Abstract
Tomopenem (formerly CS-023) is a novel 1β-methylcarbapenem with broad-spectrum coverage of gram-positive and gram-negative pathogens. Its antibacterial activity against European clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa was compared with those of imipenem and meropenem. The MICs of tomopenem against MRSA and P. aeruginosa at which 90% of the isolates tested were inhibited were 8 and 4 μg/ml, respectively, and were equal to or more than fourfold lower than those of imipenem and meropenem. The antibacterial activity of tomopenem against MRSA was correlated with a higher affinity for the penicillin-binding protein (PBP) 2a. Its activity against laboratory mutants of P. aeruginosa with (i) overproduction of chromosomally coded AmpC β-lactamase; (ii) overproduction of the multidrug efflux pumps MexAB-OprM, MexCD-OprJ, and MexEF-OprN; (iii) deficiency in OprD; and (iv) various combinations of AmpC overproduction, MexAB-OprM overproduction, and OprD deficiency were tested. The increases in the MIC of tomopenem against each single mutant compared with that against its parent strain were within a fourfold range. Tomopenem exhibited antibacterial activity against all mutants, with an observed MIC range of 0.5 to 8 μg/ml. These results suggest that the antibacterial activity of tomopenem against the clinical isolates of MRSA and P. aeruginosa should be ascribed to its high affinity for PBP 2a and its activity against the mutants of P. aeruginosa, respectively.
Patients with serious bacterial infections, such as nosocomial pneumonia, intra-abdominal infections, and complicated skin and soft tissue infections, are often treated empirically, because a delay in initiation of appropriate antimicrobial therapy has been shown to significantly increase morbidity and mortality (4, 11, 12, 21). The causative pathogens in these infections are a variety of gram-positive and gram-negative aerobes and anaerobes such as methicillin-resistant Staphylococcus aureus (MRSA), Enterobacteriaceae species, Pseudomonas aeruginosa, and Bacteroides fragilis (5, 6, 34). Treatment guidelines for these infections recommend specific antibiotics or combinations of antibiotics against these pathogens (1, 28). Of all the β-lactams, carbapenems have the broadest spectra of antibacterial activity; tomopenem (formerly CS-023), a novel 1β-methylcarbapenem, exhibits activity against diverse hospital pathogens, including MRSA and P. aeruginosa isolated in the United States and Japan (9, 33). In a previous clinical trial in healthy volunteers, tomopenem (27) showed a longer half-life (1.7 h) than imipenem (IPM)-cilastatin (1.0 h) (22) and meropenem (MEM) (0.9 h) (26). This study evaluated the antibacterial activity of tomopenem against MRSA and P. aeruginosa in Europe and investigated the mechanisms of its antibacterial activity against MRSA and P. aeruginosa. MRSA is characterized by the expression of a special penicillin-binding protein (PBP), PBP 2a. This PBP is not efficiently inhibited by commercially available carbapenems such as IPM and MEM (13, 30). The recently reported carbapenems SM-216601 (36) and ME1036 (13), which exhibit activity against MRSA, have weak activities against P. aeruginosa, which has intrinsic and acquired antibiotic resistance. This organism has several resistance mechanisms, such as outer membrane impermeability, multiple efflux systems, and a chromosomal AmpC β-lactamase (17). In this study, we evaluated the affinity of tomopenem for PBP 2a and the activity of tomopenem against P. aeruginosa laboratory mutants.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy [abstr., 1231], at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy [abstr., F-366], and at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy [abstr., F-326].)
MATERIALS AND METHODS
Antibiotics.
Tomopenem was synthesized at Daiichi Sankyo Research Laboratories, Tokyo, Japan (Fig. 1). IPM, MEM, and ceftazidime (CAZ) were obtained from the National Institute of Infectious Diseases, Tokyo, Japan. Levofloxacin was extracted at Daiichi Sankyo from a commercial formula. Amikacin (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan) and oxacillin (Sigma-Aldrich Japan K.K.) were obtained commercially.
