and replaced with new “overlay” media containing 2% fetal bovine serum and 1% human immunoglobulins. Three to four days after adsorption plaques were counted after formalin fixation and crystal violet staining. The 50% effective concentration (EC50) values were calculated as the concentrations of compound that reduced the number of plaques by 50% compared to the untreated control.
(ii) Inhibition of cytopathic effect (CPE) assay.
For evaluation of HSV, HCMV, and VZV activities, low-passage human foreskin fibroblast cells were seeded into 96-well tissue culture plates 24 h prior to use at a cell concentration of 2.5 × 105 cells/ml in 0.1 ml of minimal essential medium (MEM) supplemented with 10% fetal bovine serum. The cells were then incubated for 24 h at 37°C in a CO2 incubator. The medium was removed, and 125 μl of experimental drug was added to the first row in triplicate wells, all other wells having 100 μl of media. The drug was then serially diluted 1:5 by transferring 25 μl using a BioMek 2000 liquid handling apparatus. After 1 h of incubation, 100 μl of the appropriate virus concentration was added to each well, excluding cell control wells, which received 100 μl of MEM. For HSV-1 and HSV-2 assays the virus concentration was 1,000 PFU per well. For HCMV and VZV assays the virus concentration was 2,500 PFU per well. The plates were then incubated 3 days for HSV-1 and HSV-2, 10 days for VZV, and 14 days for HCMV. After the incubation period, the medium was aspirated, and the cells were stained with a 0.1% crystal violet solution for 4 h. The stain was then removed, and the plates were rinsed with tap water and allowed to air dry for 24 h. The plates were read on a BioTek plate reader at 630 nm, and the results were calculated by using the MacSynergy II software program.
(iii) EBV and HHV-6 DNA hybridization assay.
As previously described (28) the human T-cell lymphoblastoid line HSB-2, Molt-3, or Daudi cells were obtained from the American Type Culture Collection. The HHV-6A strain GS was propagated in HSB-2 cells, the HHV-6B strain Z29 was propagated in Molt-3 cells, and EBV strain P3H-R was prepared in Daudi cells. The assay for HHV-6 was set up in 96-well plates containing uninfected HSB-2 or Molt-3 cells (2 × 105 cells/ml) and appropriate dilutions of virus and antiviral drugs. Each drug concentration was tested in triplicate. The plates were incubated at 37°C for 7 days. DNA hybridization screening against HHV-6 was performed by dot blot analysis. Cells were lysed by the addition of denaturation buffer (1.2 M NaOH, 4.5 M NaCl), and 50 μl of the DNA was applied under vacuum to a positively charged nylon membrane. Hybridization was carried out using an HHV-6- or EBV-specific digoxigenin-labeled probe, and detection of this probe was performed by using a digoxigenin detection kit (Roche Molecular Biochemicals). Blot profiles were developed by using CDP-Star. EBV DNA synthesis was monitored by using a Simply Sensitive Horseradish Peroxidase-AEG in situ detection system (Enzo Diagnostics, Farmingdale, NY) according to the manufacturer's instructions.
In vivo antiviral activity.
As previously reported, female Swiss-Webster mice weighing 18 to 21 g (Harlan) were administered a 0.1-ml suspension containing 3 mg of medroxyprogesterone acetate (Upjohn Pharmacia) by subcutaneous injection 7 and 1 days prior to viral challenge to increase susceptibility to vaginal HSV-2 infection (2). The vaginal vault was swabbed twice, first with a moistened type 1 calcium alginate-tipped swab (Fisher Scientific) and then with a dry swab. Animals were treated with 15 μl of either the randomers dissolved in PBS or a placebo (PBS) control by using a positive displacement pipetter. Five to sixty minutes later, the animals were inoculated by instillation of 15 μl of a suspension containing 104 PFU of HSV-2 strain 186 into the vagina. Vaginal swab samples were collected from all animals on day 2 after inoculation and stored frozen (−80°C) until assayed for the presence of virus by culture. Mice were evaluated daily up to day 21 after inoculation for evidence of symptomatic infection that can include (i) hair loss and erythema around the perineum, (ii) chronic urinary incontinence, (iii) hind-limb paralysis, and (iv) mortality. Animals that did not develop symptoms were defined as infected if virus was isolated from vaginal swab samples collected on day 2 after inoculation. All animals that developed disease had virus isolated from the vagina, while on rare occasions an infected animal did not develop symptoms.
