Abstract
Peripheral blood mononuclear cells (PBMCs) from healthy individuals can be infected by human T-lymphotropic virus type 1 (HTLV-1) upon cocultivation of the PBMCs with irradiated HTLV-1-transformed human MT-2 cells. This model system closely mimics HTLV-1 transmission through cell-to-cell contact. Carbohydrate-binding agents (CBAs) such as the α(1,3)/α(1,6)mannose-specific Hippeastrum hybrid agglutinin and the GlcNAc-specific Urtica dioica agglutinin, and also the small, nonpeptidic α(1,2)-mannose-specific antibiotic pradimicin A, were able to efficiently prevent cell-to-cell HTLV-1 transmission at nontoxic concentrations, as evidenced by the lack of appearance of virus-specific mRNA and of the viral protein Tax in the acceptor cells. Consistently, antivirally active doses of CBAs fully prevented HTLV-1-induced stimulation of PBMC growth. The inhibitory effects of CBAs on HTLV-1 transmission were also evident when HTLV-1-infected C5MJ cells were used in place of MT-2 cells as a virus donor cell line. The anti-HTLV-1 properties of the CBAs highlight the importance of the envelope glycans in events underlying HTLV-1 passage from cell to cell and indicate that CBAs should be further investigated for their potential to prevent HTLV-1 infection, including mother-to-child virus transmission by cell-to-cell contact through breast milk feeding.
Human T-lymphotropic virus type 1 (HTLV-1) is a member of the genus Deltaretrovirus of the family Retroviridae and is associated with various pathologies (35). The most common characterized pathologies are oncological transformation (i.e., adult T-cell leukemia [ATL] and cutaneous T-cell lymphoma) and neurological abnormalities (i.e., tropic spastic paraparesis). The virus is spread in areas of endemicity in Japan, Central America, and South America. Its worldwide spread encompasses around 20 million infected individuals (12, 13, 26). The main routes of HTLV-1 infection in vivo consist of mother-to-infant transmission (mainly through breast-feeding), parenteral infection, and viral transmission through sexual intercourse (20, 26, 27). HTLV-1 infection occurs predominantly by infection of CD4+ T lymphocytes, followed by random integration of the provirus into the cellular genome (11). HTLV-1 infection in vivo results in a lifelong infection that is characterized by low, if any, viremia. Therefore, in the absence of a sustained extracellular phase during the replication cycle of HTLV-1, survival and propagation of the virus within the host seem to be mainly ensured by two modalities: (i) the reverse transcriptase (RT)-independent mitotic pathway and (ii) cell-to-cell contacts allowing the virus to migrate from infected to uninfected cells through an RT-dependent route. However, the mechanisms involved in the entry of HTLV-1 into the cell have not been fully elucidated. HTLV-1 transmission benefits from the formation of cellular conjugates through the generation of biological synapses and involvement of the cytoskeleton (17). Although the glucose transporter GLUT-1 has been identified as a cellular receptor for HTLV-1 (22), other studies indicate that the interaction with this receptor is not sufficient by itself for viral entry into the cell (18, 29). The structural component of HTLV-1 presumably required for receptor recognition is composed of the envelope glycoprotein (gp46) and a noncovalently associated transmembrane anchor protein (gp21), derived from a common gp63 precursor (21). gp46 is among the smallest retroviral envelope proteins known, and it exhibits little sequence variability (19). Studies have revealed that all four potential N-glycosylation sites (28) in gp46 are used and that the glycans are mannose rich and/or hybrid in composition (1).
Therapeutic treatments for HTLV-1-related pathologies are still limited and await further investigation (30). The classical chemotherapeutic approach has failed in HTLV-1-related disorders (32). Antiretroviral therapy in tropic spastic paraparesis patients has so far proven to be poorly effective or not effective at all (31), while combination therapy with antiretrovirals and immunomodulants in ATL patients has provided moderate activity (15). Recently, carbohydrate-binding agents (CBAs) have been proposed as potential new anti-human immunodeficiency virus (HIV) microbicide candidate drugs, since they were able to inhibit not only cell-free virus infection of T lymphocytes and macrophages, but also virus transmission from persistently HIV type 1 (HIV-1)-infected to uninfected target cells, virus capture by DC-SIGN-expressing cells, and subsequent virus transmission to uninfected T lymphocytes (4, 9, 14, 34). The CBAs are a heterogeneous group of agents that likely act by inhibiting HIV entry through binding to glycan residues of the HIV gp120 envelope (4). Although the effects of CBAs in HTLV-1 infection have not been truly investigated, it has recently been reported that the β-galactoside-binding mammalian lectin galectine was able to affect proliferation of ATL cells in vitro by inhibiting antiapoptotic gene expression through suppression of NF-κB activation (24). However, there is no evidence yet of a prominent inhibitory role played by lectins in inhibiting HTLV-1 transmission in vitro. Given the properties of CBAs to inhibit virus transmission from HIV-infected cells to uninfected lymphocytes, they could be good candidates for investigation in HTLV-1 infection, where cell-free virus release is limited and cell-to-cell contact is the predominant pathway of infection. Indeed, a number of in vitro studies have shown that target cells for HTLV-1 infection, mimicking the in vivo situation, are highly susceptible to virus transmission through cocultivation with virus-infected donor cell lines (3). Therefore, it can be hypothesized that drugs that interact with envelope surface glycans could interfere with HTLV-1 entry in vitro, blocking HTLV-1 transmission at a very early step in the transmission process.
In this study, the effects of the α(1,3)/α(1,6)-mannose-specific Hippeastrum hybrid agglutinin (HHA) and the GlcNAc-specific Urtica dioica agglutinin (UDA) plant lectins, and also the small α(1,2)-mannose-specific nonpeptidic antibiotic pradimicin A (PRM-A), on HTLV-1 infection were investigated upon cocultivation of mononuclear cells from healthy donors with two chronically HTLV-1-infected cell lines. The results show that CBAs efficiently inhibit infection, transmission, and immortalization of cells exposed to HTLV-1 at concentrations devoid of cytotoxic activity. These findings highlight the pivotal role of glycans as chemotherapeutic targets for inhibition of HTLV-1 cell-to-cell transmission.
MATERIALS AND METHODS
Compounds.
The CBAs HHA, UDA, and PRM-A were isolated and purified (23, 25, 33) and kindly provided by E. Van Damme and W. Peumans (University of Ghent, Ghent, Belgium) and T. Oki and Y. Igarashi (Toyama University, Toyama, Japan).
HTLV-1 infection, treatment, and cell growth evaluation.