FIG. 1.
Chemical structure of tomopenem.
Bacterial strains.
MRSA and P. aeruginosa used in the susceptibility tests were isolated from clinical specimens in European hospitals from 2001 to 2002 and from 2000 to 2002, respectively, and were stored frozen at −80°C. Sixty strains of MRSA were isolated in Germany, and 138 strains of P. aeruginosa were isolated in Germany (87 strains), France (19 strains), Italy (12 strains), Spain (7 strains), Ukraine (5 strains), Poland (4 strains), Russia (2 strains), Croatia (1 strain), and Lithuania (1 strain). S. aureus ATCC 29213 and P. aeruginosa ATCC 27853 were used as the quality control strains. The clinical isolates of MRSA 123-1 and 12386-1, which were isolated by culturing Japanese clinical isolates of MRSA 123 and 12386 from which a penicillinase-encoding plasmid had been removed, were used for PBP 2a affinity tests. The P. aeruginosa mutants (N043, MR08, OCR1, N044, N045, N041, COR6, and N092) used in this study are described in Table 1. Highly CAZ-resistant mutant N043 was isolated by plating a CAZ-resistant mutant, which was isolated on Mueller-Hinton agar (MHA; Becton Dickinson and Company, Sparks, MD) containing 6.25 μg of CAZ per ml from PAO1, on MHA containing 50 μg of CAZ per ml. β-Lactamase produced in N043 was determined by UV spectrophotometry to have 80-fold more activity than that in a CAZ-resistant mutant. IPM-resistant mutants N041 and N044 were isolated by plating OCR1 and N043 on MHA containing 3 μg of IPM per ml, respectively. The outer membrane protein OprD was not detected in N041 and N044 by Western blot analysis with a rabbit anti-OprD antibody. Ofloxacin-MEM-resistant mutant N045 was isolated by plating N043 on MHA containing 0.8 μg of ofloxacin and 0.8 μg of MEM per ml. Ofloxacin-IPM-resistant mutant N092 was isolated by plating another PAO1 strain (a generous gift from T. Köhler) onto MHA containing 0.5 μg of ofloxacin and 1 μg of IPM per ml. A decrease in the amount of the outer membrane protein OprD and an increase in the amount of the outer membrane protein OprN in N092 were observed compared with the results seen with N091 by Western blot analysis with a rabbit anti-OprN antibody. Metallo-β-lactamase production was screened with modified Hodge and EDTA disk synergy tests (15).
TABLE 1.
MICs of tomopenem against various mutants of Pseudomonas aeruginosa
| Strain | Phenotype(s) (genotype) | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|---|
| Tomopenem | IPM | MEM | CAZ | AMK | LVFX | ||
| PAO1b | Parent | 0.25 | 1 | 0.5 | 2 | 4 | 0.5 |
| N043c | β-Lactamase-overproducing mutant | 0.5 (2) | 1 (1) | 1 (2) | 128 (64) | 8 (2) | 0.25 (1/2) |
| MR08d | OprD-deficient mutant | 1 (4) | 16 (16) | 4 (8) | 2 (1) | 4 (1) | 0.5 (1) |
| OCR1d | MexA-MexB-OprM-overproducing mutant (nalB mutant) | 0.5 (2) | 1 (1) | 2 (4) | 8 (4) | 4 (1) | 4 (8) |
| N044c | β-Lactamase-overproducing OprD-deficient mutant and OprD-deficient mutant | 8 (32) | 32 (32) | 16 (32) | 128 (64) | 8 (2) | 0.25 (1/2) |
| N045c | β-Lactamase- and MexA-MexB-OprM-overproducing mutant | 1 (4) | 1 (1) | 2 (4) | 128 (64) | 8 (2) | 2 (4) |
| N041c | MexA-MexB-OprM-overproducing OprD-deficient mutant | 2 (8) | 8 (8) | 16 (32) | 8 (4) | 4 (1) | 4 (8) |
| COR6e | MexC-MexD-OprJ-producing mutant (nfxB mutant) | 0.25 (1) | 0.5 (1/2) | 0.5 (1) | 1 (1/2) | 0.5 (1/8) | 8 (16) |
| N091f | Parent PAO1 | 0.25 | 1 | 0.5 | 2 | 4 | 0.5 |
| N092c | MexE-MexF-OprN-producing low-OprD-producing mutant of N091 (nfxC mutant) | 0.5 (2) | 4 (4) | 1 (2) | 1 (1/2) | 2 (1/2) | 8 (16) |
The values in parentheses represent the severalfold increases and decreases (fractional number) in MIC compared to the MIC for a parent strain. AMK, amikacin; LVFX, levofloxacin.
