Abstract
We analyzed the effects of temperature on the interaction of Legionella pneumophila with Acanthamoeba castellanii. At <20°C, overexpression of type 1 metacaspase, a stimulator of A. castellanii encystation, was associated with a reduced number of bacteria within amoeba. At low temperatures, A. castellanii seems to eliminate L. pneumophila by encystation and digestion.
The intracellular pathogen Legionella pneumophila causes Legionnaires’ disease and exploits aquatic protozoa for replication. L. pneumophila is more frequently isolated from man-made water systems with high water temperature (9, 17) than from relatively cold freshwater environments (4, 16). This fact suggests that thermal conditions affect the relationship between L. pneumophila and protozoa, a notion supported by some reports (1, 3, 7, 11). The trophozoite of protozoa transforms into a cyst under harmful environments such as starvation, cold, and certain chemicals used in medical treatment. The effect of protozoal encystation on Legionella infection is not well understood although it seems that encystation is enhanced in a freshwater environment at low temperature. With these considerations in mind, we investigated whether the host-parasite relationship between L. pneumophila and protozoa is temperature dependent.
Two strains of L. pneumophila serogroup 1, Suzuki and Lp01, and Acanthamoeba castellanii ATCC 30234 were used in the present study. Intracellular growth kinetics assays using A. castellanii were performed as described previously (15). Reverse transcription and quantitative reverse transcription-PCR analyses were performed to examine (i) the expression of virulence- and growth-related genes (pmrA, cpxR, csrA) following transfer of L. pneumophila Lp01 growing in the post-exponential phase in buffered yeast extract α (BYEα) broth to fresh medium and incubation at 15°C or 35°C for 24 h (as an in vitro model of intracellular growth of L. pneumophila in A. castellanii at 15 and 35°C [13]) and (ii) the expression of the type 1 metacaspase gene, which was recently implicated in the encystation of A. castellanii (20), in A. castellanii buffer at 15 and 35°C with and without infection of L. pneumophila Lp01. The comparative ΔΔCT approximation method was used to analyze relative changes in gene expression (10).
Total RNA was purified using an RNeasy minikit (Qiagen, Valencia, CA) according to the instructions provided by the manufacturer. Total RNA was reverse transcribed into cDNA using an RNA PCR kit (Takara Bio Inc., Shiga, Japan). Real-time PCR with Sybr greenER (Invitrogen Life Sciences) was performed using the ABI Prism 7000 system (Applied Biosystems, Foster City, CA). Table 1 lists the primer pairs for target and internal control genes. Data were analyzed by Student's t test, and a two-tailed P value of <0.01 was considered significant.
TABLE 1.
Primer sets used in the present study
| Organism | Gene or rRNAa | Primer sequences (forward, reverse) (5′-3′) |
|---|---|---|
| L. pneumophila Lp01 | cpxR (target gene; lpg1483) | CCTCTTTGGAAAATGCTTGC, AAAATCCCAACTCCCAAACC |
| pmrA (target gene; lpg1292) | CCTGGCCACTGTTTTCAAGT, AGATAGCGGTGCTGACGATT | |
| 16S rRNA (internal control; lpg0569) | ACGGCAGCATTGTCTAGCTT, TGAGTTTCCCCAAGTTGTCC | |
| A. castellanii ATCC 30234 | mcasp1 (target gene) | CGCTCTTCCTTCATTTCTCG, GTAGACGTAGGGCAGGTCCA |
| 18S rRNA (internal control) | GCATCTGCCAAGGATGTTTT, TCACAAGCTGCTGCTAGGGGAGT |
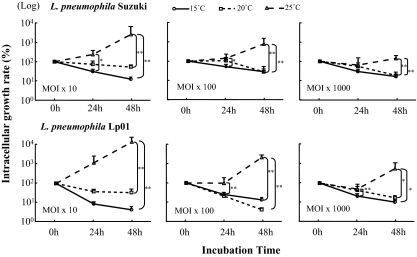
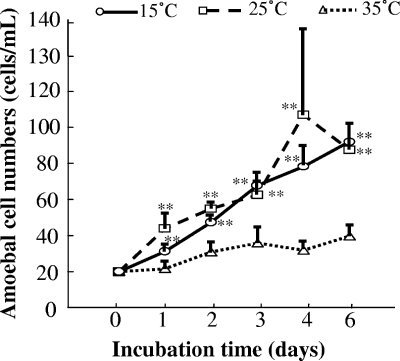
L. pneumophila showed rapid increase in growth in protozoa or in broth at temperatures above 35°C (data not shown). However, an intracellular kinetics assay of A. castellanii using different strains and doses showed decreases of intracellular counts of both L. pneumophila Suzuki and Lp01 at temperatures below 20°C, with significant differences to 25°C (Fig. 1). A. castellanii in peptone-yeast extract-glucose medium (PYG) grew actively at 15°C but not at 35°C (Fig. 2). On the other hand, L. pneumophila did not multiply at 15°C in BCYEα broth (data not shown). These findings suggest that A. castellanii can kill L. pneumophila at low temperature.
FIG. 1.