Peripheral blood mononuclear cells (PBMCs) were harvested from healthy adult donors who were seronegative for HIV and hepatitis B/C viruses. Mononuclear cells were separated by Ficoll-Hypaque density gradient (Cederlane, Hornby, Ontario, Canada). The cells were then washed twice in RPMI 1640 medium (Gibco-Invitrogen Co., Paisley, Scotland, United Kingdom). HTLV-1 infection was performed by coculturing PBMCs with lethally irradiated (120 Ci from a cesium gamma cell irradiator 1000; Canada Atomic Energy Ltd., Canada) MT-2 (irrMT-2) or C5MJ (irrC5MJ) cells at a ratio of five PBMCs to one virus donor cell. Both MT-2 and C5MJ are chronically HTLV-1-infected cell lines derived from cord blood mononuclear cells exposed to HTLV-1 from leukemic patients. PBMC/irrMT-2 or PBMC/irrC5MJ cocultures were maintained at 0.8 × 106 cells/ml in RPMI 1640 medium (Gibco) supplemented with 12% fetal bovine serum, glutamine, penicillin-streptomycin (Gibco-Invitrogen) (hereafter referred as complete medium), and 20 U/ml of recombinant interleukin 2 (IL-2) (Proleukin; Chiron Co., Holland). The CBAs were investigated at 1 and 10 μg/ml (1.2 and 12 μM) for PRM-A, 1 and 10 μg/ml (0.02 and 0.2 μM) for HHA, and 1 and 10 μg/ml (0.11 and 1.1 μM) for UDA. The highest concentrations (10 μg/ml) for each compound were able to markedly inhibit HIV-1(IIIB) replication in CEM cell cultures. The activities of the compounds were examined following two different modalities of treatment: (i) pretreatment, i.e., compounds were added to PBMCs (PBMCpt) and/or to irrMT-2 (MT-2pt) or irrC5MJ (C5MJpt) cells about 16 h before cocultivation; (ii) cotreatment, i.e., compounds were added to PBMCs at the time of cocultivation, immediately before their exposure to irrMT-2 (PBMC/MT-2cot) or to irrC5MJ (PBMC/C5MJcot). Successively, the compounds were readded at half of the initial concentration 3, 7, and 10 days postinfection. To test the effects of the compounds in the medium-term period on the growth of uninfected PBMCs, the same culture conditions were used for normal PBMCs alone. PBMC cultures, infected or uninfected, treated either with different compounds or with control medium, were split weekly, and cell growth was monitored weekly by evaluating living cells using the trypan blue dye exclusion assay. The number of living cells was calculated as the mean of two independent evaluations. After the cells were counted, the cell concentration was readjusted to 0.8 × 106/ml. The results of cell growth were expressed as a total cell number (TCN) calculated from living-cell counts as follows: for the first week, the TCN was the actual number of cells, expressed as millions of viable cells, detected before the first adjustment, while in the succeeding weeks, the TCN was calculated theoretically as the TCN in the previous week multiplied by the cell concentration, expressed as millions of viable cells per milliliter, detected before weekly adjustment. IrrMT-2 and irrC5MJ cells, utilized as HTLV-1 donor cells for infection, were kept separately in culture for the duration of the experiments and showed no evidence of growth.
Extraction of RNA and RT-PCR analysis.
RNA isolation was performed using a NucleoSpin RNA kit, and to remove possible DNA contamination, RNA was treated with RNase-free DNase according to the manufacturer's instructions (Machenery-Nagel, Dueren, Germany). The quantity and the quality of all RNA preparations were assessed by gel electrophoresis and optical density at 260 nm/optical density at 280 nm ratios.
Total RNA from infected and uninfected cells was reverse transcribed into cDNA in a 25-μl reaction mixture containing 0.5 μg of RNA, 1× RT buffer, 1 mM deoxynucleoside triphosphates (Promega, Madison, WI), 1.5 μg oligo(dT) (New England Biolabs, Beverly, MA), 50 U recombinant RNase inhibitor (Promega), 10 mM dithiothreitol (Sigma, St. Louis, MO), 25 U Moloney murine leukemia virus RT (New England Biolabs) for 1 h at 37°C. The reaction mixture was incubated at 95°C for 5 min in order to inactivate the RT and then chilled on ice. Two hundred nanograms of cDNA was amplified by PCR in a total volume of 50 μl. Amplifications with 0.5 μM of primer pairs specific for the Tax/Rex region of HTLV-1 (RPX3 and RPX4) (2) or, as an internal control, with primers specific for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward primer, 5′ TGGTATCGTGAAAGGACT 3′; reverse primer, 5′ ATGCAAGTGAGCTTCCCGTTC 3′). Samples were subjected to 30 cycles of PCR amplification, each cycle consisting of 30 s at 94°C, 30 s at 55°C, and 45 s at 72°C on a Eppendorf MasterCycler personal (Eppendorf, Hamburg, German). Following the final cycle, samples were incubated at 72°C for 7 min to ensure completion of the final extension step. Amplified DNA (fragments of 149 bp for Tax/Rex and 119 bp for GAPDH) was visualized on a 1.5% agarose gel containing 10 μg/ml ethidium bromide in 1× Tris-acetate-EDTA buffer. The antiviral effects of the compounds were expressed as the minimum concentration at which 100% of viral RNA detection was inhibited (RNAC100). The limit of detection of our assay was 0.02 pg of total RNA initially extracted from PBMCs efficiently infected by coculture with irrMT-2 cells at 4 weeks following infection (2). This limit of detection corresponds to RNA extracted from 0.005 infected cell.
Real-time PCR for quantitative evaluation of viral tax RNA and of tax mRNA expression.
Real-time quantitative PCR (RQ-PCR) was performed on an ABI Prism 7300 instrument (PE Applied Biosystems, Foster City, CA) with gene-specific primers and the Sybr green chemistry. The primers for evaluation of viral tax RNA were designed with Primer Express Software (AY563953; forward primer, 5′-GGATACCCAGTCTACGTGTTTGG-3′, and reverse primer, 5′-CCGAACATAGTCCCCCAGAGA-3′). For quantitative analysis of tax mRNA, primers RPXPR1 and RPX4 were used (2). All primers were purchased from Invitrogen (Carlsbad, CA). A 0.5-μg amount of total RNA from each sample was reverse transcribed in a total volume of 20 μl with high-capacity cDNA reverse transcription kits (Applied Biosystems) according to the manufacturer's instructions. Amplification of specific PCR products was detected using the Brilliant II Sybr green QPCR Master Mix (Stratagene, La Jolla, CA). The RQ-PCR was performed in triplicate in a total reaction volume of 25 μl containing 1× Sybr green QPCR Master Mix, 150 nM forward and reverse primers, and 200 ng cDNA as a template. Samples were heated for 10 min at 95°C and were subjected to 40 cycles of PCR amplification, each cycle consisting of 15 s at 95°C and 60 s at 60°C. Within each experiment, no-template control and the glucuronidase beta (GUSB) housekeeping gene (NM_000181; forward primer, 5′-CAGTTCCCTCCAGCTTCAATG-3′, and reverse primer, 5′-ACCCAGCCGACAAAATGC-3′), used as a reference, were run in parallel to verify any contamination and to determine the amplification efficiency. Each run was completed with a melting-curve analysis to confirm the specificity of amplification and lack of nonspecific products and primer dimers. Quantification was performed using the threshold cycle (CT) comparative method. Relative gene mRNA levels were calculated as follows: 2−[ΔCt(sample) − ΔCt(calibrator)] = 2−ΔΔCt, where ΔCT(sample) = [CT(tax gene) − CT(GUSB)] represents the difference, in the CT number, between the genes and GUSB.
