Abstract
We compared Etest with broth microdilution testing for isavuconazole activity against 92 Cryptococcus isolates. A 97.8% agreement was found between these methods, without major discrepancies (>2-well dilution difference). Our findings support the use of the Etest methodology as a reliable method for the determination of MICs against Cryptococcus spp.
The Clinical and Laboratory Standards Institute (CLSI), formerly the National Committee for Clinical Laboratory Standards (NCCLS), has developed and published guidelines for broth microdilution (BMD) antifungal susceptibility testing against yeast (6). These guidelines serve as a reference standard to facilitate interlaboratory consistency for antifungal susceptibility testing. Because this method is labor-intensive, other methods, such as Etest (AB Biodisk, Solna, Sweden), which employs an antimicrobial gradient on an agar surface, have been developed to assist in determination of MICs.
Isavuconazole (formerly BAL4815) is a new triazole with proven in vitro activity against Aspergillus, Fusarium, Scedosporium, and Candida spp.; the zygomycetes; and Cryptococcus neoformans (3, 4, 9, 11). In vivo efficacy in a murine model of invasive aspergillosis caused by Aspergillus flavus has also been demonstrated (11). Oral and intravenous formulations have been developed, providing advantages over similar triazoles, such as posaconazole, which is currently available only in oral solution, and voriconazole, which has little activity against the zygomycetes (7). The intravenous formulation of isavuconazole does not require the addition of cyclodextrin to achieve solubility, as is required for itraconazole and voriconazole, thereby eliminating concerns regarding nephrotoxicity due to the cyclodextrin component of intravenous solutions (8). These factors make isavuconazole a potentially attractive choice during the care of invasive mycoses.
The isavuconazole Etest has previously been evaluated only for Aspergillus and Candida spp. (3). Concordance rates of 96% and 93% were found between the Etest and BMD methods for these species. However, the isavuconazole Etest method has yet to be evaluated for use with Cryptococcus spp. In this study, we evaluated the performance of isavuconazole by comparison of Etest to BMD in accordance with CLSI methodology.
Ninety-two nonduplicate clinical and environmental Cryptococcus isolates (courtesy of B. L. Wickes) were tested (57 C. neoformans and 35 C. gattii isolates), including 67 clinical strains, 24 environmental strains, and 1 recombinant strain collected from the United States, Australia, France, Denmark, Italy, Thailand, and Zaire over the last 15 years. Prior to testing, isolates were maintained in sterile water or frozen in glycerol-supplemented broth. Each isolate was subcultured at least twice on Sabouraud dextrose agar (Remel, Inc., Lenexa, KS) prior to in vitro susceptibility testing. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality controls in accordance with NCCLS document M27-A2 (6).
Isavuconazole powder and isavuconazole Etest strips were supplied by Basilea Pharmaceutica Ltd. For BMD testing, an isavuconazole stock solution was prepared in dimethyl sulfoxide and further diluted in RPMI 1640 medium with l-glutamine and without bicarbonate, buffered to a pH of 7.0 with MOPS (morpholinepropanesulfonic acid). Aliquots (0.1 ml) of isavuconazole at 2× concentrations were dispensed into 96-well microdilution trays. BMDs were performed in accordance with the M27-A2 reference method. Inocula containing 0.5 × 103 to 2.5 × 103 cells/ml were prepared spectrophotometrically to the optical density equivalent of a 0.5 McFarland standard. The inocula were added, and the trays were incubated at 35°C. The final isavuconazole concentrations ranged from 0.015 to 8 μg/ml. The MICs were read as 50% reductions in turbidity compared to the levels for the drug-free control tube at 48 and 72 h. This method has been shown to have >95% intralaboratory reproducibility (2).
For the Etest MIC determination, 90-mm-diameter plates containing RPMI agar (1.5% [vol/vol] agarose) supplemented with 2% glucose (RPG medium) were used. The agar surface was inoculated with isolates prepared as described above, using a nontoxic swab dipped in the cell suspension. Isavuconazole Etest strips were then applied to each plate. The plates were incubated at 35°C and read at 48 and 72 h. Comparison of MICs at 48 and 72 h revealed infrequent changes, with all isolates' 72-h MICs within 2 dilutions of the recorded 48-h values. For 24 of the isolates, the 48-h MICs were 1 dilution lower than the corresponding 72-h MICs, while 7 isolates had 48-h MICs 2 dilutions lower than those recorded at 72 h; no isolates had higher MICs at 48 h than at 72 h. The Etest MICs were read as the points at which a prominent reduction of growth intersected the Etest strip (5). MICs obtained from Etest were raised to the next twofold level of dilution to account for the continuous gradient of concentrations provided by this modality of testing. Differences of more than 2 dilutions between results for the BMD and Etest methods were defined as discrepant.
