Abstract
Staphylococcus aureus invades eukaryotic cells. When methicillin-resistant S. aureus (MRSA) ATCC 33591 is phagocytized by human THP-1 macrophages, complete restoration of susceptibility to cloxacillin and meropenem is shown and the strain becomes indistinguishable from MSSA ATCC 25923 due to the acid pH prevailing in phagolysosomes (S. Lemaire et al., Antimicrob. Agents Chemother. 51:1627-1632, 2007). We examined whether this observation can be extended to (i) strains of current clinical and epidemiological interest (three hospital-acquired MRSA [HA-MRSA] strains, two community-acquired MRSA [CA-MRSA] strains, two HA-MRSA strains with the vancomycin-intermediate phenotype, one HA-MRSA strain with the vancomycin-resistant phenotype, and one animal [porcine] MRSA strain), (ii) activated THP-1 cells and nonprofessional phagocytes (keratinocytes, Calu-3 bronchial epithelial cells), and (iii) other β-lactams (imipenem, oxacillin, cefuroxime, cefepime). All strains showed (i) a marked reduction in MICs in broth at pH 5.5 compared with the MIC at pH 7.4 and (ii) sigmoidal dose-response curves with cloxacillin (0.01× to 100× MIC, 24 h of incubation) after phagocytosis by THP-1 macrophages that were indistinguishable from each other and from the dose-response curve for methicillin-susceptible S. aureus (MSSA) ATCC 25923 (relative potency [50% effect], 6.09× MIC [95% confidence interval {CI}, 4.50 to 8.25]; relative efficacy [change in bacterial counts over the original inoculum for an infinitely large cloxacillin concentration, or maximal effect], −0.69 log CFU [95% CI, −0.79 to −0.58]). Similar dose-response curves for cloxacillin were also observed with MSSA ATCC 25923 and MRSA ATCC 33591 after phagocytosis by activated THP-1 macrophages, keratinocytes, and Calu-3 cells. By contrast, there was a lower level of restoration of susceptibility of MRSA ATCC 33591 to cefuroxime and cefepime after phagocytosis by THP-1 macrophages, even when the data were normalized for differences in MICs. We conclude that the restoration of MRSA susceptibility to β-lactams after phagocytosis is independent of the strain and the types of cells but varies between β-lactams.
Methicillin-resistant Staphylococcus aureus (MRSA) is becoming an increasingly problematic pathogen. While it has long been confined to the hospital setting or closely related environments such as nursing homes, this phenotype is now emerging in the community as well as in food and pet animals and their human contacts (1, 2, 8, 11, 16, 18, 20, 33, 42, 44, 45). MRSA has thus become a true epidemiological scourge with serious therapeutic implications as well as a potential cause of great economic loss. Beyond the creation of immediate local and general damage (by the expression of various virulence factors), staphylococcal infections recur and relapse at high rates, sometimes even at intervals of years. This has been ascribed, at least in part, to the capacity of S. aureus to infect and survive in various cell types (10, 19, 27, 28, 35, 39), a situation which could be aggravated in the case of MRSA (7). A recent study has shown that S. aureus modulates its gene expression at early times after phagocytosis to promote intracellular survival (13). There is also now substantial evidence that the intracellular milieu is unfavorable to the actions of many currently registered antistaphylococcal antibiotics (4, 36), including vancomycin (3, 46), explaining why intracellular forms of S. aureus are difficult to eradicate.
In the course of systematic studies aimed at measuring the intracellular activity of various antistaphylococcal antibiotics, we made the unanticipated observation that MRSA strains phagocytized by human THP-1 macrophages show complete restoration of their susceptibility to cloxacillin and meropenem, making them virtually indistinguishable from methicillin-susceptible S. aureus (MSSA) strains in our model (23). This phenomenon could be ascribed to the acidic pH (5 to 5.5) to which intracellular S. aureus becomes exposed once it is phagocytized and transferred to phagolysosomes (23). This allowed linkage of the current observation to the well-known suppression of the intrinsic resistance of MRSA to methicillin and other penicillins at acidic pH described in the early 1970s (37). Recent studies have now shown that β-lactams are able to bind to and acylate PBP 2a at pH 5.5, thanks to an acid pH-favored conformational change of the transpeptidase catalytic site of this protein from a closed to an open state (21). In the present study, we wished to address three critical questions to further delineate the pertinence and potential applications of our observation concerning the intraphagocytic MRSA strains, namely, whether it can it be extended (i) to strains of current clinical and epidemiological interest (within the context of the present MRSA threat); (ii) to cell types other than macrophages, including nonprofessional phagocytes; and (iii) to other molecules selected among the three main classes of currently registered antistaphylococcal β-lactams. Our current data show that the suppression of methicillin resistance after phagocytosis is neither strain nor cell specific. Comparison of β-lactams, however, suggests that this effect is preferentially seen with penicillins and carbapenems as opposed to cephalosporins.
MATERIALS AND METHODS
Antibiotics and main reagents.
Oxacillin, cloxacillin, dicloxacillin, nafcillin, cefotaxime, and cephalexin were purchased from Sigma-Aldrich, St. Louis, MO. Faropenem and cefadroxil were obtained as microbiological standards from Shenzhen Haibin Pharmaceuticals, Shenzhen, Guangdong, China, and from Bristol-Myers Squibb, Princeton, NJ, respectively. The other antibiotics were obtained as the clinical products for intravenous administration to humans and in compliance with the European Pharmacopoeia (gentamicin as Geomycin [distributed in Belgium by Glaxo-SmithKline SA, Genval, Belgium], cefepime as Maxipime [Bristol-Myers Squibb, Brussels, Belgium], cefuroxime as Cefurim [Teva Pharma Belgium, Wilrijk, Belgium], meropenem as Meronem [AstraZeneca, Brussels, Belgium], imipenem as Tienam [Merck Sharp & Dohme, Brussels, Belgium]), and ertapenem as Invanz [Merck Sharp & Dohme GmbH, Haar, Germany]). Cell culture medium and serum were from Invitrogen (Life Science Technologies, Paisley, United Kingdom) or Becton Dickinson. Unless stated otherwise, all other reagents were obtained from Merck AG (Darmstadt, Germany) or Sigma-Aldrich.
Cell lines.