Western blot analysis for evaluation of HTLV-1 Tax.
Detection of the Tax protein was performed by Western blotting. Briefly, cells from treated or control samples were subjected to centrifugation on a density gradient to eliminate debris and dead cells. Two millions cells were solubilized at 4°C in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 7.4, 1% Triton-X, 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP-40, all from Sigma) containing 0.25 mM phenylmethylsulfonyl fluoride and complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The lysates were then centrifuged for 20 min at 10,000 × g. Aliquots of the supernatants were saved for determination of the protein concentration by Bradford assay (Bio-Rad, Hercules, CA), and the remaining fluid was boiled in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol). A 30-μg amount of proteins for each sample was loaded onto a 10% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred to a nitrocellulose membrane (Bio-Rad), which was subsequently stained with 0.2% Ponceau red to ensure equal protein loading and transfer. After the membrane was blocked in 10% nonfat dry milk and 3% bovine serum albumin in 20 mM Tris-HCl, pH 8.0, 0.9% NaCl, 0.03% Tween 20 (all from Sigma), the blots were incubated overnight at 4°C with a 1:500 dilution of anti-Tax monoclonal antibody (a generous gift from John Brady, National Cancer Institute, NIH, Bethesda, MD) and with a 1:1,000 dilution of anti-β-tubulin monoclonal antibody (Santa Cruz, Santa Cruz, CA). Subsequently, the blots were washed and then incubated with goat anti-mouse immunoglobulin G (H+L) conjugated to peroxidase (Bio-Rad). Binding of antibodies was detected by chemoluminescence staining using the ECL detection kit (Amersham Bioscience, Little Chalfont, United Kingdom).
Cytotoxic assays.
To determine the cytotoxic potentials of the CBAs, two assays were performed. In the first assay, proliferation of PBMCs from healthy donors was set up in complete medium following allostimulation by exposure to 2 × 105 irrMT-2 cells in the presence of IL-2 (20 U/ml). The compounds to be assayed were added to the PBMCs 16 h before exposure to the irrMT-2 cells at concentrations of 0.12, 1.2, 12, and 120 μM PRM-A; 0.002, 0.02, 0.2, and 2 μM HHA; and 0.011, 0.11, 1.1, and 11 μM UDA. The compounds were added again at half of the original concentrations 3 days postinfection. After 1 week of cultivation, [3H]thymidine (Amersham Bioscience, Little Chalfont, United Kingdom) was added at 1 μCi/well. The cultures were harvested after a further 16 h of incubation at 37°C, and incorporated radioactivity was measured. In the second assay, the antimetabolic activities of the CBAs were examined by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] colorimetric method, using a commercial kit (MTS Cell Titer 96 Aqueous One Solution; Promega). The assay was performed by seeding 1 × 104 lymphoid MOLT-3 cells in 100 μl in the presence or absence of CBAs at four different concentrations—0.12, 1.2, 12, and 120 μM PRM-A; 0.002, 0.02, 0.2, and 2 μM HHA; and 0.011, 0.11, 1.1, and 11 μM UDA—in RPMI 1640 medium supplemented with 5% fetal bovine serum, 2 mM l-glutamine, and penicillin-streptomycin for 24 h. Twenty microliters of CellTiter 96 Aqueous One Solution Reagent was added directly to the culture wells at the end of the culture, and the mixture was incubated for 1 to 4 h, after which absorbance was read at 490 nm.
Calculation of the antivirally effective concentrations.
Results from three different determinations were used to calculate the following: (i) the RNAC100, (ii) the compound concentration required to inhibit proliferation of PBMCs stimulated by exposure to MT-2 by 50% after 1 week of culture (50% proliferation cytotoxic concentration), and (iii) the compound concentration required to inhibit formazan formation (MTS assay) in MOLT-3 cells by 50% (the 50% metabolic activity cytotoxic concentration [maCC50]). The RNAC100 was the compound concentration at which no viral RNA was detected in infected PBMCs by nonquantitative RT-PCR, as revealed by values of ≤0 following subtraction of the corresponding uninfected controls through densitometry analysis of the appropriate bands. The effective 50% concentration values were calculated according to the best-fit curve, y value versus log x, where y is the value of the examined function and x is the drug concentration. The selectivity index was calculated on the basis of the ratio of maCC50 over RNAC100 for each tested compound.
Statistical analysis.
Each experiment was performed in duplicate or triplicate, and all data are presented as mean ± standard deviation or mean ± standard error, where appropriate. Data analysis was performed using the SPSS statistical software system (version 12.0 for Windows). Comparison of means of viral tax RNA between treated and control samples was carried out using a Bonferroni posthoc multiple-comparison analysis of variance test. Differences were considered significant at a P value of <0.05 and highly significant at a P value of <0.001.
RESULTS
Protective effects of HHA, UDA, and PRM-A against cell-to-cell transmission of HTLV-1 in vitro.
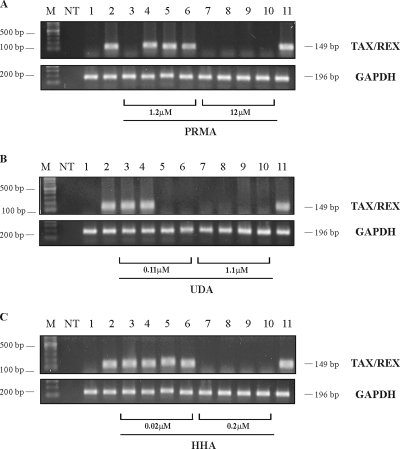
Fresh PBMCs from healthy donors were exposed to HTLV-1 infection by cocultivation with chronically HTLV-1-infected lethally irradiated MT-2 cells in the presence of 20 U/ml of IL-2 and a variety of CBA concentrations under different experimental conditions. The presence of viral (tax/rex) mRNA in the acceptor cells was recorded 4 weeks after cocultivation. The results depicted in Fig. 1 represent cocultures treated with PRM-A (Fig. 1A), UDA (Fig. 1B), and HHA (Fig. 1C). Acceptor PBMCs (lanes 3 and 7), donor irrMT-2 cells (lanes 4 and 8), or both acceptor and donor cells (lanes 5 and 9) were pretreated with CBAs overnight at 1.2 and 12 μM PRM-A, 0.11 and 1.1 μM UDA, or 0.02 and 0.2 μM HHA, respectively, before cocultivation. Alternatively, the CBAs were added at the same concentrations indicated above at the initiation of the cocultures (lanes 6 and 10). The results shown in Fig. 1 represent one representative experiment out of three independently performed assays using PBMCs from three different donors. The untreated, uninfected cell controls were negative for viral (tax/rex) mRNA (lane 1), while the infected, untreated cell controls showed an abundant presence of tax/rex mRNA after 4 weeks of culture (lane 2). PRM-A at a concentration of 1.2 μM was inhibitory only under the experimental conditions in which acceptor PBMCs had been pretreated with the CBAs (lane 3), while it was not protective under the other conditions. However, the higher PRM-A concentration of 12 μM efficiently inhibited viral mRNA appearance under each treatment condition (Fig. 1A, lanes 7 to 10). UDA at the lowest concentration of 0.11 μM was able to be fully inhibitory in the case of pretreatment of both PBMCs and irrMT-2 cells (lane 5) and in the case of drug exposure to the cells at the time of initiation of the coculture (lane 6). In contrast, at 10-fold-higher UDA concentrations, the CBA was able to completely prevent viral mRNA appearance in the acceptor cells under all experimental conditions (Fig. 1B, lanes 7 to 10). Whereas HHA was unable to inhibit virus infection of the acceptor cells at 0.02 μM (Fig. 1C, lanes 3 to 6), it completely inhibited viral mRNA appearance in the acceptor cells at a concentration of 0.2 μM under all experimental conditions (Fig. 1C, lanes 7 to 10). The comparable expression of the GAPDH housekeeping gene (Fig. 1, lanes 1 to 10) indicated that equal amounts of material were analyzed for all experimental conditions. Thus, the three CBAs evaluated were able to prevent HTLV-1 provirus transmission and subsequent expression of mRNA from infected donor cells to acceptor PBMCs, and HHA was most, and PRM-A least, effective on a molar basis.