Statistics.
Incidence data were compared by the Fisher exact test. All comparisons were two tailed.
RESULTS
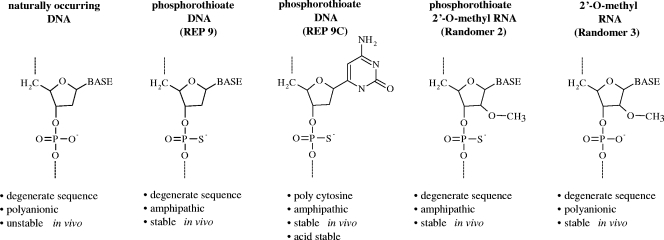
Oligonucleotides were prepared with fully degenerate sequences (27) or with defined homo- or heteropolymeric sequences that were either fully phosphorothioated, fully phosphorothioated and 2′-O methylated, or only 2′-O methylated. These different chemically modified preparations with degenerate sequences were referred to as REP 9, randomer 2, and randomer 3, respectively (see Fig. 1). The use of fully degenerate oligonucleotides avoids any antisense or sequence-specific aptameric effects that could be associated with a specific sequence. The fully degenerate nature of these compounds has been examined previously (27).
FIG. 1.
Nucleic acid chemistry used in experiments. Phosphorothioation (which stabilizes and increases the hydrophobicity of ONs) and 2′-O methylation (which only stabilizes ONs) were used to alter the chemical properties of randomers used in the present study.
Effect of phosphorothioation on the antiherpetic activity of APs.
The in vitro anti-HSV-1 activity of 40-base ONs with REP 9, randomer 2, or randomer 3 chemistries was assessed by plaque reduction assay (see Table 1). REP 9 (phosphorothioated) and randomer 2 (phosphorothioated and 2′-O-methylated) compounds had potent activity against HSV-1, while the randomer 3 ON, which was stabilized by 2′-O methylation but not phosphorothioated, had no detectable HSV-1 activity at 10 μM.
TABLE 1.
The antiviral activity of APs in HSV-1 is dependent on increased hydrophobicitya
| Compound | Chemical properties | Mean EC50 (μM) ± SDb |
|---|---|---|
| REP 9 | Polyanionic, hydrophobic | 0.136 ± 0.012 |
| Randomer 2 | Polyanionic, hydrophobic | 0.092 ± 0.017 |
| Randomer 3 | Polyanionic | >100 |
The antiviral activity was evaluated by CPE assay.
That is, the mean of three independent experiments.
Effect of sequence on antiherpetic activity of APs.
Since it was possible that the antiherpetic activity of APs could be caused by the activity of a small subset of sequence specific ONs, the in vitro anti-HSV-1 activity of a variety of 40-base, fully phosphorothioated homo- and heterpolymer APs were evaluated by plaque reduction assay (Table 2). The 40mer poly(C) and poly(T), as well as all 40mer heteropolymers tested, had an anti-HSV-1 activity similar to that of REP 9. The 40mer poly(G) had no activity, and the 40mer poly(A) had reduced activity compared to REP 9. The lack of activity of the 40mer poly(G) is potentially due to the propensity of poly(G) oligonucleotides to form G-quartet structures (21), which suggests that a “relaxed” oligonucleotide conformation may be required for antiviral activity and this may also be the case for poly(A).
TABLE 2.
The antiviral activity of APs in HSV-1 is sequence independenta
| AP sequence | Mean EC50 (μM) ± SDb |
|---|---|
| 40mer fully degenerate (N40, REP 9) | 0.043 ± 0.02 |
| 40mer poly(C) (REP 9C) | 0.097 ± 0.013 |
| 40mer poly(G) | >10 |
| 40mer poly(T) | 0.19 ± 0.011 |
| 40mer poly(A) | 0.614 ± 0.07 |
| 40mer poly(A-C) | 0.123 ± 0.009 |
| 40mer poly(T-C) | 0.199 ± 0.021 |
| 40mer poly(A-G) | 0.098 ± 0.015 |
| 40mer poly(T-G) | 0.155 ± 0.021 |
The antiviral activity evaluated by CPE assay.