This strain was provided by Dr. Gotoh (Kyoto Pharmaceutical University).
These strains were made for this study.
These strains were used for the study reported in reference 18.
This strain was used for the study reported in reference 19.
This strain was provided by T. Köhler (Centre Médical Universitaire, Geneva, Switzerland) (10).
Susceptibility tests.
The MICs were determined by a standard microdilution broth method (23). Mueller-Hinton broth (Becton Dickinson and Company, Sparks, MD) containing 25 mg of Ca2+ and 12.5 mg of Mg2+ per liter (cation-adjusted Mueller-Hinton broth) was used. The inoculum size was 4×105 CFU/ml. The MIC was defined as the lowest concentration of the compound that completely inhibited the viable growth of the organism in the microdilution wells. The determinations of the drug MIC against P. aeruginosa PAO1 and its mutants with various resistance mechanisms were performed in duplicate. Linear regression analysis (y = a + bx, where a represents the y intercept and b represents the slope) was used to correlate tomopenem log2 MICs (y) with IPM and MEM log2 MICs (x). The correlation coefficient (r) was used to describe the scatter around lines of best fit. A statistical test was performed to assess the differences in the correlation coefficients (r). All P values were two-sided. The same analysis of Japanese P. aeruginosa isolates (9) was also performed.
Affinity for PBP 2a.
The affinities of tomopenem, IPM, and MEM for PBP 2a were determined by a competition assay using [14C]benzylpenicillin (Amersham Japan Co., Ltd., Tokyo, Japan) as described previously (29, 31). Membrane fractions were collected by sequential centrifugations (5,000 × g for 10 min and 100,000 × g for 60 min) of enzymatically (100 μg of lysostaphin and 1 μg of DNase per ml) and sonically disrupted cells of MRSA 123-1 and MRSA 12386-1 in 50 mM sodium phosphate buffer containing 10 mM MgCl2 (pH 7.0). The protein concentrations of the membrane fractions were adjusted to the final concentration of 8 mg/ml after protein quantitation with bovine serum albumin as the standard. The binding reactions were done for 30 min with test compounds at each concentration followed by 30 min with [14C]benzylpenicillin at 37°C. The concentration required to prevent 50% of the binding of [14C]benzylpenicillin (50% inhibitory concentration [IC50]) to PBP 2a were determined using a BAS 2000 imaging analyzer (Fuji Chemical Co. Ltd.).
RESULTS
Antibacterial activity of tomopenem against MRSA and P. aeruginosa.