FIG. 1.
Effects of CBAs on HTLV-1 (TAX/REX) mRNA expression were evaluated 4 weeks after in vitro HTLV-1 infection of human PBMCs from healthy donors through exposure to HTLV-1-infected irrMT-2 cells. The lane numbers for panels A (PRM-A), B (UDA), and C (HHA) represent following experimental conditions. Lane 1, uninfected PBMCs; lane 2, untreated HTLV-1-infected human PBMCs; lane 3, PBMCs pretreated with 1.2 μM PRM-A before cocultivation with irrMT-2 cells; lane 4, irrMT-2 cells pretreated with 1.2 μM PRM-A before cocultivation with PBMCs; lane 5, PBMCs and MT-2 cells pretreated with 1.2 μM PRM-A before cocultivation; lane 6, PBMCs and MT-2 cells treated with 1.2 μM PRM-A at the initiation of the cocultures; lanes 7 to 10, conditions identical to lanes 3 to 6 but treated with 10-fold-higher CBA concentrations (12 μM PRM-A); lane 11, chronically HTLV-1-infected MT-2 cells. The control lanes in panels B and C are in the same order, and in panel B, lanes 3 to 6 and 7 to 11 represent 0.11 and 1.1 μM UDA, while in panel C, lanes 3 to 6 and 7 to 11 represent 0.02 and 0.2 μM HHA. The mRNA expression of the GAPDH housekeeping gene is shown for each assayed compound. Lane M, 1-kb (1.5 to 0.1 kb) molecular ruler (Bio-Rad). NT, no template.
Evaluation of the protective effect of UDA through real-time quantitative PCR.
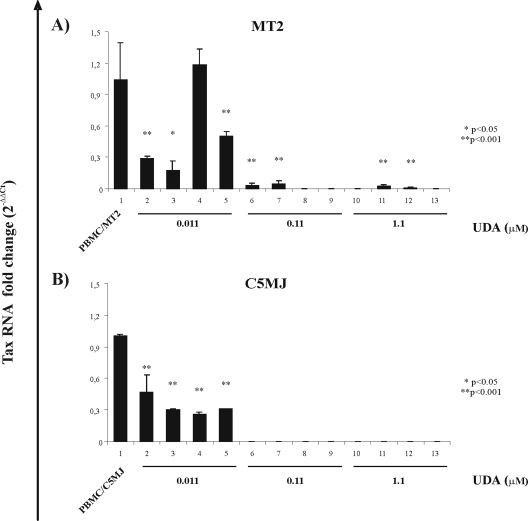
In order to more quantitatively assess the protective effects of CBAs on HTLV-1 transmission, a protection assay based on real-time PCR was utilized. Moreover, to verify whether the anti-HTLV-1 activities of CBAs were also exerted against a source of virus transmission other than MT-2 cells, in parallel experiments, C5MJ cells were also utilized as HTLV-1 donor cells. For these purposes, fresh PBMCs exposed to lethally irradiated MT-2 and/or irradiated C5MJ cells in the presence of 20 U/ml IL-2 were treated with the presumably most active CBA compound, i.e., UDA, at concentrations of 0.01, 0.1, and 1.1 μM under different experimental conditions and analyzed by real-time PCR after 4 weeks of culture. Figure 2 shows the mean values ± standard errors of viral tax RNA change with respect to untreated infected PBMCs from one experiment performed in triplicate using MT-2 (Fig. 2A) and C5MJ (Fig. 2B) as HTLV-1 donor cell lines. Note that, due to the primer set utilized in this assay, only unspliced tax RNA was detected. The highest UDA concentration of 1.1 μM fully protected PBMCs from HTLV-1 infection under all experimental conditions in the case of virus transmission by MT-2 cells, as well as by another HTLV-1-infected cell line, C5MJ. Actually, in the case of MT-2 cells, only after pretreatment with 1.1 μM UDA (Fig. 2A, bar 11) was a very small amount of tax-specific amplified product detected. Also in this case, however, the decrease in the tax RNA level was highly significant in comparison with values obtained in the untreated, infected control. In addition, at a 10-times-lower concentration, UDA highly significantly, if not completely, inhibited the level of tax RNA in pretreated PBMCs (bar 6) exposed to irrMT-2 cells (Fig. 2A) and in the case of pretreatment of MT-2 cells (bar 7) but, conversely, still fully protected PBMCs exposed to C5MJ cells (Fig. 2B) under all experimental conditions. The lowest UDA concentration assayed by real-time PCR, i.e., 0.011 μM, decreased by 70- to 80-fold the HTLV-1 infection of PBMCs when they were pretreated with the CBA (Fig. 2A, bar 2) or when they were exposed to CBA-pretreated MT-2 cells (Fig. 2A, bar 3). The lowest dose of UDA also still highly significantly protected, by 50-fold, PBMCs in the case of cotreatment (bar 5) while, conversely, it did not exert any protective effect in the case of separate pretreatment of PBMCs or MT-2 cells (bar 4). On the other hand, 0.011 μM UDA highly significantly decreased tax RNA, by 50-fold, in PBMCs exposed to C5MJ cells (Fig. 2B) in the case of PBMC pretreatment (bar 2) or by 60-fold both in the case of pretreatment of C5MJ cells and when PBMCs and C5MJ cells were singly pre- or cotreated (bars 3, 4, and 5, respectively) in comparison with the untreated, infected control. Thus, RQ-PCR analysis completely confirmed the qualitative data obtained by nonquantitative RT-PCR (Fig. 1) in the case of PBMC infection by cocultivation with MT-2 donor cell lines. Moreover, interestingly, RQ-PCR also revealed partial protection by the lowest UDA concentration, under most experimental conditions with MT-2 cells, which was not detectable by nonquantitative RNA PCR and under all the experimental conditions using irrC5MJ cells.