That is, the mean of three independent experiments.
Effect of APs on other herpesviruses.
The in vitro activity against several other herpesviruses was next examined. The antiviral activities of REP 9 against HSV-1, HSV-2, HCMV, VZV, EBV, and HHV-6A and HHV-6B are presented in Table 3. The antiviral activity of REP 9 was shown for each virus with an EC50 ranging from <0.02 μM against VZV to 14.7 μM against EBV.
TABLE 3.
REP 9 has broad-spectrum antiherpetic activity in vitro
| Virus | Straina | Randomer 1 (REP 9) CC50 (μM)b | Mean EC50 (μM) ± SDc
|
||||
|---|---|---|---|---|---|---|---|
| Randomer 1 | CDV | ACV | FOS | GCV | |||
| HSV-1 | KOS* | >100 | 0.14 ± 0.02 | - | 3.2 | 163.7 | - |
| E-377† | >100 | 0.2 | - | 1.3 | - | - | |
| HSV-2 | MS2* | >100 | 0.06 ± 0.01 | - | 2.8 | 51.7 | - |
| MS† | >100 | 0.02 | - | 0.4 | - | - | |
| CMV | AD169* | >100 | 0.13 ± 0.02 | 1.61 | - | 59.4 | 5.9 |
| HCMV | >100 | 0.02 | - | - | - | 3.9 | |
| VZV | Ellen† | >100 | <0.02 | - | 0.1 | - | - |
| EBV | P3H-R† | >100 | 14.7 ± 3.7 | - | 6.4 | - | - |
| HHV-6A | GS† | >100 | 10.2 ± 1.3 | 2.7 | - | - | - |
| HHV-6B | Z29† | >100 | 2.9 ± 1.1 | 4.1 | - | - | - |
*, Determined by plaque reduction assay (mean of three independent experiments or the results of one experiment when no standard deviation value is given is given); †, determined by CPE assay (the mean of two independent experiments or the results of one experiment when no standard deviation value is given); †, determined by DNA hybridization assay (the mean of two independent experiments or the results of one experiment when no standard deviation value is given).
CC50, concentration that results in 50% toxicity in uninfected cells compared to untreated, uninfected controls.
ACV, acyclovir; FOS, foscarnet; GCV, ganciclovir; CDV, cidofovir. -, not tested.
To further explore the clinical potential of APs, the in vitro activities of REP 9 against several acyclovir- and foscarnet-resistant clinical isolates of HSV-1 and HSV-2 strains were also examined by plaque reduction assay. The activity of REP 9 in all of the drug-resistant strains evaluated was comparable to its activity against the wild-type HSV strains, as shown in Table 4.
TABLE 4.
REP 9 has equivalent activity against wild-type and drug-resistant strains of HSV-1 and HSV-2 in vitro
| Virus | Straina | Resistance | EC50 (μM)b
|
||
|---|---|---|---|---|---|
| Randomer 1 (REP 9) | ACV | FOS | |||
| HSV-1 | KOS* | Wild type | 0.14 | 3.24 | 163.7 |
| C88894 | Wild type | 0.093 | 3.77 | 6.57 | |
| A1† | ACV | 0.032 | 49.7 | 63.4 | |
| O24† | ACV | 0.034 | 50.4 | 67.5 | |
| 920062* | FOS | 0.23 | 1.42 | 254.9 | |
| 920068* | FOS | 0.36 | 0.62 | 194.0 | |
| HSV-2 | MS2* | Wild type | 0.06 | 2.78 | 51.74 |
| C74708 | Wild type | 0.229 | 0.97 | 63.15 | |
| 91670* | ACV | 0.102 | 92.9 | 120.3 | |
| 890540* | ACV | 0.135 | 114.9 | 152.6 | |
| 920023* | FOS | 0.215 | 5.32 | 432.5 | |
| 890546* | FOS | 0.138 | 7.87 | 419.5 | |
Effect of REP 9 in vivo against HSV-2 (microbicide activity).