Effects of temperature, inoculum size, and strain on multiplication of L. pneumophila in A. castellanii ATCC 30234. The numbers of Legionella cells in amoeba vacuoles after 24 and 48 h of incubation were expressed as percentages of that at 0 h (bacterial number in amoebas when Legionella and amoebas were coincubated for 3 h at 35°C and for a further 1 h with 50 μg/ml of gentamicin). Data are means ± SD. *, P < 0.05; **, P < 0.01. MOI, multiplicity of infection; numbers indicate bacterium/amoeba cell ratios.
FIG. 2.
Growth rate of A. castellanii ATCC 30234 in PYG at different temperatures. Data are means ± SD. **, P < 0.01, compared with the growth rate at 35°C.
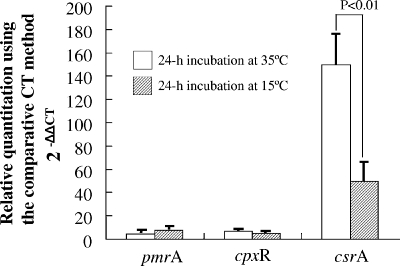
The pmrA and cprX genes comprise a two-component system and play a major role in regulating the icm and dot genes, respectively, which are components of a major virulence system in L. pneumophila (6, 21), while CsrA is a global repressor of the L. pneumophila transmission phenotype and essential activator of intracellular replication (5, 14). Although the expression of pmrA and cpxR was not markedly upregulated at both 15 and 35°C shifts, the fact that csrA expression was significantly higher at 35°C than at 15°C explains the active proliferation of L. pneumophila within Acanthamoeba. Conversely, csrA expression was reduced by the shift to 15°C, highlighting the inability of L. pneumophila to resist digestion by Acanthamoeba at 15°C (Fig. 3).
FIG. 3.
Expression of virulence- and intracellular-growth-related genes (pmrA, cpxR, and csrA) using an in vitro model of intracellular growth of L. pneumophila Lp01 in A. castellanii ATCC 30234 at 15 and 35°C. L. pneumophila cells grown aerobically to post-exponential phase at 35°C in BYEα broth were transferred to a fresh broth at a final density of 1 × 107 CFU/ml and incubated aerobically at 15°C or 35°C for 24 h. Data are means ± SD. CT, threshold cycle.
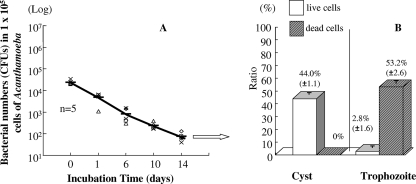
Greub and Raoult (8) proposed that encystment is the main process by which an amoeba escapes Legionella infection. Figure 4 shows that the numbers of L. pneumophila cells decreased by more than 3 log units after incubation for 14 days at 15°C in A. castellanii buffer containing amoebas. Furthermore, the percentage of cysts to cells at day 17 of this experiment was 44.0 ± 1.1 (mean ± standard deviation [SD]) in the microcosm, and most trophozoites were dead, as determined by trypan blue staining. In addition, the infectivity of L. pneumophila for cysts in the population (75.4%) following encystment treatment was significantly lower than that for the trophozoite component (data not shown).
FIG. 4.
Survival of L. pneumophila Suzuki in A. castellanii ATCC 30234 after 14 days of incubation at 15°C (A) and ratio of trophozoites to cysts at day 17 (B). Data are means ± SD.
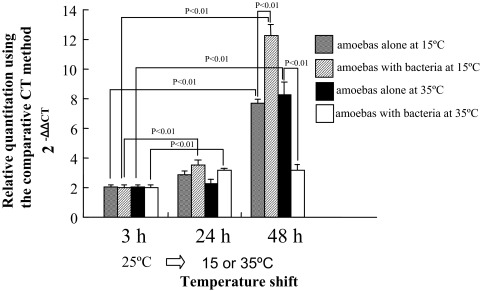
The expression level of the type 1 metacaspase gene in A. castellanii increased with incubation time at both 15 and 35°C. In both amoebas alone and amoebas infected with L. pneumophila after 24 h of incubation, the increases in expression were nearly the same, although the expression levels in the bacterium-infected population were slightly higher than in noninfected amoebas. However, the difference was more marked after a 48-h incubation. In short, the expression level of the type 1 metacaspase gene at 15°C was approximately 12 times higher in amoebas with bacteria than in the amoebas at baseline (amoebas grown in PYG at 25°C; data not shown in figure), while the gene upregulation at 15°C was approximately eightfold in noninfected populations relative to infected populations. The significantly low expression in infected amoebas after 48 h at 35°C was probably due to L. pneumophila-induced cell disruption (Fig. 5). Our results suggest that encystation is prompted both by stimulation at 15°C and by L. pneumophila infection and that encystment serves to protect amoeba populations infected by L. pneumophila. However, the mechanism by which L. pneumophila induces type 1 metacaspase gene expression remains elusive; L. pneumophila could perhaps activate the Icm/IcDot type IV secretion system, which in turn triggers caspase-3 activation (12).
FIG. 5.