FIG. 2.
Effects of UDA on HTLV-1 tax RNA expression evaluated in real-time PCR 4 weeks after in vitro PBMC exposure to irrMT-2 cells (A) or irrC5MJ cells (B). (A) Bar 1, PBMC/MT-2, infected, untreated control; bar 2, PBMCpt, PBMCs pretreated with 0.011 UDA; bar 3, MT-2pt, MT-2 pretreated with 0.011 UDA; bar 4, PBMCpt/MT-2pt, both PBMCs and MT-2 cell lines singly pretreated with 0.011 UDA; bar 5, PBMC/MT-cot, UDA at concentrations indicated above was added at the time of cocultivation and exposed to irrMT-2 cells. For bars 6, 7, 8, and 9, conditions were as indicated above but in the presence of 0.11 μM UDA. For bars 10, 11, 12, and 13, conditions were as above but in the presence of 1.1 μM UDA. The error bars indicate standard errors. (B) For C5MJ cells, the same culture conditions were applied: bar 1, PBMC/C5MJ, infected untreated control; bar 2, PBMCpt, PBMCs pretreated with 0.011 UDA; bar 3, C5MJpt, C5MJ pretreated with 0.011 UDA; bar 4, PBMCpt/C5MJpt, both PBMCs and C5MJ cell lines singly pretreated with 0.011 UDA; bar 5, PBMC/C5MJcot, UDA at concentrations as indicated above was added at the time of cocultivation and exposed to irradiated C5MJ cells. For bars 6, 7, 8, and 9, conditions were as indicated above but in the presence of 0.11 μM UDA. For bars 10, 11, 12, and 13, conditions were as above but in the presence of 1.1 μM UDA. *, P value significant, P < 0.05; **, P value highly significant, P < 0.001.
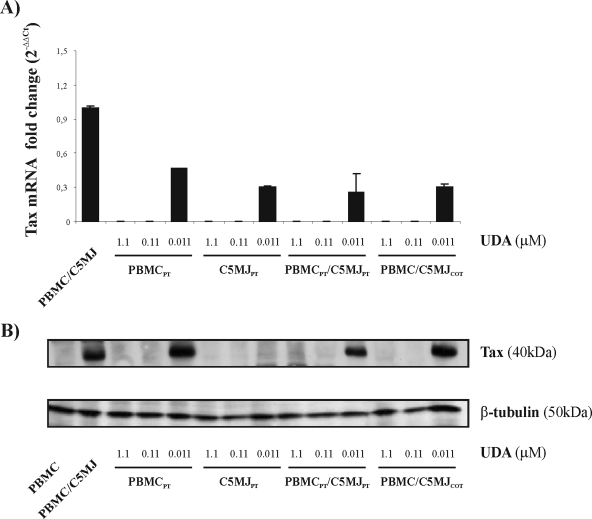
Inhibition of tax mRNA and Tax protein expression in HTLV-1-infected cultures treated with UDA.
Having observed the anti-HTLV-1 activities of CBAs at total viral-RNA level, we also wanted to confirm the effects of these compounds by investigating in further detail the abilities of CBAs to specifically interfere with both viral tax gene and viral Tax protein expression. To this end, we analyzed the expression of HTLV-1 spliced tax mRNA and Tax protein in PBMCs exposed to C5MJ cells, either treated with UDA or untreated. Fresh PBMCs were exposed to irradiated C5MJ cells in the presence of IL-2 and 0.011, 0.11, and 1.1 μM UDA under different experimental conditions, and after 4 weeks, tax mRNA and Tax protein were assessed by RQ-PCR and immunoblot analysis, respectively. In order to evaluate the effect of UDA on tax mRNA by RQ-PCR, we used a primer specific for the splicing site of the tax/rex region. The results in Fig. 3A show the changes in respect to the untreated, infected control obtained in one experiment performed in triplicate. Concentrations of 1.1 and 0.11 μM UDA completely inhibited tax mRNA in PBMCs exposed to irrC5MJ cells under the different culture conditions, while the lowest concentration of 0.011 μM UDA decreased tax mRNA expression by 50 to 70%. Immunoblot analysis, shown in Fig. 3B, indicated that both 1.1 and 0.11 μM UDA homogeneously inhibited Tax expression under the different culture conditions used. The lowest concentration of 0.011 μM UDA did not inhibit Tax expression under the experimental conditions in which PBMCpt, PBMCpt/C5MJpt, and PBMC/C5MJcot were used, while it completely suppressed Tax in the case of PBMCs exposed to C5MJpt. These data are in full agreement with results obtained by the RQ-PCR assay, in which UDA only partially protected PBMCs from infection at the lowest dose, except for the experimental conditions reported above, in which, although tax mRNA was detected by RQ-PCR, no Tax expression was detected by immunoblot analysis. However, this could be easily explained by higher sensitivity for the former assay or by a possible posttranslation mechanism of Tax inhibition. Equal expression of the housekeeping β-tubulin gene ensured that the same amount of sample was analyzed for each condition in immunoblot analysis.
FIG. 3.
Effects of UDA on tax mRNA and protein expression evaluated through immunoblot analysis after 4 weeks of culture. PBMCs were exposed to irrC5MJ cells under different experimental conditions (in the presence of UDA at 1.1, 0.11, and 0.011 μM). (A) Tax mRNA was expressed as change under different culture conditions in respect to the untreated, infected control. The error bars indicate standard deviations. (B) Whole-cell extracts were prepared, and 30 μg protein for each sample was loaded for analysis. Immunoblotting of β-tubulin indicated that equal amounts of protein were applied in all lanes.
Effects of HHA, UDA, and PRM-A on the growth of HTLV-1-infected cells.