Experiments were performed with (i) THP-1 cells (ATCC TIB-202; American Type Culture Collection, Manassas, VA), a human myelomonocytic cell line that displays macrophage-like activity and that was maintained as described previously (6); (ii) activated THP-1 cells (reported to show increased phagocytic activity, HLA-DR expression, and enhanced constitutive production of interleukin 1β [40]) prepared from unactivated cells by a 24-h culture in the presence of 2 mg/liter phorbol myristate acetate (12); (iii) primary cultures of human skin keratinocytes (obtained from Invitrogen SA, Merelbeke, Belgium); and (iv) the Calu-3 human bronchial epithelial cell line (ATCC HTB-55), which was maintained as described previously (15) but without coating of the culture flasks.
Bacterial strains and susceptibility testing.
The strains used in this study, their main characteristics, and their origins are shown in Table 1. They were obtained from the American Type Culture Collection, the Network on Antimicrobial Resistance in Staphylococcus aureus Program (operated by Eurofins Medinet, Inc., Hendon, VA; supported under NIAID/NIH contract HHSN2722007 00055C), or from the collections of two of us (P.C.A. and Y.G.). MICs were measured by the microdilution method, as described earlier (23). The genotype of each MRSA strain was confirmed by the detection of mecA by PCR, as described previously (23), and the staphylococcal chromosomal cassette mec (SCCmec) subgroup of each strain was established by a multiplex PCR assay (47).
TABLE 1.
Strains used in this study (phenotypes and origins, SCCmec subgroup, and cloxacillin MIC in broth (at pH 7.4 and 5.5)
| Strain no. | Phenotypea | Type of strain and origin | SCCmec groupb | MIC (mg/liter)
|
|
|---|---|---|---|---|---|
| pH 7.4 | pH 5.5 | ||||
| ATCC 25923 | MSSA (β-lactamase negative) | Laboratory standardc | NA | 0.125 | 0.06 |
| ATCC 33591 | HA-MRSA (inducible) | Laboratory standardc | III | 16 | 0.06 |
| NRS100 (COL) | MRSA (constitutively β-lactamase negative) | Clinical (operation theater; historical)d (9, 31) | I | >512 | 0.25 |
| N4120032 | HA-MRSA | Clinical (wound infection)e | I | 1 | 0.06 |
| N4120210 | HA-MRSA | Clinical (urinary tract infection)e | ND | 2 | 0.06 |
| HMC546 | HA-MRSA | Clinical (endocardits [aortic value])f (17) | IVa | 16 | 0.25 |
| N4090440 | CA-MRSA (PVL positive) | Clinical (wound infection)e | IVa | 0.5-1 | 0.03 |
| N4042228 | CA-MRSA (PVL positive) | Clinical (septicemia secondary to soft tissue abscess)e | IVa | 0.5 | 0.03 |
| NRS192 | CA-MRSA (PVL positive) | Clinical (pneumonia, septic arthritis)d | IVa | 1 | 0.06 |
| NRS18 | HA-MRSA/VISA | Clinical (wound, skin and soft tissue infections)d | II | 8 | 0.06 |
| NRS126 | HA-MRSA/VISA | Clinical (bloodstream infection)d | II | 16 | 0.06 |
| VRS2 | HA-MRSA/VRSA | Clinical (wound, skin and soft tissue infections)c | II | 32 | 0.06 |
| N7112046 | Animal MRSA | Carriage (food animal caregiver)e | ND | 512 | 0.125 |
Abbreviations: HA, hospital acquired; CA, community acquired; PVL, Panton-Valentine leukocidin; VISA, vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus.
Detection of mecA by PCR and SCCmec by multiplex PCR. NA, not applicable; ND, not detected.
From the American Tissue Culture Collection (Manassas, VA).
From the Network on Antimicrobial Resistance in Staphylococcus aureus Program (operated by Eurofins Medinet, Inc., Hendon, VA; supported under NIAID/NIH contract HHSN2722007 00055C); details on each is strain are available from www.narsa.net.
Clinical collection (Y.G.).
Clinical collection (P.C.A.).
Cells, cell infection, and assessment of intracellular activities of antibiotics.
Infection of unactivated THP-1 cells was performed exactly as described previously (4, 23, 38), and the same procedure was followed for activated THP-1 cells. Infection of human skin keratinocytes and the bronchial epithelial cell line was performed as described previously for J774 macrophages (38), except that S. aureus internalization was allowed to take place for 2 h and an initial inoculum of 5 × 107 to 1 × 108 bacteria/ml was used (28). For both cell types, the postphagocytosis inoculum comprised from 1.5 × 106 to 3.0 × 106 CFU per mg of cell protein, a value close to that used for the THP-1 macrophages. The data generated from experiments in which the concentration of the antibiotics was varied over a wide range were analyzed by using the classical dose-response model used in pharmacological studies (based on the Hill equation) to calculate the corresponding pertinent descriptors, namely, relative efficacy (change in bacterial counts over the original inoculum for an infinitely large cloxacillin concentration, or maximal effect [Emax]), relative potency (cloxacillin concentration in multiples of the MIC measured at pH 5.5 causing a change in bacterial counts halfway between that observed for an infinitely low cloxacillin concentration [Emin] and Emax, or the 50% effective concentration [EC50]), and the apparent static concentration, as explained in detail in a previous publication (4).
Assay of cell-associated carbapenems (imipenem, meropenem) and isoxazoylpenicillins (oxacillin, cloxacillin).
Cell lysates were prepared after 24 h of incubation and were used for microbiological assay with MSSA ATCC 25923 by the general method described previously (see references 4, 5, and 24 for details). The cell antibiotic content was expressed as a function of the total sample protein content, and the ratio of the cell antibiotic concentration over the extracellular concentration was calculated on the basis of a cell volume of 5 μl per mg protein.
Analysis of carbapenem and isoxazoylpenicillin stability at neutral and acid pHs.
The stabilities of the carbapenems and the isoxazoylpenicillins at neutral and acid pHs were analyzed by monitoring the disappearance of the corresponding signal by high-pressure liquid chromatography upon incubation of the drugs in buffered medium by use of the protocols described previously (24) for testing of the stability of ertapenem and meropenem in culture medium (linear response over concentration ranges of 1 to 500 mg/liter [R2 = 0.999] and 0.19 to 200 mg/liter [R2 = 0.999] for imipenem and meropenem, respectively) and with adaptations (mobile phase, 25 mM phosphate buffer, pH 6.7, and acetonitrile [75:25; vol/vol]) for oxacillin and cloxacillin (linear response over a concentration range of 16 to 1,000 mg/liter for both antibiotics [R2 ≥ 0.999]).
