Abstract
Multiple outbreaks of food-borne gastroenteritis caused by the coccidian parasite Cyclospora cayetanensis have been reported annually in North America since 1995. Detection of C. cayetanensis contamination typically relies on laborious and subjective microscopic examination of produce washes. Molecular detection methods based on nested PCR, restriction fragment length polymorphism, or multiplex PCR have been developed for C. cayetanensis; however, they have not been adequately validated for use on food products. Further challenges include reliably extracting DNA from coccidian oocysts since their tough outer wall is resistant to lysis and overcoming PCR inhibitors in sample matrices. We describe preliminary validation of a reliable DNA extraction method for C. cayetanensis oocysts and a sensitive and specific novel PCR assay. The sensitivity and repeatability of the developed methods were evaluated by multiple DNA extractions and PCR amplifications using 1,000-, 100-, 10-, or 1-ooycst aliquots of C. cayetanensis oocysts in water or basil wash sediment. Successful PCR amplification was achieved on 15 and 5 replicates extracted from aliquots containing 1,000 oocysts in water and basil wash, respectively. All 45 replicates of the 100-oocyst aliquots in water and 5 in basil wash were amplified successfully, as were 43/45 and 41/45 of the 10- and 1-oocyst aliquots in water and 9/15 and 2/15 in basil wash, respectively. The developed primers showed no cross-reactivity when tested against bacteria, nematodes, and protozoans, including Eimeria, Giardia, and Cryptosporidium. Our results indicate that these methods are specific, can reliably detect a single oocyst, and overcome many of the limitations of microscopic diagnosis.
Cyclospora cayetanensis is a coccidian protozoan parasite that causes severe gastroenteritis in both immunocompromised (16) and immunocompetent (20) humans. The oocysts are shed unsporulated with the feces of infected individuals and can take from one to several weeks to become fully sporulated and infectious. Therefore, C. cayetanensis infections are unlikely to be passed directly from person to person but rather occur through the ingestion of water or food contaminated with feces (11). Multiple food-borne outbreaks linked to fresh imported produce such as raspberries and basil have occurred annually in North America since 1995 (6) and have been reported in Europe (4). C. cayetanensis infections have also been found in travelers visiting developing tropical and subtropical countries such as Nepal, Peru, Indonesia, and Guatemala, where the parasite is endemic (1, 8).
Sensitive and specific molecular detection of C. cayetanensis remains a challenge since current methods for DNA extraction from oocysts can be inefficient, labor-intensive, and unreliable (11). In addition, successful PCR detection of coccidian oocysts is dependent on the method used to extract DNA (3, 12). There are several PCR assays currently available for detection of C. cayetanensis DNA, although they rely on nested amplification (15, 17) and restriction fragment length polymorphism (RFLP) (7, 18) to achieve the theoretically useful levels of sensitivity and specificity necessary for use with fresh produce or human clinical samples. Sensitivity estimates are often determined by extracting DNA from a large number of oocysts, followed by serial dilution of the DNA to calculate “oocyst equivalents” (14), or are based on the proportional quantity of DNA added to the PCR (21). Both techniques assume DNA extraction to be 100% efficient, which is unlikely and results in misleading reports of assay sensitivity. For the purposes of diagnostic testing of produce, a more desirable approach would be to base estimates of PCR assay sensitivity on actual extraction of DNA from a stock of oocysts where numbers have been validated by repeated counts or flow cytometry. A further challenge for molecular detection of Cyclospora lies in efficiently recovering the oocysts from sample matrices such as feces or produce washes which contain PCR inhibitors. Since an immunomagnetic separation (IMS) assay for Cyclospora is not yet available, DNA extraction methods and PCR assays used in clinical or food testing must be robust enough to perform reliably when inhibitors are present.
The objectives of this study were (i) to develop a novel DNA extraction method and PCR assay for the detection of as few as one C. cayetanensis oocyst using a single-round amplification, (ii) to generate preliminary validation data to demonstrate the sensitivity and reproducibility of the DNA extraction method and PCR amplification using both purified oocysts and oocysts spiked into basil wash sediment, and (iii) to demonstrate specificity of the developed PCR primers for C. cayetanensis DNA.
MATERIALS AND METHODS
Oocysts.