In the initial experiments, doses of 10 and 50 mg/ml (10 mg = 758 μM) of REP 9 were evaluated, and neither dose provided significant protection against the HSV-2 challenge when administered 5 min prior to challenge (data not shown). However, when the dose was increased to 100 mg/ml, mice were protected against disease and infection (Table 5). The duration of protection using 100 and 275 mg of REP 9/ml was examined next. As seen in Table 4, a dose of 100 mg of REP 9/ml protected 100% of the animals when challenged 5 min after dosing (P < 0.001 compared to control) but only 33% of animals when challenged 30 min after dosing (not significant [NS]). When the dose was further increased to 275 mg/ml, significant protection was seen against challenge at 30 min: 75% against disease and infection (P < 0.005). It should also be noted that in the second experiment a dose of 100 mg/ml was less effective compared than the initial trial (50% at 5 min [NS]) and that the 275-mg/ml dose also protected 75% of the animals when challenged 5 min after dosing.
TABLE 5.
Effect of dose and time on microbicide activity of REP 9 against HSV-2 challenge
| Expt and group | Treatment | Concn | Time (min) | No. of animals tested | No. of animals (%) protecteda against:
|
|
|---|---|---|---|---|---|---|
| Disease | Infectionb | |||||
| Expt 1 | ||||||
| 1 | REP 9 | 100 mg/ml | 5 | 12 | 8 (67)A | 8 (67)A |
| 2 | PRO 2000 | 4% gel | 5 | 12 | 12 (100)B | 12 (100)B |
| 3 | PBS | NAc | 5 | 12 | 0 (0) | 0 (0) |
| Expt 2 | ||||||
| 1 | REP 9 | 100 mg/ml | 5 | 12 | 6 (50) | 6 (50) |
| 2 | REP 9 | 275 mg/ml | 5 | 12 | 9 (75)C | 9 (75) |
| 3 | REP 9 | 275 mg/ml | 30 | 12 | 9 (75)C | 9 (75)C |
| 4 | PRO 2000 | 4% gel | 30 | 12 | 12 (100)D | 12 (100)D |
| 5 | PRO 2000 | 4% gel | 5 | 8 | 7 (88)D | 7 (88)D |
| 6 | PBS | NA | 5 | 12 | 1 (8) | 1 (8) |
Significance as determined by the Fisher exact test compared to the PBS control group is indicated by superscript letters as follows: A, P < 0.001; B, P < 0.001; C, P < 0.005; and D, P < 0.001 (not significant compared to REP 9 at 275 mg/ml).
Animals without symptoms were defined as infected if virus was isolated from swabs obtained on day 2 after inoculation.
NA, not applicable.
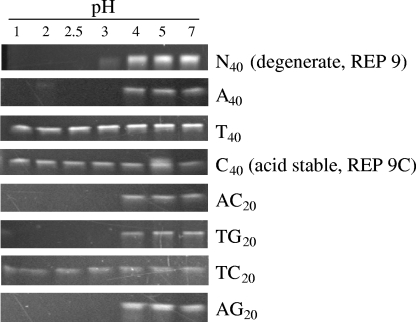
Because it was felt that some of the variability of protection could be due to degradation of the product at the acid pH found in the vagina, the homo- and heteropolymeric analogs of REP 9 that were active in Table 3 were tested for their pH stability. As seen in Fig. 2, there were significant differences in the acid stability of different hetero- and homopolymeric sequences. It was observed that polypyrimidine sequences (T40, C40, or TC20) were stable to acid hydrolysis at pH 1 for 24 h, while all other sequences showed comparable pH stability (stable at pH 4 or greater) to REP 9.
FIG. 2.
Stability of various homo- and heteropolymer PS-ON sequences to low pH. 40mer PS-ONs with different homo- and heteropolymeric sequences (indicated on the right) were incubated for 24 h in PBS buffered to various pH levels (indicated at the top). These preparations were subsequently neutralized and run on urea-sodium dodecyl sulfate page gels, and PS-ONs were detected with ethidium-bromide staining. Only polypyrimidine sequences [poly(C), poly(T), or poly(TC)] showed resistance to degradation at pH <4, and these oligonucleotides were fully stable after 24 h of incubation at pH 1.