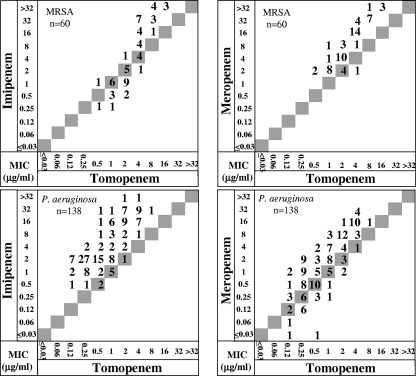
Table 2 shows the antibacterial activity of tomopenem against European clinical isolates of MRSA and P. aeruginosa. The MICs at which 90% of the isolates tested were inhibited (MIC90s) of tomopenem, IPM, and MEM against 60 strains of MRSA were 8, >32, and 32 μg/ml, respectively. The MIC90s of tomopenem, IPM, and MEM against 138 strains of P. aeruginosa were 4, 32, and 16 μg/ml, respectively. Scattergrams comparing the MICs of tomopenem with those of IPM and MEM for MRSA and P. aeruginosa are shown in Fig. 2. Against MRSA, the MIC of tomopenem was twofold to fourfold higher than that of IPM when the MICs of tomopenem were less than or equal to 2 μg/ml against 16 strains out of 29 strains, while it was twofold to eightfold lower than that of IPM when the MICs of tomopenem were more than 2 μg/ml against 26 strains out of 31 strains. The MIC of tomopenem was equal to or more than twofold lower than that of MEM against the MRSA isolates tested, except for 1 strain. Tomopenem inhibited the growth of all P. aeruginosa strains, including IPM-resistant (MIC of IPM ≥ 16 μg/ml) and MEM-resistant (MIC of MEM ≥ 16 μg/ml) strains, at 8 μg/ml or lower. Tomopenem inhibited 98 percent of IPM-resistant P. aeruginosa strains (MIC of IPM ≥ 16 μg/ml), and 94% of MEM-resistant P. aeruginosa strains (MIC of MEM ≥ 16 μg/ml) showed MICs of tomopenem below 8 at 4 μg/ml or lower. Against European P. aeruginosa isolates, there was no significant difference (P = 0.536) between the activities of tomopenem and MEM (correlation coefficient, r = 0.803) and the activities of tomopenem and IPM (correlation coefficient, r = 0.774). Against Japanese P. aeruginosa isolates, the correlation between tomopenem and MEM (correlation coefficient, r = 0.867) was significantly higher (P = 0.0290) than that between tomopenem and IPM (correlation coefficient, r = 0.765). No metallo-β-lactamase producing strains were found (data not shown).
TABLE 2.
Antibacterial activity of tomopenem against MRSA and P. aeruginosa clinical isolates
| Organism (no. of strains) and compound | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| MRSA (60) | |||
| Tomopenem | 0.5-16 | 4 | 8 |
| Imipenem | 0.25->32 | 4 | >32 |
| Meropenem | 2->32 | 8 | 32 |
| P. aeruginosa (138) | |||
| Tomopenem | 0.12-8 | 0.5 | 4 |
| Imipenem | 0.5->32 | 2 | 32 |
| Meropenem | <0.03-32 | 1 | 16 |
FIG. 2.
Scattergrams comparing the MICs of tomopenem with the MICs of IPM and MEM. The figure shows the number of strains with the corresponding MICs.
Affinities of tomopenem for PBP 2a of MRSA.
Table 3 shows the affinities of tomopenem, IPM, and MEM for PBP 2a of two strains of MRSA. The MIC of tomopenem against MRSA 123-1 and 12386-1 was 8 μg/ml, that of IPM and MEM against MRSA 123-1 was 32 μg/ml, and that of IPM and MEM against MRSA 12386-1 was 16 μg/ml. Tomopenem exhibited improved affinity which was more than 25-fold as high as that for IPM and more than 15-fold as high as that for MEM.
TABLE 3.
Affinities of tomopenem for PBP 2a of MRSA strains
| Strain and compound | IC50 (μg/ml) | MIC (μg/ml) |
|---|---|---|
| 123-1 | ||
| Tomopenem | 5.3 | 8 |
| Imipenem | 170 | 32 |
| Meropenem | 130 | 32 |
| 12386-1 | ||
| Tomopenem | 2.9 | 8 |
| Imipenem | 76 | 16 |
| Meropenem | 53 | 16 |
Activity of tomopenem against P. aeruginosa with various resistance mechanisms.