Oocysts of C. cayetanensis were obtained from human fecal samples collected in Nepal and stored in 2.5% potassium dichromate solution at 4°C for less than 2 months. The majority of oocysts were unsporulated. Prior to use, oocysts of C. cayetanensis were isolated from feces using a modified sucrose flotation method (5). Briefly, approximately 5 ml of feces was filtered and washed two times with 50 ml water by centrifugation (2,056 × g) at room temperature for 20 min to remove potassium dichromate. The resulting 2 ml of washed fecal suspension was then mixed with sucrose solution (specific gravity, 1.26) (9) in a 16- by 125-mm glass tube, covered with a coverslip, and centrifuged at room temperature for 10 min at 327 × g using a swing-out rotor. Coverslips were removed onto a standard microscope slide and examined for C. cayetanensis oocysts using a compound microscope at a magnification of ×200. Oocysts were then collected by placing the coverslip and slide into a 50-ml plastic centrifuge tube and rinsing with approximately 50 ml of NANOpure water. The oocysts were concentrated by centrifugation (2,056 × g) at room temperature for 20 min and stored in 2% sulfuric acid or NANOpure water at 4°C.
DNA extraction from oocysts.
DNA was extracted from oocysts using either the QIAamp DNA microkit (<100 oocysts; Qiagen, Mississauga, Canada) or the DNeasy blood and tissue kit (≥100 oocysts; Qiagen, Mississauga, Canada) with the following modifications to the manufacturer's protocols: (i) oocysts were first suspended in 300 μl of ATL buffer (Qiagen, Mississauga, Canada) and subjected to 8 cycles of freezing in liquid nitrogen for 1 min followed by thawing in a 95°C water bath for 1 min; (ii) lysed oocyst suspensions were then incubated with 20 μl proteinase K (20 mg/ml; Qiagen, Mississauga, Canada) for 3 h at 56°C, followed by incubation with 300 μl AL buffer (Qiagen, Mississauga, Canada) at 70°C for 10 min with vortexing for 10 s every 3 min. DNA was purified through the columns according to the manufacturer's protocol and eluted in 20 μl (<100 oocysts) or 50 μl (≥100 oocysts) of the supplied AE buffer and stored at −20°C.
Primer design.
Signature Oligo software (LifeIntel Software, Inc., Port Moody, Canada) was employed to identify regions of nucleotide sequence that were unique to C. cayetanensis ribosomal DNA (rDNA) (a consensus of GenBank accession no. AF301386 to AF301391) (13) when compared to rDNA sequences of related coccidia (representatives of Cryptosporidium, Eimeria, Sarcocystis, Hammondia, Toxoplasma, Neospora, Atoxoplasma, Isospora, and Besnoitia spp.) and other potential environmental contaminants originating from plants, bacteria, fungi, or mammals. Diagnostic PCR primers CCITS2-F (5′-GCAGTCACAGGAGGCATATATCC-3′) and CCITS2-R (5′-ATGAGAGACCTCACAGCCAAAC-3′) were then designed to anneal at these unique signature sequence locations using Beacon Designer software (version 6; Premier Biosoft International). The primers were checked for sequence specificity by comparison to the GenBank nucleotide sequence database (http://www.ncbi.nlm.nih.gov) using BLASTN and were synthesized by Invitrogen (Burlington, Canada).
PCR amplification.
PCR amplification was performed using a thermal cycler (iCycler; Bio-Rad Laboratories, Hercules, CA). The 25-μl PCRs were carried out using GoTaq Flexi DNA polymerase buffer (Promega Corporation, Madison, WI), 2 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate (dNTP), 1 U of GoTaq Flexi DNA polymerase, 400 nM of each primer, and 2 μl of template DNA or water as a negative control. The specificity of the CCITS2-F/CCITS2-R primers was tested by PCR amplification of C. cayetanensis DNA extracted from oocysts isolated from five individual human fecal samples and of a mixed panel of DNA with (MP+) or without (MP−) C. cayetanensis DNA. The MP− sample contained equal volumes of DNA from organisms obtained from a stored research and diagnostic collection. They included Eimeria vermiformis, Eimeria ahsata, Eimeria zuernii, Eimeria falciformis, Eimeria tenella, Sarcocystis cruzi, Cryptosporidium parvum, Toxoplasma gondii, Giardia lamblia, Escherichia coli, Trichinella spiralis, Saccharomyces cerevisiae, and a mixture of plants and microorganisms in ovine fecal DNA; the MP+ sample also included an equal volume of C. cayetanensis DNA. The PCR protocol consisted of 2 min at 95°C followed by 40 cycles of denaturing at 95°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s and a final extension for 5 min at 72°C. PCR products were subjected to electrophoresis in a 2.5% (wt/vol) agarose-TBE (89 mM Tris-borate, 2 mM EDTA [pH 8.3]) gel, stained with Sybr gold (Invitrogen, Burlington, Canada), and photographed with UV transillumination using the AlphaImager 2000 gel documentation system (Alpha Innotech Corporation, San Leandro, CA). To verify the identity of the amplified target, bands were excised and purified using the QiaQuick gel extraction kit (Qiagen, Mississauga, Canada) according to the manufacturer's instructions and sequenced (GenBank accession no. EU719102 to EU719106).