Statistical analyses.
Curve-fitting analyses were performed with Prism software (version 4.02 for Windows; GraphPad Prism Software, San Diego, CA). Analysis of variance (ANOVA) was performed with Instat software (version 3.06; GraphPad Prism Software).
RESULTS
Intracellular susceptibilities of MRSA strains of clinical and epidemiological interest to cloxacillin.
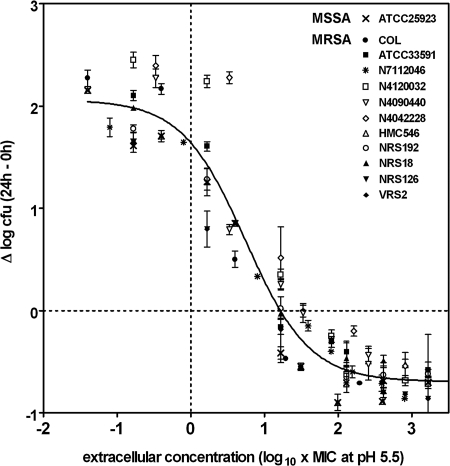
In the first series of experiments, we examined whether the suppression of methicillin resistance originally seen with laboratory MRSA strain ATCC 33591 when it was growing in THP-1 macrophages could be generalized to other MRSA strains of defined epidemiological and clinical interest. For this purpose, THP-1 macrophages were infected with the different strains listed in Table 1. The intracellular growth of each strain measured after 24 h and in the absence of added cloxacillin (but in the presence of gentamicin [0.5 mg/liter], to prevent extracellular growth [4]) was essentially similar to that of ATCC 33591 (about 2 log10 CFU increase in 24 h; data not shown), indicating a similar level of growth permissivity. Experiments were then conducted in the presence of cloxacillin at increasing extracellular concentrations from about 1/100- to 100-fold its MIC (as measured in broth at pH 5.5) to analyze the full dose-response effect of the antibiotic. In all cases, typical concentration-dependent effects were observed, which allowed us to use the pharmacological model based on the Hill equation, which had been validated for use in this type of study in a previous study (4). Statistical analysis of the individual dose-response curves, together with that of the pertinent quantitative parameters for each strain (relative efficacy [Emax], relative potency [EC50], and apparent static concentration; see the information in the supplemental material), revealed no significant differences or only minor differences between strains (including MSSA ATCC 25923). All data were therefore pooled, and the global results, together with the corresponding Hill functions, are shown in Fig. 1 (see the legend to Fig. 1 for the pertinent regression parameters). This shows that (i) a mean static effect was obtained for an external concentration of about 19-fold the MIC (measured at pH 5.5; corresponding to concentrations roughly from 0.5 to 4 mg/liter); (ii) the activity neared a maximum for an extracellular concentration of about 1,000-fold the MIC (30 to 250 mg/liter); and (iii) the maximal reduction of CFU (Emax) was about 0.7 log10 compared to the original inoculum, whatever strain was examined (including MSSA ATCC 25923).
FIG. 1.
Global dose-response curve for cloxacillin against the intracellular forms of the 13 S. aureus strains described in Table 1 after phagocytosis by THP-1 macrophages (see the supplemental material for the dose-responses of each strain). The ordinate shows the change in the number of CFU (means ± standard deviations [n = 3]; several standard deviation bars are smaller than the symbols) per mg of cell protein. The abscissa is the log10 of the cloxacillin extracellular concentration (log10 of the multiples of its MIC measured for the corresponding strain at pH 5.5; Table 1). Data for all strains were used to fit one single sigmoidal function with a Hill coefficient described previously (30) and with the following parameters: Emin, 2.18 log10 CFU (95% CI, 2.01 to 2.35 log10 CFU); Emax, −0.68 log10 CFU (95% CI, −0.79 to −0.58 log10 CFU); EC50, 6.09 (95% CI, 4.50 to 8.25); goodness of fit (R2), 0.925. The apparent static concentration (no apparent change from the original inoculum; dotted horizontal line) is ∼19.1× the MIC measured at pH 5.5 (see the supplemental material for the parameters observed with each strain).
Susceptibility of MRSA ATCC 33591 to cloxacillin after phagocytosis by unstimulated and stimulated macrophages, keratinocytes, and bronchial epithelial cell lines.
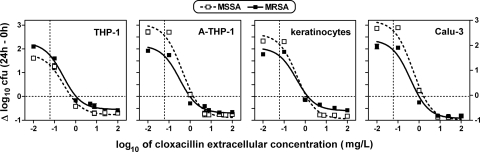
In the second series of experiments, we examined whether the results obtained with THP-1 macrophages were dependent on the lack of activation of these cells and whether the results could be extended to other cell types of interest in the context of persistent staphylococcal infections. Thus, we compared the susceptibility of fully susceptible strain ATCC 25923 to that of MRSA strain ATCC 33591 after phagocytosis by unstimulated and activated forms of THP-1 cells and by human skin keratinocytes and human Calu-3 bronchial epithelial cells, using the same model (dose-response study at 24 h) as in the previous experiments. The results are shown graphically in Fig. 2, with the pertinent numerical and statistical data presented in Table 2. The intracellular growth of MSSA in the absence of antibiotic (as estimated by extrapolation for an infinitely low cloxacillin concentration) was more pronounced after phagocytosis by activated THP-1 macrophages, keratinocytes, and Calu-3 cells (in that order) than after phagocytosis by unstimulated THP-1 macrophages. This was not the case for MRSA, which grew more extensively than MSSA in unstimulated THP-1 macrophages but less extensively in activated THP-1 macrophages, keratinocytes, and Calu-3 cells. Despite these differences in apparent growth, the pharmacological responses of both strains to cloxacillin (in terms of the EC50 and Emax) were nevertheless similar in the four types of cells. Of note is the fact that the static concentrations of cloxacillin never exceeded 0.6 to 1.6 mg/liter, i.e., concentrations about 10- to 30-fold less than its MIC at pH 7.4.
FIG. 2.