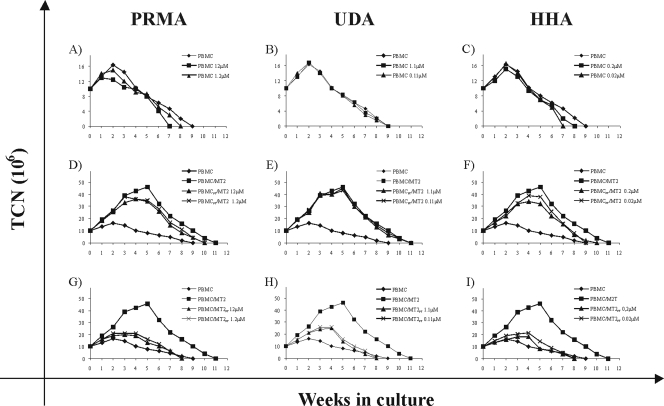
In addition to their direct antiviral activities, the effects of PRM-A, UDA, and HHA on the growth of HTLV-1-exposed PBMCs was investigated. PBMCs, cultured in the presence of IL-2 under different experimental conditions, were assayed weekly for cell viability by trypan blue dye exclusion. In Fig. 4, the results obtained during the first 5 weeks of cultivation are shown for one out of three representative experiments. Untreated PBMCs showed their highest cell numbers at week 2 postcultivation. From then on, the viability of the cell cultures gradually decreased. Addition of PRM-A, UDA, and HHA did not affect the numbers of viable uninfected PBMCs during the whole observation period (Fig. 4A to C). When PBMCs were exposed to, and cocultured with, HTLV-1-infected irrMT-2 cells in the absence of CBAs, cell growth was markedly stimulated (Fig. 4D to I, PBMCs/irrMT-2), reaching a peak by week 5. After this initial burst of cell growth, the cell numbers gradually decreased again (data not shown). As shown in Fig. 4D to F, pretreatment of PBMCs with PRM-A, UDA, and HHA before cocultivation with irrMT-2 cells did not influence the growth slopes of the cell cultures. In contrast, under all experimental conditions in which HTLV-1-infected irrMT-2 cells had been pretreated with PRM-A, UDA, and HHA before cocultivation with PBMCs, the cocultures resulted in a slight but limited stimulation of cell growth (Fig. 4G to I), closely resembling the growth pattern of the uninfected PBMCs. These observations disclose a poor ability of PBMC allostimulation by HTLV-1-infected MT-2 cells after being treated with CBAs. The growth curve under the culturing conditions in which both PBMCs and irrMT-2 cells were pretreated before cocultivation was similar to that of cocultures in which only irrMT-2 cell cultures were pretreated (data not shown). When the treatment with CBAs was initiated at the onset of the cocultures, virus-exposed cultures showed a growth curve that was in between the curves obtained under the above-mentioned treatment conditions (data not shown).
FIG. 4.
Effects of PRM-A, UDA, and HHA on the growth of uninfected (PBMC) or HTLV-1-infected cell (co)cultures (PBMC/MT-2). The growth values on the y axis were expressed as TCN, calculated from living-cell counts. For the first week, TCN was the actual number of cells, expressed as millions of viable cells, detected before the first adjustment, while in the succeeding weeks, TCN was calculated theoretically as the TCN in the previous week multiplied by the cell concentration, expressed as millions of viable cells per milliliter, detected before weekly adjustment. (A to C) Growth curves of uninfected PBMCs kept in IL-2 in the presence of PRM-A at 12 and 1.2 μM, UDA at 1.1 and 0.11 μM, and HHA at 0.2 μM and 0.02 μM or in the absence of CBAs (PBMC). (D to F) Growth curves of PBMCs pretreated with PRM-A at 12 and 1.2 μM, UDA at 1.1 and 0.11 μM, and HHA at 0.2 and 0.02 μM and cocultivated with irrMT-2 cells in the presence of IL-2. (G to I) Growth curves of PBMCs cocultivated with HTLV-1-infected irrMT-2 cells kept in IL-2 and pretreated with PRM-A at 12 and 1.2 μM, UDA at 1.1 and 0.11 μM, and HHA at 0.2 and 0.02 μM. An experiment representative of three performed with different donors is shown.
Cytotoxic activities of the CBAs.
The cytotoxic activities of the CBAs were investigated under two different experimental conditions. In the first assay, PBMCs were exposed to different concentrations of the CBAs, after which they were stimulated to proliferate by exposure to irrMT-2 cells. PBMC proliferation was not inhibited at the highest CBA concentrations tested (Table 1). In the second assay, the CBAs were examined for their inhibition of the metabolic activity of exponentially growing T-lymphocyte MOLT-3 cells by an MTS assay. HHA showed an maCC50 of 1.8 μM, whereas PRM-A and UDA were not inhibitory at concentrations of 120 and 11 μM, respectively, as measured by the MTS dye method. Thus, the latter assay was shown to be slightly more sensitive than the former in measuring potential side effects of the compounds and was utilized for calculation of the selectivity index (SI). However, although the SI was presented, the duration of the antiviral assay in respect to the MTS assay was not the same. Since the antiviral activities of different CBAs in PBMCs, expressed as the RNAC100, were 12, 1.1, and 0.2 μM for PRM-A, UDA, and HHA, respectively, the SIs (the maCC50/RNAC100 ratios) we calculated to compare the potential anti-HTLV-1 efficacies of different CBAs were >10 for PRM-A and UDA and 9 for HHA. These results indicated that CBA concentrations endowed with full inhibitory activity against HTLV-1 transmission were significantly below the cytotoxic potentials of the test compounds.
TABLE 1.
Anti-HTLV-1 and cytotoxic activities of CBAs
| CBA | RNAC100a (μM) | pCC50b (μM) (PBMCs) | maCC50c (μM) (MOLT-3) |
|---|---|---|---|
| PRM-A | 12 | >120 | >120 |
| UDA | 1.1 | >11 | >11 |
| HHA | 0.2 | >2 | 1.8 (±0.1) |
Anti-HTLV-1 activity evaluated as the compound concentration at which no detectable tax/rex mRNA product was observed by RT-PCR at 4 weeks postinfection in cultures of PBMCs exposed to HTLV-1 by cocultivation with irrMT-2 cells and subsequently treated with the compounds under different experimental conditions.
Cytotoxic activity evaluated as the compound concentration required to cause proliferation inhibition by 50% in 1-week PBMC cultures. PBMCs from three donors were pretreated overnight with four different concentrations of the compounds, after which irrMT-2 cells were added as an allostimulus.
Cytotoxic activity evaluated as the compound concentration able to cause reduction in formazan product formation (MTS assay) by 50% in exponentially growing MOLT-3 cell cultures treated with four different concentrations of the compounds for 24 h. The data represent results derived from three different experiments.
DISCUSSION
The present study investigated for the first time the effects of CBAs on HTLV-1 infection through cell-to-cell transmission. Using the experimental model of HTLV-1 infection of PBMCs upon cocultivation with two different irradiated HTLV-1-transformed cell lines, we could demonstrate that CBAs can efficiently prevent virus transmission through cell-to-cell contact. The results obtained by real-time quantitative assays showed that UDA, utilized as a representative CBA compound, was able to efficiently inhibit both total tax RNA and spliced tax mRNA expression. In particular, while the higher concentrations of the compound tested completely inhibited tax RNA under all coculture conditions, independently for the virus donor cells, the potency of the lowest UDA concentration (0.011 μM) seemed to be influenced by the type of virus donor cells used. Actually, 0.011 μM UDA inhibited by 50-fold tax RNA expression under all the experimental conditions of coculture using C5MJ cells as donor cells. Conversely, when both PBMCs and irrMT-2 cells were pretreated with 0.011 μM UDA, tax RNA expression was not inhibited, while it was suppressed in the case of single pretreatment. Considering that both MT-2 and C5MJ produce viral particles equally, a possible hypothesis is that the difference in the inhibitory effect at the lowest UDA concentration used might depend on a qualitative or quantitative difference in the structural targets involved in virus cell-to-cell transmission on the surfaces of the two HTLV-1-infected cell lines.