Table 1 shows the MICs of tomopenem and comparators against P. aeruginosa PAO1 and its mutants with various resistance mechanisms. Against PAO1, a parent strain, the MIC of tomopenem was fourfold lower than that of IPM and twofold lower than that of MEM. Although the antibacterial activity of tomopenem against a mutant with deficiency in OprD (MR08), a double mutant with overproduction of β-lactamase and overproduction of MexAB-OprM (N045), a double mutant with deficiency in OprD and overproduction of ΜexΑΒ-OprM (N041), and a double mutant with overproduction of β-lactamase and deficiency in OprD (N044) was reduced 4- to 32-fold, that of tomopenem against other mutants was almost the same as that against PAO1. Tomopenem exhibited antibacterial activity against all mutants, with an observed MIC range of 0.5 to 8 μg/ml.
DISCUSSION
Tomopenem showed antibacterial activity with MRSA and P. aeruginosa in European clinical isolates and laboratory mutants. The commercially available carbapenems such as IPM and MEM are insufficiently active against MRSA. Therefore, a number of carbapenems that target MRSA and other resistant gram-positive organisms have been investigated. However, so far carbapenems with both anti-MRSA activity and anti-P. aeruginosa activity have not been launched. Tomopenem exhibited improved activity against MRSA and P. aeruginosa. There have been tomopenem phase II clinical trials conducted for complicated skin and skin structure infections in the United States and the European Union at doses of 750 mg three times a day (t.i.d.) and 1,500 mg t.i.d. J. L. Kuti et al. reported that the doses of 750 mg t.i.d. and 1,500 mg t.i.d. would achieve bactericidal exposures at breakpoints of 8 and 16 μg/ml, respectively, based on pharmacodynamic modeling (14). These proposed breakpoints would be differentiated clinically from existing carbapenems.
The MIC50 of tomopenem and IPM against MRSA was 4 μg/ml, and the MIC90s of tomopenem and IPM were 8 and >32 μg/ml, respectively. According to the scattergrams, the MIC of tomopenem was equal to or more than twofold higher than that of IPM against most of the MRSA strains when the MICs of tomopenem were less than or equal to 2 μg/ml. However, it was equal to or more than twofold lower than that of IPM when the MICs of tomopenem were more than 2 μg/ml. The MIC of tomopenem was equal to or more than twofold lower than that of MEM against most of the strains. In this study, all the MRSA strains were isolated in Germany, and therefore this result may not be representative of Europe. We determined the IC50 values for tomopenem, IPM, and MEM for PBP 2a with two strains of MRSA. The IC50s of tomopenem were more than 15-fold lower than those of IPM and MEM. Since the major mechanism of resistance to β-lactams in MRSA is the low affinity to PBP 2a, there would be a good correlation between the MIC of each carbapenem and the binding affinity for PBP 2a. This correlation is consistent with other new carbapenems (13, 35). The affinity of tomopenem for PBP 2a might be higher than that of IPM at the boundary of a MIC of tomopenem of more than 2 μg/ml. This hypothesis needs further evaluation, utilizing more strains with various MICs of tomopenem. The differences in MICs may be ascribed to the differences in the affinities for PBP 2a. However, the effects of other presumed factors influencing β-lactam resistance in MRSA, such as cell wall precursor formation and turnover, regulation, transport, and signal transduction (2), remain to be elucidated.
Recently, several cephalosporins and carbapenems that bind to PBP 2a with higher affinity than those of available β-lactams have been developed (7, 13, 32, 35). These compounds have in common a significantly longer side chain than commercially available β-lactams, which would be expected to increase their interactions with the active-site groove of PBP 2a (3, 16). The anti-MRSA activity of tomopenem may be related to structural aspects, such as a new side chain, a 2-guanidinoacetylamino pyrrolidine moiety at position 2. Another group has also reported that the introduction of guanidine moieties to a pyrrolidine-3-ylthio group at the C-2 position in the carbapenem skeleton showed potent and well-balanced antibacterial activity, including anti-MRSA activity (24). This structural feature would permit the molecule to be positioned within the groove in such a way that the acylation reaction would proceed at a more rapid rate than that in commercially available carbapenems.