Dose-response curves for cloxacillin against the intracellular forms of S. aureus ATCC 25923 (MSSA; open symbols and dotted lines) and S. aureus ATCC 33591 (MRSA; closed symbols and continuous line) after phagocytosis by human unstimulated human THP-1 macrophages, human activated THP-1 macrophages, human skin keratinocytes, and human bronchial epithelial (Calu-3) cells (from left to right, respectively). The ordinate shows the change in the number of CFU per mg of cell protein (means ± standard deviations [n = 3]; most standard deviation bars are smaller than the symbols). The abscissa is the log10 of the cloxacillin extracellular concentration (in mg/liter). The horizontal dotted lines correspond to an apparent static effect. The vertical dotted lines correspond to the MIC of cloxacillin for the two strains used in these experiments when they were tested in broth at pH 5.5 (0.06 mg/liter). All data were used to fit single sigmoidal functions, as indicated in the legend to Fig. 1. See Table 2 for goodness-of-fit and pertinent regression parameters.
TABLE 2.
Susceptibility of MSSA ATCC 25923 and MRSA ATCC 33591 to cloxacillin after phagocytosis by THP-1 macrophages, activated THP-1 macrophages, keratinocytes, and Calu-3 cellsa
| Cell line | MSSAb
|
MRSAb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eminc (95% CId) | Emaxe (95% CI) | EC50f (95% CI) | Cstaticg | R2 | E0c (95% CI) | Emax (95% CI) | EC50 (95% CI) | Cstatic | R2 | |
| THP-1 | 1.79 (1.23 to 2.35) a,A | −0.77 (−1.08 to −0.47) a,A | 0.27 (0.09 to 0.82) a,A | 0.6 | 0.987 | 2.27 (1.73 to 2.81) a,A+ | −0.55 (−0.86 to −0.24) a,A+ | 0.26 (0.09 to 0.70) a,A | 0.9 | 0.990 |
| THP-1 macrophages | 3.05 (2.42 to 3.69) b,A | −0.90 (−1.24 to −0.56) a,A | 0.43 (0.18 to 1.02) a,A | 1.3 | 0.981 | 2.15 (1.52 to 2.79) a,B | −0.70 (−1.04 to −0.36) a,A+ | 0.32 (0.09 to 1.04) a,A | 1.0 | 0.977 |
| Keratinocytes | 2.64 (1.94 to 3.34) a+,b,A | −0.95 (−1.37 to −0.53) a,A | 0.42 (0.14 to 1.23) a,A | 1.1 | 0.980 | 2.06 (1.10 to 3.02) a,A | −0.60 (−1.22 to 0.02) a,A | 0.39 (0.05 to 2.86) a,A | 1.3 | 0.959 |
| Calu-3 | 3.19 (2.28 to 4.11) b,A | −0.99 (−1.63 to −0.36) a,A | 0.52 (0.15 to 1.77) a,A | 1.6 | 0.983 | 2.39 (1.49 to 3.29) a,A+ | −0.93 (−1.49 to −0.37) a,A | 0.39 (0.08 to 1.71) a,A | 1.0 | 0.977 |
The parameters described in the table were derived from analysis of the data shown in Fig. 2. The results of statistical analyses were as follows: global analysis of the Hill functions describing the dose-responses of the MSSA strain versus those of the MRSA strain for each cell type (unpaired t test, two-tailed, with all data points) showed no significant difference, and comparison of cell types (one-way ANOVA by the Tukey test for multiple comparisons) showed no significant differences. By analysis of the parameters in each column (one-way ANOVA by the Tukey test for multiple comparisons), values with different lowercase letters are significantly different from each other (P < 0.01; when the same letter has a plus (+), the difference between the values is significant at P values between 0.01 and 0.05); for analysis of the parameters in each row (unpaired, two-tailed t test between the corresponding parameters for MSSA and MRSA), values with different uppercase letters are significantly different from each other (P < 0.01; when the same letter has a plus (+), the difference between values is significant at P values between 0.01 and 0.05). Statistical analysis of the EC50s was performed with the corresponding log10 values.
Cloxacillin has the same MIC (0.06 mg/liter) for both strains when it is tested at pH 5.5 (Table 1).
Emin, minimum effect, expressed as the change in the numbers of CFU (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for an infinitely low cloxacillin concentration.
CI, confidence interval.
Changes in the numbers of CFU (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for an infinitely high antibiotic concentration.
Concentration (mg/liter; total drug) causing a reduction of the inoculum halfway between the initial (E0) and the maximal (Emax) values, as obtained from the Hill equation (by using a slope factor of 1).
Cstatic, concentration (mg/liter; total drug) resulting in no apparent bacterial growth (the number of CFU was identical to that of the original inoculum), as determined by graphical interpolation.
Susceptibility of MRSA ATCC 33591 to various β-lactams after phagocytosis by THP-1 macrophages.
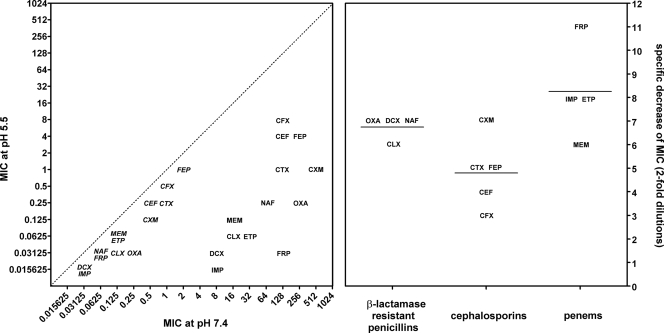
Our last series of experiments was aimed at examining whether restoration of the susceptibility of phagocytized MRSA to cloxacillin (and meropenem (25) could also be observed for other currently used registered antistaphylococcal β-lactams. Figure 3 (left panel) shows the changes in the MICs observed for both MSSA ATCC 25923 and MRSA ATCC 33591 when they were tested at pH 5.5 versus pH 7.4 with four β-lactamase-resistant penicillins, five cephalosporins, and four penems. Whereas only modest (about 2 dilutions) but constant decreases in MICs were noted with MSSA ATCC 25923, large reductions were observed for MRSA ATCC 33591. These, however, were much more variable between drugs. Thus, while the values recorded at pH 5.5 reached 8 mg/liter for cefotaxime, they were as low as 0.0156 mg/liter for imipenem. Closer analysis of the results allowed us to observe that the mean specific change in the susceptibility of MRSA ATCC 33591 (after discounting the effect seen for MSSA ATCC 25923, which was considered a general effect unrelated to the modulation of methicillin resistance [25]) was globally the lowest for the cephalosporins (4.8 dilutions), intermediate for β-lactamase-resistant penicillins (6.75 dilutions), and the highest for the penems (8.25 dilutions) examined in this study.