The CBAs could interfere with several sites during HTLV-1 infection. They may block viral-particle entry into the acceptor cells through binding and cross-linking of glycans present on the virus envelope glycoproteins. It has been shown that the CBAs, such as HHA and PRM-A, strongly bind to gp120 of HIV-1 (7). They do not block binding of HIV to its CD4 receptor but prevent (one of) the subsequent steps during gp120/gp41-directed virus fusion with the target cell membrane. The CBAs have previously been shown to prevent fusion and virus transmission between persistently HIV-1-infected cells and uninfected T lymphocytes (6-8), as well as DC-SIGN-directed capture of HIV-1 particles by DC-SIGN-expressing cells and subsequent virus transmission to T lymphocytes (9). As a result, CBAs have been suggested as potential microbicide candidate drugs against HIV (5). Given the glycosylated nature of the HTLV-1 gp46 and the mode of transmission through cell-to-cell contact, CBAs were well suited to exert a mechanism of inhibition of HTLV-1 transmission as they do for HIV. Moreover, the finding that HTLV-1, like HIV, contains high-mannose-type glycans on its envelope means that the virus is highly vulnerable to a specific interaction with mannose-specific CBAs, because high-mannose-type glycans rarely occur in glycans of mammalian glycoproteins (7). Besides specifically interacting with viral glycoproteins, CBAs may also inhibit HTLV-1 transmission by binding to cell surface proteoglycans. Interestingly, recent data indicate that the cell surface molecule GLUT-1 could efficiently act as an HTLV-1 receptor, enhancing virus transmission, only when associated to heparan sulfate proteoglycans (18, 29). Thus, it can be hypothesized that binding of CBAs to heparan sulfate could prevent or disturb its correct association with GLUT-1, hindering viral entry through this receptor. Interestingly, it has been reported that anti-ICAM-2 and anti-ICAM-3 monoclonal antibodies inhibited fusion events between HTLV-1-infected cells and dendritic cells expressing the mannose-specific surface lectin DC-SIGN (10). Thus, another mechanism by which CBAs could exert their anti-HTLV-1 actions is by binding mannose residues of the ICAM-2 and ICAM-3 adhesion molecules and, consequently, inhibiting intercellular contacts that play a pivotal role in cell-to-cell HTLV-1 transmission. Finally, CBAs could exert their anti-HTLV-1 action by cross-linking with glycans present on HTLV-1-associated antigens that are responsible for the sustained proliferation of recipient cells. Indeed, our data concerning the effects of CBAs on the growth of HTLV-1-infected cells show that, independently of the CBA capacity to inhibit virus transmission, pretreatment of virus-transformed donor cells, but not of acceptor PBMCs, by CBAs remarkably reduced the burst in the growth of PBMCs occurring after cocultivation with HTLV-1-donor cells. Considering that sustained proliferation capacity seems to be an important step in mechanisms involved in immortalization/transformation of HTLV-1-infected cells, CBAs could also reduce the transforming potential of HTLV-1 acting at that level.
Interestingly, the CBAs included in our studies are endowed with a low cytotoxic effect in cell culture. They are not mitogenic, do not agglutinate human red blood cells at antiviral concentrations, and do not markedly induce activation/differentiation markers or chemokines (8, 16). Therefore, HHA, PRM-A, and UDA are effective in inhibiting HTLV-1 infection at concentrations remarkably lower than their cytotoxic or antimetabolic concentrations. It should be noted, however, that the mannose-specific HHA and the GlcNAc-specific UDA were chosen for their different carbohydrate specificities in this study and acted to provide proof of the concept that CBAs are able to efficiently prevent cell-to-cell HTLV-1 transmission and subsequent infection of the target (acceptor) cells. The demonstration that low-molecular-weight (MW), nonpeptidic CBAs, such as the PRM-A antibiotic, show effects similar to those of the lectins opens a new, exciting area of discovery and exploration of the antiviral activities and specificities of new classes of synthetic, small, nonpeptidic CBAs. Whereas HHA and UDA are high-MW proteins (MW, 50,000 and 8,700, respectively), PRM-A is a nonpeptidic low-MW benzonaphtacenequinone antibiotic (MW, 838). The two types of molecules have different pharmacokinetic and pharmacodynamic behaviors. HHA and UDA have been shown to keep their carbohydrate-binding (and anti-HIV) activities after prolonged exposure to low pH (7) and thus may well survive passage through the stomach. Although such agents are not expected to have pronounced oral bioavailability, systemic uptake may not necessarily be required if these types of compounds were ever administered to infants to prevent HTLV-I infection through breast-feeding. Although PRM-A has low oral bioavailability, efforts should be devoted to searching for PRM-A derivatives or other nonpeptidic CBAs with increased oral bioavailability.
Interestingly, antivirally active concentrations of the CBAs are at least equal to, if not lower than, those required for nucleoside RT inhibitors (NRTI) to afford comparable inhibition of HTLV-1 transmission. Indeed, nucleoside analogues at low concentrations (around 0.1 μM) are unable to prevent HTLV-1 transmission when added at the onset of the coculture or after an overnight pretreatment period prior to cocultivation. Therefore, the CBAs should be considered to be among the most potent inhibitors of HTLV-1 infection/transmission reported so far. Moreover, the different mechanisms of action against HTLV-1 infection exerted by NRTI and CBAs also prompt us to hypothesize that the combination of CBAs with nucleoside analogues could lead to a superior inhibition of HTLV-1 transmission. CBAs could inhibit virus entry into target cells, while NRTI could act on those cells that escape from the CBA effect by blocking the RT-catalyzed conversion of viral RNA to DNA and, thus, the eventual incorporation of proviral DNA into the host cell genome.
In addition to new directions for anti-HTLV-1 therapy, this study also demonstrates how inhibition of cell-to-cell transmission in HTLV-1 infection by compounds capable of interacting with viral and/or cellular glycoconjugates (such as CBAs) plays a key role in limiting viral spread, although virus transmission through the mitotic pathway is evidently not affected by CBAs. This could contribute to an understanding of the yet-unresolved issue of how HTLV-1 moves from infected to uninfected cells, highlighting the importance of the carbohydrate portion of the participating molecules in the underlying molecular mechanisms involved in this event.
At present, no drugs are available to prevent HTLV-1 transmission. A preventive vaccine is also not available for HTLV-1. Therefore, inhibition of virus spread by means of nontoxic treatments would significantly improve the life expectancy of HTLV-1-exposed individuals. Moreover, since an important mode of HTLV-1 transmission occurs from mother to child through breast milk containing HTLV-1-infected cells, it would be of interest to further investigate whether CBAs can play a role in preventing virus transmission through this important pathway of infection.
Acknowledgments
This work was supported by grants from the Italian Ministry of University and Research, Research Projects of National Interest, to B.M.; from Istituto Superiore di Sanità, AIDS Project, and from the University of Rome “Tor Vergata” to B.M.; from the University of Messina to A.M.; and from the K. U. Leuven Centers of Excellence (EF-05/15), the Geconcerteerde Onderzoeksacties, and the Fonds voor Wetenschappelijk Onderzoek (G.0485.08) to J.B.
Footnotes
Published ahead of print on 27 May 2008.