The emergence of carbapenem resistance in P. aeruginosa has become a global concern, since carbapenems remain important agents for the treatment of serious infections such as septicemia, pneumonia, and abdominal and urinary tract and skin and soft tissue infections due to multidrug-resistant P. aeruginosa in hospitalized patients and since only a few drugs are active against P. aeruginosa. Tomopenem showed more potent activity against P. aeruginosa from Europe, being fourfold more active than IPM and MEM. The correlation coefficient between MEM and tomopenem with European isolates was not significantly different from that between IPM and tomopenem. The increase in the drug MIC for the single mutant was similar to that of MEM in comparison with that of IPM (Table 1), and the affinity of tomopenem for PBPs 2 and 3 in P. aeruginosa ATCC 15692 was similar to that of MEM in comparison with IPM (N. Masuda, personal data). The reason for this lack of significant difference is unclear, but the proportion of MexA-MexB-OprM-overproducing OprD-deficient mutants in clinical isolates might be higher, since the increase in the drug MIC for this double mutant was similar to that of IPM in comparison with that of MEM. On the other hand, the correlation coefficient between MEM and tomopenem with Japanese isolates was significantly higher than that between IPM and tomopenem. The difference in analysis results between the European and Japanese isolates might be due to the difference in the proportions of the strains with various resistance mechanisms. Metallo-β-lactamase-producing P. aeruginosa was not detected in this study. The absence of metallo-β-lactamase-producing P. aeruginosa would reflect the region of isolates, since more than 60% of the strains were isolated from Germany, where metallo-β-lactamases are reported to be rare (8, 37).
The mechanisms associated with acquired resistance to carbapenems include the derepression of the chromosomal AmpC β-lactamases and loss of the substrate-specific channel, OprD, and the efflux pump, MexAB-OprM (17, 20). The MICs of tomopenem against the laboratory strains with these mechanisms were determined. Against PAO1, a parent strain, the MIC of tomopenem was equal to or more than twofold lower than those of IPM and MEM. The MR08 strain, an OprD-deficient mutant, showed increased resistance to all the carbapenems tested (Table 1), suggesting that, like other carbapenems, tomopenem penetrates outer membrane via this channel. Against the OCR1 strain, a MexAB-OprM-overproducing strain, the increase in the MIC of tomopenem was twofold lower than that of MEM. This result suggests that tomopenem is a substrate for MexAB-OprM as well as MEM. Since these were single mutants for which the increases in the MICs of tomopenem and MEM were less than fourfold and eightfold, respectively, tomopenem could be considered to come under less influence from this kind of resistance mechanism than MEM. This expectation supported the observation that the MICs of tomopenem against laboratory mutants with combinations of resistance mechanisms whose strains were found in carbapenem-resistant isolates (N044, N045, and N041) were equal to or more than twofold lower than that of MEM. Against the strain N091, a MexEF-OprN-producing low-OprD-producing mutant, the increase in the MIC of tomopenem was twofold, although it is unclear whether tomopenem is a substrate for this pump or whether the association reflects the coregulation of MexEF-OprN with the low production of OprD. The lower MIC of tomopenem compared to those IPM and MEM against various combinations of resistant mutants of P. aeruginosa is suggested to be related to its strong activity against susceptible bacteria (PAO1) and to a weaker reduction in the activity of each resistance mechanism.
Carbapenem resistance in clinical isolates of P. aeruginosa is a result of the interplay between diminished production of OprD and increased activity of ampC and of several efflux systems (25). The mechanism of the antibacterial activity of tomopenem against clinical isolates of P. aeruginosa is suggested to be related to its strong activity against susceptible bacteria intrinsically and to a smaller reduction in activity by various resistant mutations.