FIG. 3.
Influence of pH on the MICs of β-lactams for MSSA ATCC 25923 (italics) or MRSA ATCC 33591 (no italics). OXA, oxacillin; CLX, cloxacillin; DCX, dicloxacillin; NFC, nafcillin; CFX, cephalexin; CEF, cefadroxil; CXM, cefuroxime; CTX, cefotaxime; FEP, cefepime; IMP, imipenem; MEM, meropenem; ETP, ertapenem; FRP, faropenem. (Left panel) Correlation between the values observed at pH 7.4 and those observed at pH 5.5 for all antibiotics (the dotted line shows equivalence); when two antibiotics had the same MIC, they are placed on top of each other and aligned on their MIC at pH 7.4. (Right panel) Difference in MIC (expressed as the change in the log2 concentration [or twofold dilution decreases]) for MRSA ATCC 33591 when it was tested at pH 5.5 and when it was tested at pH 7.4, after discounting the change observed for the corresponding antibiotic for MSSA 25923 upon the same change of pH. Data are broken down by antibiotic class. The horizontal bars are the corresponding means.
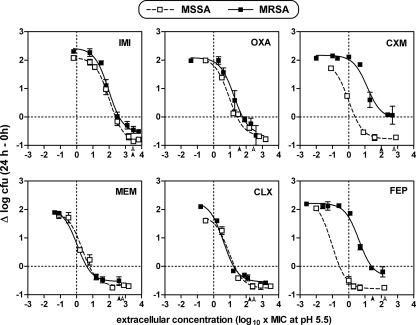
On the basis of a similar relative level of suppression of methicillin resistance at acidic pH (MIC reduction of about 7 log2 dilutions), four molecules (oxacillin, imipenem, cefuroxime, and cefepime) were selected, together with cloxacillin and meropenem, for in-depth pharmacological analysis of their activities against MRSA ATCC 33591 phagocytized by THP-1 macrophages. The results, which were normalized on the basis of the MICs measured for each antibiotic at pH 5.5, are shown graphically in Fig. 4, with pertinent numerical and statistical data shown in Table 3. In all cases, concentration-dependent activity similar to that described above was observed. Quantitative analysis showed that the dose-responses obtained for MRSA with imipenem, meropenem, oxacillin, and cloxacillin were virtually indistinguishable from those obtained with the same antibiotic and MSSA. The relative potency (EC50) of imipenem against both strains, however, was about 100-fold lower (in multiples of the MIC) than that of meropenem (also see the differences in the static concentrations). The situation was very different for cefuroxime and cefepime. First, there was a considerable separation of the dose-response curves between the MSSA and MRSA strains (with the curve for the latter being shifted toward higher concentrations), even though the data had been normalized on the basis of the MICs measured at pH 5.5 (the difference would have been even larger if the data had been expressed as a function of weight concentrations). This suggests that restoration of the susceptibility of MRSA to those two cephalosporins upon phagocytosis by THP-1 macrophages was only partial. Second, close analysis revealed that, at least for cefepime, part of the difference was due to the fact that this antibiotic had a relative potency against MSSA that was quite high (low EC50; when its MIC is taken into account) compared to the potencies of the other antibiotics tested. Third, and quite conspicuously, the relative efficacies (Emax) of both cephalosporins were significantly weaker than those of the penicillins and the penems, with cefuroxime actually being unable to achieve more than a strictly bacteriostatic effect.
FIG. 4.
Dose-response curves of two penems (left panels; IMI, imipenem; MEM, meropenem), two β-lactamase-resistant penicillins (middle panels; OXA, oxacillin; CLX, cloxacillin), and two cephalosporins (right panels CXM, cefuroxime; FEP, cefepime) against the intracellular forms of S. aureus ATCC 25923 (MSSA; open symbols and dotted lines) and S. aureus ATCC 33591 (MRSA; closed symbols and continuous lines) after phagocytosis by human THP-1 macrophages. The ordinate shows the change in the number of CFU per mg of cell protein (means ± standard deviations [n = 3]; most standard deviation bars are smaller than the symbols). The horizontal dotted lines correspond to an apparent static effect. The abscissa is the log10 of the cloxacillin extracellular concentration (log10 of the multiples of its MIC, as measured with the corresponding antibiotic and for the corresponding strain (ATCC 25923 or ATCC 33591) at pH 5.5 [Fig. 3]). The open and closed arrowheads point to the concentrations corresponding to the reported maximum concentration of the corresponding antibiotic in serum when it is administered to humans at conventional doses (imipenem, 52 mg/liter; meropenem, 50 mg/liter; oxacillin, 10 mg/liter; cloxacillin, 8 mg/liter; cefuroxime, 100 mg/liter; cefepime, 130 mg/liter), with the vertical dotted lines corresponding to the MICs. All data were used to fit sigmoidal functions, as described in the legend to Fig. 2.
TABLE 3.