When the acid-stable 40mer polycytidine amphipathic DNA polymer, REP 9C, was evaluated in the mouse model, protection against disease and infection was seen in 100% of the animals administered 100 mg/ml (100 mg = 8,264 μM), but only 75% of animals when 240 mg/ml was applied 5 min prior to challenge (Table 6). When the dose was increased to 240 mg/ml, significant protection was seen against challenge at 30 min; 83% against disease (P < 0.001) and 67% against infection (Table 5, P < 0.05). Increasing the time to challenge to 60 min decreased the effect so that 50% of the animals were protected against disease (P = 0.07), while 33% were protected against infection (NS).
TABLE 6.
Effect of REP 9C against genital HSV-2 challenge
| Expt and group | Drug | Concn (mg/ml) | Time (min) | No. of animals tested | No. of animals (%) protecteda against
|
|
|---|---|---|---|---|---|---|
| Disease | Infectionb | |||||
| Expt 1 | ||||||
| 1 | REP 9C | 240 mg/ml | 5 | 12 | 9 (75)A | 9 (75)A |
| 2 | REP 9C | 100 mg/ml | 5 | 11 | 11 (100)A | 11 (100)A |
| 3 | PBS | NAc | 5 | 12 | 0 (0) | 0 (0) |
| 4 | PRO 2000 | 4% gel | 5 | 7 | 7 (100) | 7 (100) |
| Expt 2 | ||||||
| 1 | REP 9C | 240 mg/ml | 60 | 12 | 6 (50)B | 4 (33) |
| 2 | REP 9C | 240 mg/ml | 30 | 12 | 10 (83)A | 8 (67)C |
| 3 | REP 9C | 100 mg/ml | 30 | 12 | 4 (33) | 4 (33) |
| 4 | REP 9C | 100 mg/ml | 5 | 12 | 12 (100)A | 12 (100)A |
| 5 | PBS | NA | 5 | 12 | 1 (8) | 1 (8) |
Significance as determined by the Fisher exact test(two tailed) compared to the PBS control group is indicated by superscript letters as follows: A, P < 0.001; B, P = 0.07; and C, P < 0.05.
Animals without symptoms were defined as infected if virus was isolated from swabs obtained on day 2 after inoculation.
NA, not applicable.
DISCUSSION
In vitro activity APs.
The sequence-independent, phosphorothioation-dependent antiviral activity of APs was confirmed in this report by showing a variety of homo- and heteropolymeric AP sequences were active against HSV-1 infection in vitro (13, 15, 17, 22, 28). It is important to reiterate that ONs require phosphorothioation for their antiviral activity but that this is not due simply to the stabilization of ONs by this modification, as evidenced by the lack of activity of randomer 3 ONs, which are not phosphorothioated but fully stable (21, 27). Similar activity of PS-ONs dependent on their activity as APs in HIV-1(27) and arenaviruses (22), as well as influenza virus, respiratory syncytial virus, and Ebola virus (A. Vaillant, unpublished data) has also been demonstrated. Interestingly, all of these viruses have type 1 fusion proteins, which are characterized by amphipathic alpha-helical regions. In the case of HIV-1 and influenza virus, it is widely accepted that these alpha-helices fold on each other to bring viral and host membranes in close opposition, allowing membrane fusion to take place (4, 5, 7). The specific interaction of APs with the alpha-helices of HIV-1 gp41 in the same phosphorothioation- and size-dependent manner has been previously shown (27), suggesting that direct interaction with these structures are the mechanism underlying at least part of the antiviral activity of APs in a broad spectrum of enveloped viruses. Recently, the presence and function of amphipathic alpha-helices similar to those found in HIV-1 and influenza virus were discovered in glycoprotein H (gH) of many different Herpesviridae (15, 16) and the presence of these alpha-helical structures in HSV-1 gB has also been documented (18). Therefore, it is suggested that the activity of APs in HSV is analogous to the activity we have demonstrated in HIV (28), i.e., preventing the attachment and or fusion of HSV by preventing the association of alpha-helices of gH and or gB. Further, recent evidence suggests that the activity of APs is due to more than their ability to block attachment since they also inhibit viral entry and are active even when added after virus entry (17).