In conclusion, the new parenterally administered carbapenem tomopenem is considered to be a promising compound for further evaluation in cases of nosocomial infections with MRSA and P. aeruginosa.
Acknowledgments
We thank N. Gotoh and T. Köhler for providing us with the laboratory mutants of P. aeruginosa.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.American Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 2.Berger-Bächi, B., and S. Rohrer. 2002. Factors influencing methicillin resistance in staphylococci. Arch. Microbiol. 178:165-171. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, H. F. 2003. Solving staphylococcal resistance to β-lactams. Trends Microbiol. 11:145-148. [DOI] [PubMed] [Google Scholar]
- 4.Depuydt, P., D. Benoit, D. Vogelaers, G. Claeys, G. Verschraegen, K. Vandewoude, J. Decruyenaere, and S. Blot. 2006. Outcome in bacteremia associated with nosocomial pneumonia and the impact of pathogen prediction by tracheal surveillance cultures. Intensive Care Med. 32:1173-1181. [DOI] [PubMed] [Google Scholar]
- 5.Gales, A. C., H. S. Sader, and R. N. Jones. 2002. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia in Latin America: frequency of occurrence and antimicrobial susceptibility profile: results from the SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn. Microbiol. Infect. Dis. 44:301-311. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein, E. J. 2002. Intra-abdominal anaerobic infections: bacteriology and therapeutic potential of newer antimicrobial carbapenem, fluoroquinolone, and desfluoroquinolone therapeutic agents. Clin. Infect. Dis. 35(Suppl. 1):S106-S111. [DOI] [PubMed] [Google Scholar]
- 7.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichfreise, B., I. Wiegand, K. J. Sherwood, and B. Wiedemann. 2005. Detection of VIM-2 metallo-β-lactamase in Pseudomonas aeruginosa from Germany. Antimicrob. Agents Chemother. 49:1668-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koga, T., T. Abe, H. Inoue, T. Takenouchi, A. Kitayama, T. Yoshida, N. Masuda, C. Sugihara, M. Kakuta, M. Nakagawa, Y. Utsui, T. Fukuoka, and S. Kuwahara. 2005. In vitro and in vivo antibacterial activity of CS-023 (RO4908463), a novel parenteral carbapenem. Antimicrob. Agents Chemother. 49:3239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler, T., S. F. Epp, L. K. Curty, and J. C. Pechère. 1999. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181:6300-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollef, M. H. 2004. Appropriate empiric antimicrobial therapy of nosocomial pneumonia: the role of the carbapenems. Respir. Care 49:1530-1541. [PubMed] [Google Scholar]
- 12.Krobot, K., D. Yin, Q. Zhang, S. Sen, A. Altendorf-Hofmann, J. Scheele, and W. Sendt. 2004. Effect of inappropriate initial empiric antibiotic therapy on outcome of patients with community-acquired intra-abdominal infections requiring surgery. Eur. J. Clin. Microbiol. Infect. Dis. 23:682-687. [DOI] [PubMed] [Google Scholar]
- 13.Kurazono, M., T. Ida, K. Yamada, Y. Hirai, T. Maruyama, E. Shitara, and M. Yonezawa. 2004. In vitro activities of ME1036 (CP5609), a novel parenteral carbapenem, against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 48:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuti, J. L., C. H. Nightingale, and D. P. Nicolau. 2004. Pharmacodynamics of RO4908463 (CS-023), a novel carbapenem, against Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PSA). Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 51. American Society for Microbiology, Washington, DC.