Susceptibilities of MSSA ATCC 25923 and MRSA ATCC 33591 to imipenem, meropenem, oxacillin, cloxacillin, cefuroxime, and cefepime after phagocytosis by THP-1 macrophagesa
| Antibiotic | MSSAb
|
MRSAb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emaxc (95% CId) | EC50 (95% CI)e expressed as:
|
Cstaticf expressed as:
|
R2 | Emax (95% CI) | EC50 (95% CI) expressed as:
|
Cstatic expressed as:
|
R2 | |||||
| Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | |||||
| Imipenem | −0.90 (−0.96 to −0.84) a,A | 104 (90.3 to 121) a,A | 1.63 (1.41 to 1.89) | 234 | 3.80 | 0.999 | −0.56 (−0.79 to −0.34) a,B | 94.2 (57.6 to 154) a,A | 1.47 (0.90 to 2.41) | 407 | 6.38 | 0.991 |
| Meropenem | −0.69 (−1.00 to −0.38) a,b,A | 2.17 (0.87 to 5.41) b,A | 0.14 (0.05 to 0.34) | 6.03 | 0.38 | 0.980 | −0.53 (−0.64 to −0.43) a,A | 1.10 (0.38 to 3.16) b,A+ | 0.14 (0.05 to 0.40) | 4.17 | 0.51 | 1.000 |
| Oxacillin | −0.68 (−0.95 to −0.41) b+,A | 8.82 (4.31 to 18.0) c,A | 0.28 (0.13 to 0.56) | 26.9 | 0.85 | 0.985 | −0.62 (−0.99 to −0.25) a,A | 18.0 (8.21 to 39.5) c,A+ | 4.50 (2.05 to 9.88) | 60.2 | 15.1 | 0.987 |
| Cloxacillin | −0.77 (−1.08 to −0.47) a,b,A | 8.53 (2.76 to 26.3) c,A | 0.27 (0.09 to 0.82) | 19.5 | 1.62 | 0.987 | −0.55 (−0.86 to −0.24) a,A+ | 4.08 (1.50 to 11.1) d,A | 0.26 (0.09 to 0.69) | 17.0 | 1.05 | 0.990 |
| Cefuroxime | −0.78 (−0.94 to −0.61) a,b,A | 0.99 (0.58 to 1.68) e,A | 0.12 (0.07 to 0.21) | 2.45 | 0.30 | 0.999 | −0.06 (−0.42 to +0.30) b,B | 13.6 (5.74 to 32.1) e,B | 13.6 (5.74 to 32.1) | >500 | >500 | 0.975 |
| Cefepime | −0.79 (−0.97 to −0.62) a,b,A | 0.09 (0.02 to 0.35) f,A | 0.09 (0.02 to 0.35) | −0.27 | 0.27 | 0.999 | −0.37 (−0.71 to −0.02) a,B | 4.23 (2.27 to 7.88) d,B | 16.9 (9.09 to 31.5) | 25.1 | 104 | 0.987 |
The parameters described in the table are derived from the analysis of the data shown in Fig. 4. The results of statistical analyses were as follows: global analysis of the parameters of the Hill functions describing the dose-responses of the MSSA strain versus those of the MRSA strain for each antibiotic (unpaired t test, two-tailed, with all data points) showed no significant difference for imipenem, meropenem, oxacillin, and cloxacillin; P equal to 0.047 for cefuroxime; and P equal to 0.03 for cefepime. Global analysis of the parameters of the Hill functions describing the dose-responses of all antibiotics for MSSA or MRSA (one-way ANOVA by the Tukey test for multiple comparisons) showed no significant difference between antibiotics for MSSA. By analysis of the parameters in each column (one-way ANOVA by the Tukey test for multiple comparisons), values with different lowercase letters are significantly different from each other (P < 0.01; when the same letter has a plus (+), the difference between values was significant at P values between 0.01 and 0.05). By analysis of each row (unpaired, two-tailed t test), values with different uppercase letters are significantly different from each other (P < 0.01; when the same letter has a plus (+), the difference between values was significant at P values between 0.01 and 0.05). Statistical analysis of the EC50s was performed with the corresponding log10 values.
For the MICs, see Fig. 3.
Expressed as the change in the numbers of CFU (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for an infinitely high antibiotic concentration.
CI, confidence interval.
Concentration (total drug) causing a reduction of the inoculum halfway between the initial and the maximal values, as obtained from the Hill equation (by using a slope factor of 1). The initial values ranged from 1.79 and 2.42 (there was no significant difference between individual values). Multiples of the MIC were measured in broth at pH 5.5
Cstatic, concentration (total drug) resulting in no apparent bacterial growth (the number of CFU was identical to that of the original inoculum), as determined by graphical interpolation. Multiples of the MIC were measured in broth at pH 5.5.
Stability and cell accumulation of carbapenems and isoxazolylpenicillins.
Because the experiments described above disclosed significant differences in the dose-effect relationships between imipenem and meropenem, we compared the stability and cellular accumulation of these two antibiotics, using isoxazolylpencillins as controls. Stability was assessed by measuring the amount of drug (initial concentration, 50 mg/liter) remaining after 24 h of incubation at 37°C in buffered medium at pH 7.4 and 5.5 (mimicking the extracellular and intraphagosomal environments, respectively). The proportions of imipenem remaining were 39.3% ± 2.3% and 30.5% ± 2.1% at pH 7.4 and pH 5.5, respectively, and the proportions of meropenem remaining were 49.3% ± 1.0% and 40.3% ± 0.9% at pH 7.4 and pH 5.5, respectively, whereas the proportions of oxacillin remaining were 94.2% ± 0.1% and 91.8% ± 0.1% at pH 7.4 and pH 5.5, respectively, and the proportions of cloxacillin remaining were 97.8% ± 1.4% and 93.3% ± 0.2% at pH 7.4 and pH 5.5, respectively. We then measured the apparent cellular concentration-to-extracellular concentration ratios for each antibiotic after 24 h of incubation at an extracellular concentration of 250 mg/liter (this high concentration was needed to obtain measurable cellular contents). The values were 0.05 ± 0.02, 0.20 ± 0.02, 0.20 ± 0.02, and 0.28 ± 0.01 for imipenem, meropenem, oxacillin, and cloxacillin, respectively.
DISCUSSION
The present study expands our recent observation demonstrating that MRSA strains recover susceptibility to β-lactam antibiotics when they are phagocytized by eukaryotic cells. The new findings presented here are based on an established approach aimed at obtaining a comprehensive description of the pharmacological properties of antibiotics against intracellular bacteria in terms of dose-effect relationships (4).
The first new finding is that the observations originally made with a laboratory strain of MRSA can be extended to several MRSA isolates of clinical and epidemiological interest. As these strains were of various, unrelated origins, we may reasonably assume that what is shown here is probably applicable to most MRSA strains, irrespective of their subgroup. Their common phenotypic character is the expression of PBP 2a (also referred to in the literature as PBP 2′ [14]). In association with the transglycosylase domain of the native PBP 2, the activity of which is not inhibited by β-lactams (34), PBP 2a performs the critical cross-linking of the cell wall of the bacterium and, as such, serves a vital function for MRSA. We showed previously (23) that the restoration of intraphagocytic MRSA susceptibility to β-lactams is not seen if the phagolysosomal pH is increased by incubating cells in the presence of ammonium chloride, which specifically points to the role of acid pH in this effect. We also recently showed that acidic pH allows β-lactams to acylate PBP 2a, thanks to a pH-induced conformational change (21). Increased interactions with other targets at acidic pH may also play an additive role (23). The present data provide the necessary background for further advanced studies by identifying which β-lactams are more prone to cause such effects.