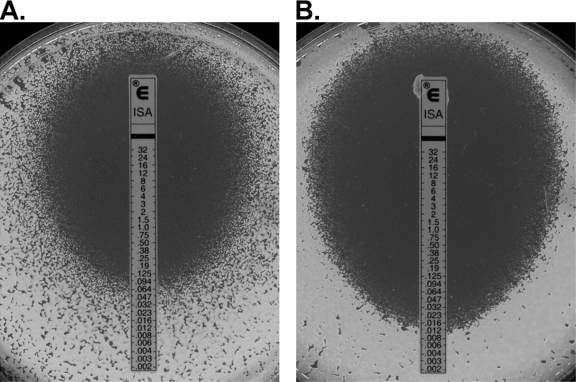
Isavuconazole MICs were consistent with previously reported results for C. neoformans (4). Table 1 summarizes the in vitro susceptibilities of 92 Cryptococcus isolates. Isavuconazole MICs did not exceed 1 μg/ml by either method, a finding consistent with previously reported susceptibility testing for extended spectrum triazoles in Cryptococcus isolates (4). Significant differences between MIC50 and MIC90 values were not observed upon comparison of C. neoformans and C. gattii isolates. Clear zones of inhibition were observed for each isolate at concentrations above the MIC with the Etest methodology (Fig. 1). The Etest and BMD results were within 2 dilutions for 90 of the 92 isolates (97.8%). The first discrepant isolate had an Etest MIC lower than its BMD MIC (0.004 μg/ml [Etest] versus 0.03 μg/ml [BMD]), a difference unlikely to affect clinical decision making or outcomes. The other discrepant isolate had an MIC reported by Etest higher than that reported by the BMD microdilution method (Etest MIC, 0.125 μg/ml, and BMD MIC, ≤0.015 μg/ml). Similarly, infrequent overestimation of MICs in Cryptococcus isolates by the Etest method have been reported for both fluconazole and voriconazole (1, 5, 10).
TABLE 1.
Antifungal activity of isavuconazole Etest and BMD against 92 isolates of C. neoformans and C. gattii
| Method | Organism(s) | GMa | MIC range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| BMD | Total | 0.025 | ≤0.015-0.5 | ≤0.015 | 0.06 |
| C. gattii | 0.027 | ≤0.015-0.25 | 0.03 | 0.06 | |
| C. neoformans | 0.024 | ≤0.015-0.5 | ≤0.015 | 0.06 | |
| Etest | Total | 0.025 | 0.002-0.064 | 0.023 | 0.064 |
| C. gattii | 0.030 | 0.003-0.064 | 0.32 | 0.125 | |
| C. neoformans | 0.022 | 0.002-0.5 | 0.023 | 0.047 |
GM, geometric mean.
FIG. 1.
Representative Etest figures demonstrating activity against C. neoformans isolates. Clear zones of inhibition were observed at isavuconazole concentrations above the MIC.
Our results illustrate the agreement of the Etest method with BMD for determining in vitro susceptibility of Cryptococcus isolates with the new triazole isavuconazole. Etest provides great assistance to the clinical laboratory for testing multiple antifungal agents concurrently in an efficient manner. The role of isavuconazole in the treatment of cryptococcosis remains to be defined; however, the in vitro efficacy determined by BMD and the Etest method suggest that isavuconazole may be a welcome addition to the growing antifungal armamentarium.
Acknowledgments
This study was funded by NIH contract no. NOI-A1-25475.
Isavuconazole was kindly provided by Basilea Pharmaceutica Ltd.
G.R.T., A.W.F, A.C.V., and B.L.W. report no conflicts of interest. N.P.W. has received research support from Pfizer and Schering-Plough. T.F.P. has received research support from Merck, Pfizer, Schering-Plough, and Nektar Therapeutics and has served on the speakers' bureau for Merck and Pfizer and as a consultant for Basilea, Merck, Nektar, Pfizer, and Toyama.
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Chen, S. C., M. L. O'Donnell, S. Gordon, and G. L. Gilbert. 1996. Antifungal susceptibility testing using the E test: comparison with the broth microdilution technique. J. Antimicrob. Chemother. 37:265-273. [DOI] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff, A., C. W. Kish, Jr., T. M. Kerkering, R. A. Fromtling, K. Bartizal, J. N. Galgiani, K. Villareal, M. A. Pfaller, T. Gerarden, M. G. Rinaldi, et al. 1992. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J. Clin. Microbiol. 30:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guinea, J., T. Pelaez, S. Recio, M. Torres-Narbona, and E. Bouza. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of Zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illnait-Zaragozi, M. T., G. F. Martinez, I. Curfs-Breuker, C. M. Fernandez, T. Boekhout, and J. F. Meis. 2008. In vitro activity of the new azole isavuconazole (BAL4815) compared with six other antifungal agents against 162 Cryptococcus neoformans isolates from Cuba. Antimicrob. Agents Chemother. 52:1580-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell, M. J., S. A. Messer, R. J. Hollis, D. J. Diekema, and M. A. Pfaller. 2003. Evaluation of Etest method for determining voriconazole and amphotericin B MICs for 162 clinical isolates of Cryptococcus neoformans. J. Clin. Microbiol. 41:97-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 7.Pasqualotto, A. C., and D. W. Denning. 2008. New and emerging treatments for fungal infections. J. Antimicrob. Chemother. 61(Suppl. 1):i19-i30. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt-Hoffmann, A., B. Roos, M. Heep, M. Schleimer, E. Weidekamm, T. Brown, M. Roehrle, and C. Beglinger. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert, H., U. Aurbach, D. Stefanik, and O. Cornely. 2007. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob. Agents Chemother. 51:1818-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simor, A. E., G. Goswell, L. Louie, M. Lee, and M. Louie. 1997. Antifungal susceptibility testing of yeast isolates from blood cultures by microbroth dilution and the E test. Eur. J. Clin. Microbiol. Infect. Dis. 16:693-697. [DOI] [PubMed] [Google Scholar]
- 11.Warn, P. A., A. Sharp, and D. W. Denning. 2006. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J. Antimicrob. Chemother. 57:135-138. [DOI] [PubMed] [Google Scholar]