Effects of incubation temperature on expression of type 1 metacaspase gene in A. castellanii ATCC 30234 with or without infection with L. pneumophila Lp01. Data are means ± SD. CT, threshold cycle.
Berk et al. (2) demonstrated abundant release of vesicles from amoebas following ingestion of live L. pneumophila, with many vesicles containing bacteria. Most of the vesicles appeared in the medium just prior to encystment. This observation was supported by reports that Acanthamoeba spp. expel food vacuoles prior to encystment rather than retaining them within the cyst (18, 19). We also observed vesicles expelled from amoebas in the present study (data not shown).
In conclusion, L. pneumophila infects the trophozoite form of free-living amoebas and replicates intracellularly by activating the global regulator gene csrA at temperatures over 25°C. In contrast, at temperatures below 20°C, L. pneumophila is actively digested by A. castellanii, and the process of encystation eliminates L. pneumophila from the host, transmitting it to the extracellular environment.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Anand, C. M., A. R. Skinner, A. Malic, and J. B. Kurtz. 1983. Interaction of Legionella pneumophila and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. 91:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk, S. G., R. S. Ting, G. W. Turner, and R. J. Ashburn. 1998. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 64:279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birtles, R. J., T. J. Rowbotham, R. Michel, D. G. Pitcher, B. Lascola, S. Alexiou-Daniel, and D. Raoult. 2000. ‘Candidatus Odyssella thessalonicensis’ gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int. J. Syst. Evol. Microbiol. 50(Pt. 1):63-72. [DOI] [PubMed] [Google Scholar]
- 4.Fliermans, C. B., W. B. Cherry, L. H. Orrison, S. J. Smith, D. L. Tison, and D. H. Pope. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsbach-Birk, V., T. McNealy, C. Shi, D. Lynch, and R. Marre. 2004. Reduced expression of the global regulator protein CsrA in Legionella pneumophila affects virulence-associated regulators and growth in Acanthamoeba castellanii. Int. J. Med. Microbiol. 294:15-25. [DOI] [PubMed] [Google Scholar]
- 6.Gal-Mor, O., and G. Segal. 2003. Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185:4908-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greub, G., B. La Scola, and D. Raoult. 2003. Parachlamydia acanthamoeba is endosymbiotic or lytic for Acanthamoeba polyphaga depending on the incubation temperature. Ann. N. Y. Acad. Sci. 990:628-634. [DOI] [PubMed] [Google Scholar]
- 8.Greub, G., and D. Raoult. 2003. Morphology of Legionella pneumophila according to their location within Hartmanella vermiformis. Res. Microbiol. 154:619-621. [DOI] [PubMed] [Google Scholar]
- 9.Hsu, B. M., C. H. Chen, M. T. Wan, and H. W. Cheng. 2006. Legionella prevalence in hot spring recreation areas of Taiwan. Water Res. 40:3267-3273. [DOI] [PubMed] [Google Scholar]
- 10.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 11.Moffat, J. F., and L. S. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molmeret, M., S. D. Zink, L. Han, A. Abu-Zant, R. Asari, D. M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell. Microbiol. 6:33-48. [DOI] [PubMed] [Google Scholar]
- 13.Molofsky, A. B., and M. S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29-40. [DOI] [PubMed] [Google Scholar]
- 14.Molofsky, A. B., and M. S. Swanson. 2003. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50:445-461. [DOI] [PubMed] [Google Scholar]
- 15.Ohno, A., N. Kato, K. Yamada, and K. Yamaguchi. 2003. Factors influencing survival of Legionella pneumophila serotype 1 in hot spring water and tap water. Appl. Environ. Microbiol. 69:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer, C. J., Y. L. Tsai, C. Paszko-Kolva, C. Mayer, and L. R. Sangermano. 1993. Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent-antibody, and plate culture methods. Appl. Environ. Microbiol. 59:3618-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabria, M., J. Alvarez, A. Dominguez, A. Pedrol, G. Sauca, L. Salleras, A. Lopez, M. A. Garcia-Nunez, I. Parron, and M. P. Barrufet. 2006. A community outbreak of Legionnaires’ disease: evidence of a cooling tower as the source. Clin. Microbiol. Infect. 12:642-647. [DOI] [PubMed] [Google Scholar]
- 18.Schuster, F. 1979. Small amebas and amoeboflagellates, p. 215-285. In S. H. Hutner (ed.), Biochemistry and physiology of protozoa, vol. 1. Academic Press, New York, NY. [Google Scholar]
- 19.Stewart, J. R., and R. A. Weisman. 1972. Exocytosis of latex beads during the encystment of Acanthamoeba. J. Cell Biol. 52:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trzyna, W. C., X. D. Legras, and J. S. Cordingley. 3 August 2006. A type-1 metacaspase from Acanthamoeba castellanii. Microbiol. Res. [Epub ahead of print.] doi: 10.1016/j.micres.2006.06.017. [DOI] [PubMed]
- 21.Zusman, T., G. Aloni, E. Halperin, H. Kotzer, E. Degtyar, M. Feldman, and G. Segal. 2007. The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol. Microbiol. 63:1508-1523. [DOI] [PubMed] [Google Scholar]