The second key finding is that the suppression of methicillin resistance in phagocytized MRSA is not cell specific and, therefore, is probably not primarily dependent on cell defense mechanisms. This opens interesting perspectives for the assessment of such a phenomenon in vivo, with special attention to tissues such as skin and bronchial epithelia, where the survival of intracellular S. aureus may play a critical role (26, 27). Our conclusions, however, should be clearly limited to those cells in which S. aureus gains access to and sojourns in acidic compartments. While this may be the case for many cells, including most professional phagocytes and epithelial cells, such as those evaluated in the present study, this may not be the case for other cell types, such as endothelial cells, where S. aureus appears to access and thrive in the cytosol (29), thus remaining at a neutral pH. Studies combining subcellular localization approaches with an evaluation of the modulation of methicillin resistance would be rewarding in this context.
A third but unanticipated finding from the present study is that the suppression of methicillin resistance in phagocytized bacteria does not seem to be obtained at the same level by all β-lactams, with the two cephalosporins tested in detail being obviously at a disadvantage in this context, even when the results were compared after normalization for differences in MICs at acid pH. This is most likely related to the way in which cells handle these cephalosporins because pH seems to influence their activity largely to the same extent as it influences the activity of other β-lactams. Further studies (i) examining a larger number of molecules to obtain more comprehensive structure-effect relationship data, (ii) comparing the cell uptake and disposition properties of cephalosporins to those of penicillins and penems, (iii) and analyzing the behavior of the novel anti-MRSA cephalosporins could help clarify the picture. The weaker relative potency (larger EC50) of imipenem compared to the potencies of meropenem and the isoxazolylpenicillins was also puzzling at first glance. It could not possibly be related to a difference in the restoration of susceptibility of MRSA to imipenem, since the dose-response curves of MRSA and MSSA were both shifted to high values compared to the value for meropenem or the isoxazolylpenicillins and were indistinguishable from each other. A partial explanation of a pharmacokinetic nature is probably the lower level of cellular accumulation of imipenem compared to the levels for meropenem and the isoxazolylpenicillins, coupled with the slightly greater instability of imipenem compared to that of meropenem. The instability of carbapenems in aqueous medium has already largely been documented (41). Our data suggest that more detailed studies aimed at defining how and to what extent the intracellular degradation of carbapenems or other pharmacodynamic factors could decrease their potencies against intracellular S. aureus are warranted.
The biological and chemotherapeutic significance of the present observations also deserve attention. Restoration of the susceptibility of MRSA to β-lactams by acidic pH was discovered in the early 1970s (37) but was not considered of clinical importance by its discoverers. However, S. aureus shows a high level of tolerance to pH variations, allowing its survival within acidic habitats (43). This survival is probably a key determinant in the relapsing and recurrent character of many staphylococcal infections. It is therefore somewhat ironic that a diagnosis of an MRSA infection invariably leads the clinician to discontinue β-lactams in favor of vancomycin or linezolid, two antibiotics which are known to be poorly effective against the intracellular forms of S. aureus (3, 4, 22). While this could call for recommendation of the use of β-lactams for the treatment of intracellular MRSA infections, our findings and the model used have obvious limits. First, the intraphagocytic activities of β-lactams against S. aureus remain quantitatively limited in terms of the Emax (about 1 log10 CFU at the most) in comparison with what can be obtained with fluoroquinolones or the novel lipoglycopeptides (2 to 3 log10 CFU [3, 4]). Thus, β-lactams would by no means qualify as being bactericidal against intracellular S. aureus, according to the general definition of a bactericidal effect proposed by the National Committee for Clinical Laboratory Standards (a 3-log10 CFU decrease) (32). Yet, what we see with β-lactams is at least as important as what is seen with other drugs recommended for use against multiresistant bacteria, such as linezolid (4). The data, however, suggest that fairly high concentrations of β-lactams maintained for prolonged periods of time will be needed to obtain a maximal effect. Indeed, β-lactams act only slowly on intracellular S. aureus (24). Second, our study was limited to a single 24-h time point, used constant antibiotic concentrations, and did not allow human protein binding effects to be modeled (see the discussions in references 3 and 24). These limitations are largely imposed by experimental constraints and will need to be addressed in future experiments. Third, conventional β-lactams will obviously be ineffective against the extracellular forms of MRSA. Their use to fight intracellular MRSA infections should therefore always be combined with the use of other agents that act against these extracellular forms. Carefully designed in vitro and in vivo studies examining this new type of combination therapy to control MRSA infections could be useful in this context.
Supplementary Material
Acknowledgments
We are grateful to Marie-Claire Cambier for dedicated technical assistance.
S.L. is boursière of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture, and F.V.B. is maître de recherches of the Belgian Fonds National de la Recherche Scientifique.
This work was supported by the Fonds de la Recherche Scientifique Médicale (grant 3.4.597.06) and the Belgian Federal Science Policy Office (research project P5/33; research action P5).