Previous reports have demonstrated the size-dependent nature of AP activity, with 40 length oligonucleotides showing potent HSV activity (17). In the report presented here we also demonstrate in vitro antiviral activity of 40mer APs against HCMV, VZV, EBV, HHV-6A, and HHV-6B. Thus, APs have broad antiherpetic activity, providing advantages to currently available therapies. Further, the demonstrated in vitro activity against acyclovir- and foscarnet-resistant HSV-1 and HSV-2 strains provides further rationale for developing these compounds for use in populations in which resistant herpesviruses can be a problem.
To demonstrate the in vivo activity of APs, a murine model of genital herpes was used. We chose to demonstrate the activity using a microbicide model, where the drug is applied prior to the virus challenge, because of the urgent need to develop more microbicide candidates that are safe and effective against a range of STI pathogens. It is important to note, however, that we have also demonstrated activity in a murine model of murine CMV using systemic administration of APs (R. Cardin, unpublished data).
Our evaluations revealed a dose-dependent effect, with doses of 10 and 50 mg not providing protection, while doses of 100 mg or greater provided protection from HSV-2 infection and disease. When used at a concentration of 275 mg, significant protection was shown when the drug was applied 5 or even 30 min prior to intravaginal HSV-2 challenge. This is similar to the activity seen with dendrimers, a candidate microbicide currently in clinical trials (2, 3), and our published reports of PRO 2000, another candidate microbicide currently in clinical trials. When used as a positive control in the experiments presented here, PRO 2000 protected 100% of the animals when used as late as 30 min prechallenge. In a recent report, however, the efficacy of PRO 2000 was significantly decreased when used in the presence of seminal plasma (23), whereas activity was maintained with REP 9 (17).
Because it is important to maintain the acidic environment of the vagina as a natural protective mechanism (1, 26) and in order to determine whether efficacy could be improved by the use of an acid-stable AP, the acid stability of several APs was examined, and a more acid-stable AP was evaluated. This acid-stable AP, a 40mer polycytidine amphipathic DNA polymer (REP 9C), provided 100% protection when used at a dose of 100 mg given 5 min prior to infection. The reason for the 75% protection with 240 mg/ml given at 5 min prior to infection is unknown but likely is related to an unoptimized formulation since the 240-mg/ml dose was more effective than the 100-mg/ml dose with longer intervals between treatment and infection. Using this formulation protection, against disease but not infection was extended out to 60 min.
Since degenerate ONs (i.e., REP 9) theoretically contain a small fraction of CpG motif content, there exists the potential that some portion of the activity of long PS-ONs in vivo could be derived from a TLR-9-mediated mechanism. In fact, a PS-ON containing a CpG motif has been shown to be protective topically against HSV-2 in vitro (24). However, the potent activity of the 40mer poly(C) PS-ON described above precludes this possibility because the poly(C) PS-ON has no CpG motifs present. Thus, this argues that the antiviral activity of PS-ONs in vivo (in the presence or absence of CpG motifs) is not derived from stimulation of the immune system but more likely due to direct hydrophobic interactions preventing the attachment and fusion of virions with the host cell.
In summary, we have shown here that APs have broad spectrum in vitro antiherpesvirus activity and are active as microbicides in an animal model of genital herpes. Advantages over other microbicides that have recently been evaluated include the retention of activity in the presence of seminal plasma (17) and the expected lack of vaginal irritation produced by surfactant type microbicides (19). APs, including REP 9 and REP 9C, should be further evaluated as microbicides, as well as systemic therapies for a variety of herpesvirus infections.
Acknowledgments
This study was supported in part by the NIH/NIAID antiviral testing program (National Institute of Health contracts AI 15438 to Cincinnati Children's Hospital Medical Center and AI-30049 to the University of Alabama at Birmingham).
A.V. and J.-M.J. are employed by REPLICor, Inc.
Footnotes
Published ahead of print on 27 May 2008.