REFERENCES
- 1.Arp, J., M. Levatte, J. Rowe, S. Perkins, E. King, C. Leystra-Lantz, S. K. H. Foung, and G. A. Dekaban. 1996. A source of glycosylated human T-cell lymphotropic virus type 1 envelope protein: expression of gp46 by the vaccinia virus/T7 polymerase system. J. Virol. 70:7349-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balestrieri, E., C. Matteucci, A. Ascolani, R. Romeo, G. Romeo, U. Chiacchio, A. Mastino, and B. Macchi. 2008. Effect of phosphonated carbocyclic 2′-oxa-3′-aza-nucleoside on HTLV-1 infection in vitro. Antimicrob. Agents Chemother. 52:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balestrieri, E., M. T. Sciortino, A. Mastino, and B. Macchi. 2005. Protective effect of the acyclic nucleoside phosphonate tenofovir toward human T-cell leukaemia/lymphotropic virus type 1 infection of human peripheral blood mononuclear cells in vitro. Antivir. Res. 68:154-162. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J. 2007. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 5:583-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini, J., K. Van Laethem, S. Hatse, M. Froeyen, W. Peumans, E. Van Damme, and D. Schols. 2005. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120: a new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 280:41005-41014. [DOI] [PubMed] [Google Scholar]
- 7.Balzarini, J., K. Van Laethem, D. Daelemans, S. Hatse, A. Bugatti, M. Rusnati, Y. Igarashi, T. Oki, and D. Schols. 2007. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J. Virol. 81:362-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzarini, J., S. Hatse, K. Vermeire, K. Princen, S. Aquaro, C. F. Perno, E. De Clercq, H. Egberink, G. Vanden Mooter, W. Peumans, E. Vandamme, and D. Schols. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48:3858-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balzarini, J., Y. Van Herrewege, K. Vermeire, G. Vanham, and D. Schols. 2007. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T-lymphocytes. Mol. Pharmacol. 71:3-11. [DOI] [PubMed] [Google Scholar]
- 10.Ceccaldi, P. E., F. Delebecque, M. C. Prevost, A. Moris, J. P. Abastado, A. Gessain, O. Schwartz, and S. Ozden. 2006. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J. Virol. 80:4771-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derse, D., B. Crise, Y. Li, G. Princler, N. Lum, C. Stewart, C. F. McGrath, S. H. Hughes, D. J. Munroe, and X. Wu. 2007. Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses. J. Virol. 81:6731-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edlich, R. F., J. A. Arnett, and F. M. Williams. 2000. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-1). J. Emerg. Med. 18:109-119. [DOI] [PubMed] [Google Scholar]
- 13.Franchini, G., C. Nicot, and J. M. Johnson. 2003. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv. Cancer Res. 89:69-132. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 15.Hermine, O., H. Dombret, J. Poupon, B. Arnulf, F. Lefrere, P. Rousselot, and G. Damaj. 2004. Phase III trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol. J. 5:130-134. [DOI] [PubMed] [Google Scholar]
- 16.Huskens, D., K. Princen, M. Schreiber, and D. Schols. 2007. The role of N-glycosylation sites on the CXCR4 receptor for CXCL-12 binding and signalling and X4 HIV-1 viral infectivity. Virology 363:280-287. [DOI] [PubMed] [Google Scholar]
- 17.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 18.Jones, K. S., C. Petrow-Sadowski, D. C. Bertolette, Y. Huang, and F. W. Ruscetti. 2005. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 79:12692-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komurian, F., F. Pelloquin, and G. de Thé. 1991. In vivo genomic variability of human T-cell leukemia virus type I depends more upon geography than upon pathologies. J. Virol. 65:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, H. C., R. J. Biggar, W. J. Miley, E. M. Maloney, B. Cranston, B. Hanchard, and M. Hisada. 2004. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J. Infect. Dis. 190:1275-1278. [DOI] [PubMed] [Google Scholar]
- 21.Lillehoj, E., C. Tal, A. Nguyen, and S. Alexander. 1989. Characterization of env and tax encoded polypeptides of human T-cell leukemia virus type I. Clin. Biotechnol. 1:27-41. [Google Scholar]
- 22.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 23.Oki, T., M. Komishi, K. Tomatsu, K. Tomita, K. I. Saitoh, M. Tsunakawa, M. Nishio, T. Miyaki, and H. Kawagachi. 1988. Pradimicin, a novel class of potent antifungal antibiotics. J. Antibiot. 41:1701-1704. [DOI] [PubMed] [Google Scholar]
- 24.Okudaira, T., M. Hirashima, C. Ishikawa, S. Makishi, M. Tomita, T. Matsuda, H. Kawakami, N. Taira, K. Ohshiro, M. Masuda, N. Takasu, and N. A. Mori. 2007. NA modified version of galectin-9 suppresses cell growth and induces apoptosis of human T-cell leukemia virus type I-infected T-cell lines. Int. J. Cancer 120:2251-2261. [DOI] [PubMed] [Google Scholar]
- 25.Peumans, W. J., M. De Ley, and W. F. Broekaert. 1984. An unusual lectin from stinging nettle (Urtica dioica) rhizomes. FEBS Lett. 177:99-103. [Google Scholar]
- 26.Proietti, F. A., A. B. Carneiro-Proietti, B. C. Catalan-Soares, and E. L. Murphy. 2005. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24:6058-6068. [DOI] [PubMed] [Google Scholar]
- 27.Roucoux, D. F., B. Wang, D. Smith, C. C. Nass, J. Smith, S. T. Hutching, B. Newman, T.-H. Lee, D. M. Chafets, and E. L. Murphy for the HTLV Outcome Study Investigation. 2005. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J. Infect. Dis. 191:1490-1497. [DOI] [PubMed] [Google Scholar]
- 28.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 80:3618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takenouchi, N., K. S. Jones, I. Lisinski, K. Fugo, K. Yao, S. W. Cushman, F. W. Ruscetti, and S. Jacobson. 2007. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1.J. Virol. 81:1506-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, G. P., and M. Matsuoka. 2005. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 24:6047-6057. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, G. P., S. E. Hall, S. Navarrete, C. A. Michie, R. Davis, A. D. Witkover, M. Rossor, M. A. Nowak, P. Rudge, E. Matutes, C. R. Bangham, and J. N. Weber. 1999. Effect of lamivudine on human T-cell leukemia virus type I (HTLV-1) DNA copy number, T-cell phenotype, and anti-Tax cytotoxic T-cell frequency in patients with HTLV-1 associated myelopathy. J. Virol. 73:10289-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda, H., K. Takatsuki, R. Ohno, T. Masaoka, K. Okada, S. Shirakawa, Y. Ohashi, and K. Ota. 1994. Treatment of adult T-cell leukemia-lymphoma with irinotecano hydrochloride (CP11). Br. J. Cancer 70:771-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Damme, E. J. M., A. K. Allen, and W. J. Peumans. 1988. Related mannose-specific lectins from different species of the family Amaryllidaceae. Plant Physiol. 73:52-57. [Google Scholar]
- 34.Van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanisms for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, M. 2005. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insight into pathogenesis. Oncogene 24:5931-5937. [DOI] [PubMed] [Google Scholar]