Footnotes
Published ahead of print on 2 June 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Anderson, D. J., D. J. Sexton, Z. A. Kanafani, G. Auten, and K. S. Kaye. 2007. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 28:1047-1053. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 2006. MRSA—the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2):3-10. [DOI] [PubMed] [Google Scholar]
- 3.Barcia-Macay, M., S. Lemaire, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:1177-1184. [DOI] [PubMed] [Google Scholar]
- 4.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baudoux, P., N. Bles, S. Lemaire, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2007. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J. Antimicrob. Chemother. 59:246-253. [DOI] [PubMed] [Google Scholar]
- 6.Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dancer, S. J. 2008. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 61:246-253. [DOI] [PubMed] [Google Scholar]
- 8.de Neeling, A. J., M. J. van den Broek, E. C. Spalburg, M. G. Santen-Verheuvel, W. D. Dam-Deisz, H. C. Boshuizen, A. W. van de Giessen, E. van Duijkeren, and X. W. Huijsdens. 2007. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet. Microbiol. 122:366-372. [DOI] [PubMed] [Google Scholar]
- 9.Dyke, K. G., M. P. Jevons, and M. T. Parker. 1966. Penicillinase production and intrinsic resistance to penicillins in Staphylococcus aures. Lancet i:835-838. [DOI] [PubMed] [Google Scholar]
- 10.Ellington, J. K., M. Harris, L. Webb, B. Smith, T. Smith, K. Tan, and M. Hudson. 2003. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J. Bone Joint Surg. Br. 85:918-921. [PubMed] [Google Scholar]
- 11.File, T. M., Jr. 2007. Impact of community-acquired methicillin-resistant Staphylococcus aureus in the hospital setting. Cleve. Clin. J. Med. 74(Suppl. 4):S6-S11. [DOI] [PubMed] [Google Scholar]
- 12.Gadd, S. J., O. Majdic, W. Kasinrerk, H. Stockinger, D. Maurer, R. Eher, and W. Knapp. 1990. M5, a phosphoinositol-linked human myelomonocytic activation-associated antigen. Clin. Exp. Immunol. 80:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzoni, C., P. Francois, A. Huyghe, S. Couzinet, C. Tapparel, Y. Charbonnier, A. Renzoni, S. Lucchini, D. P. Lew, P. Vaudaux, W. L. Kelley, and J. Schrenzel. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgopapadakou, N. H., S. A. Smith, and D. P. Bonner. 1982. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob. Agents Chemother. 22:172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godding, V., Y. Sibille, P. P. Massion, M. Delos, C. Sibille, P. Thurion, D. Giffroy, A. Langendries, and J. P. Vaerman. 1998. Secretory component production by human bronchial epithelial cells is upregulated by interferon gamma. Eur. Respir. J. 11:1043-1052. [DOI] [PubMed] [Google Scholar]
- 16.Huijsdens, X. W., B. J. van Dijke, E. Spalburg, M. G. Santen-Verheuvel, M. E. Heck, G. N. Pluister, A. Voss, W. J. Wannet, and A. J. de Neeling. 2006. Community-acquired MRSA and pig-farming. Ann. Clin. Microbiol. Antimicrob. 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 19.Krut, O., H. Sommer, and M. Kronke. 2004. Antibiotic-induced persistence of cytotoxic Staphylococcus aureus in non-phagocytic cells. J. Antimicrob. Chemother. 53:167-173. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. H. 2003. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 69:6489-6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaire, S., C. Fuda, F. Van Bambeke, P. M. Tulkens, and S. Mobashery. 2008. Restoration of susceptibility of methicillin-resistant Staphylococcus aureus to β-lactam antibiotics by acidic pH: role of penicillin-binding protein PBP 2a. J. Biol. Chem. 283:12769-12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemaire, S., K. Kosowska-Shick, K. Julian, P. M. Tulkens, F. Van Bambeke, and P. C. Appelbaum. Clin. Microbiol. Infect., in press. [DOI] [PubMed]
- 23.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, Y. Glupczynski, and P. M. Tulkens. 2007. Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin. Antimicrob. Agents Chemother. 51:1627-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Activity of three β-lactams (ertapenem, meropenem and ampicillin) against intraphagocytic Listeria monocytogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 55:897-904. [DOI] [PubMed] [Google Scholar]
- 25.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2007. Modulation of the cellular accumulation and intracellular activity of daptomycin towards phagocytized Staphylococcus aureus by the P-glycoprotein (MDR1) efflux transporter in human THP-1 macrophages and Madin-Darby canine kidney cells. Antimicrob. Agents Chemother. 51:2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 27.Lowy, F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-343. [DOI] [PubMed] [Google Scholar]
- 28.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 29.Menzies, B. E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motulsky, H. J. 2005. Analyzing data with GraphPad Prism. GraphPad Software Inc., San Diego, CA.
- 31.Murakami, K., and A. Tomasz. 1989. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J. Bacteriol. 171:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. 1998. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline (M26A). National Committee for Clinical Laboratory Standards, Wayne, PA.
- 33.Normanno, G., M. Corrente, G. La Salandra, A. Dambrosio, N. C. Quaglia, A. Parisi, G. Greco, A. L. Bellacicco, S. Virgilio, and G. V. Celano. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int. J. Food Microbiol. 117:219-222. [DOI] [PubMed] [Google Scholar]
- 34.Pinho, M. G., H. de Lencastre, and A. Tomasz. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA 98:10886-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plouin-Gaudon, I., S. Clement, E. Huggler, C. Chaponnier, P. Francois, D. Lew, J. Schrenzel, P. Vaudaux, and J. S. Lacroix. 2006. Intracellular residency is frequently associated with recurrent Staphylococcus aureus rhinosinusitis. Rhinology 44:249-254. [PubMed] [Google Scholar]
- 36.Qazi, S. N., S. E. Harrison, T. Self, P. Williams, and P. J. Hill. 2004. Real-time monitoring of intracellular Staphylococcus aureus replication. J. Bacteriol. 186:1065-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabath, L. D., S. J. Wallace, and D. A. Gerstein. 1972. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob. Agents Chemother. 2:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266-277. [DOI] [PubMed] [Google Scholar]
- 40.Theus, S. A., M. D. Cave, and K. D. Eisenach. 2004. Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 72:1169-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viaene, E., H. Chanteux, H. Servais, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 46:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weese, J. S. 2005. Methicillin-resistant Staphylococcus aureus: an emerging pathogen in small animals. J. Am. Anim. Hosp. Assoc. 41:150-157. [DOI] [PubMed] [Google Scholar]
- 43.Weinrick, B., P. M. Dunman, F. McAleese, E. Murphy, S. J. Projan, Y. Fang, and R. P. Novick. 2004. Effect of mild acid on gene expression in Staphylococcus aureus. J. Bacteriol. 186:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wijaya, L., L. Y. Hsu, and A. Kurup. 2006. Community-associated methicillin-resistant Staphylococcus aureus: overview and local situation. Ann. Acad. Med. Singapore 35:479-486. [PubMed] [Google Scholar]
- 45.Wulf, M. W., M. Sorum, A. van Nes, R. Skov, W. J. Melchers, C. H. Klaassen, and A. Voss. 2008. Prevalence of methicillin-resistant Staphylococcus aureus among veterinarians: an international study. Clin. Microbiol. Infect. 14:29-34. [DOI] [PubMed] [Google Scholar]
- 46.Yamaoka, T. 2007. The bactericidal effects of anti-MRSA agents with rifampicin and sulfamethoxazole-trimethoprim against intracellular phagocytized MRSA. J. Infect. Chemother. 13:141-146. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, K., J. A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.