REFERENCES
- 1.Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, D. I., L. R. Stanberry, S. Sacks, N. K. Ayisi, Y. H. Gong, J. Ireland, R. J. Mumper, G. Holan, B. Matthews, T. McCarthy, and N. Bourne. 2003. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47:3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne, N., L. R. Stanberry, E. R. Kern, G. Holan, B. Matthews, and D. I. Bernstein. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44:2471-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 5.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 6.Check, E. 2007. Scientists rethink approach to HIV gels. Nature 446:12. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J., J. J. Skehel, and D. C. Wiley. 1999. N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc. Natl. Acad. Sci. USA 96:8967-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune. Defic. Syndr. 35:435-445. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan, D., and K. H. Mayer. 2006. Microbicides to prevent HIV transmission: overcoming obstacles to chemical barrier protection. J. Infect. Dis. 193:36-44. [DOI] [PubMed] [Google Scholar]
- 10.Fennewald, S. M., S. Mustain, J. Ojwang, and R. F. Rando. 1995. Inhibition of herpes simplex virus in culture by oligonucleotides composed entirely of deoxyguanosine and thymidine. Antivir. Res. 26:37-54. [DOI] [PubMed] [Google Scholar]
- 11.Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73-83. [DOI] [PubMed] [Google Scholar]
- 12.Gao, W. Y., R. N. Hanes, M. A. Vazquez-Padua, C. A. Stein, J. S. Cohen, and Y. C. Cheng. 1990. Inhibition of herpes simplex virus type 2 growth by phosphorothioate oligodeoxynucleotides. Antimicrob. Agents Chemother. 34:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, W. Y., J. W. Jaroszewski, J. S. Cohen, and Y. C. Cheng. 1990. Mechanisms of inhibition of herpes simplex virus type 2 growth by 28-mer phosphorothioate oligodeoxycytidine. J. Biol. Chem. 265:20172-20178. [PubMed] [Google Scholar]
- 14.Gaudreau, A., E. Hill, H. H. Balfour, Jr., A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 15.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus 1 gH, located downstream of the alpha-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman, E. M., N. Cheshenko, V. Shende, M. J. Keller, N. Goyette, J. M. Juteau, G. Boivin, A. Vaillant, and B. C. Herold. 2007. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir. Ther. 12:1147-1156. [PubMed] [Google Scholar]
- 18.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 19.Keller, M. J., and B. C. Herold. 2006. Impact of microbicides and sexually transmitted infections on mucosal immunity in the female genital tract. Am. J. Reprod. Immunol. 56:356-363. [DOI] [PubMed] [Google Scholar]
- 20.Keller, M. J., A. Tuyama, M. J. Carlucci, and B. C. Herold. 2005. Topical microbicides for the prevention of genital herpes infection. J. Antimicrob. Chemother. 55:420-423. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J., C. Cheong, and P. B. Moore. 1991. Tetramerization of an RNA oligonucleotide containing a GGGG sequence. Nature 351:331-332. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A. M., J. M. Rojek, A. Gundersen, U. Stroher, J. M. Juteau, A. Vaillant, and S. Kunz. 2008. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 372:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel, S., E. Hazrati, N. Cheshenko, B. Galen, H. Yang, E. Guzman, R. Wang, B. C. Herold, and M. J. Keller. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 196:1394-1402. [DOI] [PubMed] [Google Scholar]
- 24.Sajic, D., A. A. Ashkar, A. J. Patrick, M. J. McCluskie, H. L. Davis, K. L. Levine, R. Holl, and K. L. Rosenthal. 2003. Parameters of CpG oligodeoxynucleotide-induced protection against intravaginal HSV-2 challenge. J. Med. Virol. 71:561-568. [DOI] [PubMed] [Google Scholar]
- 25.Schmit, I., and G. Boivin. 1999. Characterization of the DNA polymerase and thymidine kinase genes of herpes simplex virus isolates from AIDS patients in whom acyclovir and foscarnet therapy sequentially failed. J. Infect. Dis. 180:487-490. [DOI] [PubMed] [Google Scholar]
- 26.Tuyama, A. C., N. Cheshenko, M. J. Carlucci, J. H. Li, C. L. Goldberg, D. P. Waller, R. A. Anderson, A. T. Profy, M. E. Klotman, M. J. Keller, and B. C. Herold. 2006. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: optimal candidate for microbicide combinations. J. Infect. Dis. 194:795-803. [DOI] [PubMed] [Google Scholar]
- 27.Vaillant, A., J. M. Juteau, H. Lu, S. Liu, C. Lackman-Smith, R. Ptak, and S. Jiang. 2006. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob. Agents Chemother. 50:1393-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams-Aziz, S. L., C. B. Hartline, E. A. Harden, S. L. Daily, M. N. Prichard, N. L. Kushner, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2005. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]