- 15.Lee, K., Y. Chong, H. B. Shin, Y. A. Kim, D. Yong, and J. H. Yum. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88-91. [DOI] [PubMed] [Google Scholar]
- 16.Lim, D., and N. C. Strynadka. 2002. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 9:870-876. [DOI] [PubMed] [Google Scholar]
- 17.Livermore, D. M. 2001. Of Pseudomonas, porins, pumps and carbapenems. J. Antimicrob. Chemother. 47:247-250. [DOI] [PubMed] [Google Scholar]
- 18.Masuda, N., and S. Ohya. 1992. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda, N., N. Gotoh, S. Ohya, and T. Nishino. 1996. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 40:909-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda, N., N. Gotoh, C. Ishii, E. Sakagawa, S. Ohya, and T. Nishino. 1999. Interplay between chromosomal beta-lactamase and the MexAB-OprM efflux system in intrinsic resistance to β-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlino, J. I., and M. A. Malangoni. 2007. Complicated skin and soft-tissue infections: diagnostic approach and empiric treatment options. Cleve. Clin. J. Med. 74(Suppl. 4):S21-S28. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa, K., and M. Koyama. 1985. Imipenem, cilastatin sodium, imipenem/cilastatin sodium clinical phase I study. Chemotherapy (Tokyo) 33(Suppl. 4):357-378. [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 5th ed.: approved standard M7-A5. NCCLS, Wayne, PA.
- 24.Oh, C. H., S. C. Lee, and J. H. Cho. 2003. Synthesis and biological activity of 1β-methyl-2-[5-(2-N-substituted aminoethylcarbamoyl) pyrrolidin-3-ylthio] carbapenem derivatives. Eur. J. Med. Chem. 38:841-850. [DOI] [PubMed] [Google Scholar]
- 25.Pai, H., J. Kim, J. Kim, J. H. Lee, K. W. Choe, and N. Gotoh. 2001. Carbapenem resistance mechanisms in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 45:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito, A. 1992. Pharmacokinetic study on meropenem. Chemotherapy (Tokyo) 40(Suppl. 1):276-282. [Google Scholar]
- 27.Shibayama, T., Y. Matsushita, T. Hirota, T. Ikeda, and S. Kuwahara. 2006. Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers. Antimicrob. Agents Chemother. 50:4186-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomkin, J. S., J. E. Mazuski, E. J. Baron, R. G. Sawyer, A. B. Nathens, J. T. DiPiro, T. Buchman, E. P. Dellinger, J. Jernigan, S. Gorbach, A. W. Chow, and J. Bartlett for the Infectious Diseases Society of America. 2003. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin. Infect. Dis. 37:997-1005. [DOI] [PubMed] [Google Scholar]
- 29.Spratt, B. G. 1975. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 72:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumita, Y., and S. Mitsuhashi. 1991. In vitro synergistic activity between meropenem and other beta-lactams against methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 10:77-84. [DOI] [PubMed] [Google Scholar]
- 31.Sumita, Y., M. Fukasawa, S. Mitsuhashi, and M. Inoue. 1995. Binding affinities of β-lactam antibiotics for penicillin-binding protein 2′ in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 35:473-481. [DOI] [PubMed] [Google Scholar]
- 32.Talbot, G. H., D. Thye, A. Das, and Y. Ge. 2007. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:3612-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson, K. S., and E. S. Moland. 2004. CS-023 (R-115685), a novel carbapenem with enhanced in vitro activity against oxacillin-resistant staphylococci and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 54:557-562. [DOI] [PubMed] [Google Scholar]
- 34.Trouillet, J. L., J. Chastre, A. Vuagnat, M. L. Joly-Guillou, D. Combaux, M. C. Dombret, and C. Gibert. 1998. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 157:531-539. [DOI] [PubMed] [Google Scholar]
- 35.Ueda, Y., and M. Sunagawa. 2003. In vitro and in vivo activities of novel 2-(thiazol-2-ylthio)-1β-methylcarbapenems with potent activities against multiresistant gram-positive bacteria. Antimicrob. Agents Chemother. 47:2471-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda, Y., K. Kanazawa, K. Eguchi, K. Takemoto, Y. Eriguchi, and M. Sunagawa. 2005. In vitro and in vivo antibacterial activities of SM-216601, a new broad-spectrum parenteral carbapenem. Antimicrob. Agents Chemother. 49:4185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]