Sequence analysis.
Forward and reverse sequence segments were overlapped using PreGap4 and Gap4 (Staden Package), and the primer sequences were removed. Elucidated nucleotide sequences were compared to the GenBank collection of sequences using BLASTN and were identified based on the degree of sequence identity. A consensus of sequences from the five individual human isolates of C. cayetanensis was calculated using software available on the EMBOSS explorer (http://emboss.imb.nrc.ca/).
Preparation and quantification of oocyst stocks.
The number of oocysts in all prepared stock solutions was verified by triplicate counts to ensure accuracy. Briefly, 5 μl (for stocks containing 1,000 oocysts per aliquot), 25 μl (for stocks containing 100 oocysts per aliquot), or 50 μl (for stocks containing 10 or 1 oocyst per aliquot) of the stock solution was dried onto three 9-mm-well slides (Dynal Spot-On; Invitrogen, Burlington, Canada), mounted with one drop of mounting medium (Waterborne, Inc., New Orleans, LA), and examined at a magnification of ×200 using a fluorescence microscope (Olympus BX51; 330- to 385-nm excitation dichromatic filter). The mean number of oocysts per 50-μl aliquot was calculated based on the triplicate counts, and the aliquot volume was adjusted appropriately to contain 1,000, 100, 10, or 1 oocyst. The oocysts in all stock solutions were diluted in water and stored at 4°C until used.
Repeatability and sensitivity.
To determine the repeatability and sensitivity of the developed DNA extraction method and PCR assay, DNA was extracted from 1,000, 100, 10, or 1 C. cayetanensis oocyst and amplified using primers CCITS2-F and CCITS2-R. One stock solution containing 1,000 oocysts per 50-μl aliquot was prepared on three separate occasions. DNA was extracted from five aliquots of each of the three prepared stocks and amplified by PCR, resulting in 15 replicates. Three stock solutions containing 100, 10, or 1 oocyst per 50-μl aliquot were prepared on three separate occasions. DNA was extracted from five aliquots of each of the nine prepared stocks (total of 45 replicates) and amplified by PCR.
Cyclospora oocyst detection in produce washes.
To determine the suitability of the developed primers and DNA extraction method for sensitive and specific detection of Cyclospora oocysts spiked in produce washes, 30 g of fresh basil leaves (grown in-house) was processed by stomaching in 200 ml 1 M glycine buffer (pH 5.5) as described previously (2). Five aliquots from freshly prepared 1,000- and 100-oocyst stocks and 15 (five samples tested on three separate occasions) aliquots from prepared 10- or 1-oocyst stocks (quantified as described above) were spiked into 50 μl of the concentrated basil wash sediment, and DNA was extracted from the spiked samples and amplified by PCR as described above. No attempt was made to specifically remove plant material prior to PCR.
RESULTS
Specificity.
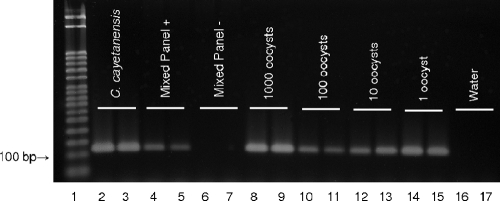
A novel pair of oligonucleotide primers was designed to amplify a 116-bp segment of the internal transcribed spacer 2 (ITS-2) region of C. cayetanensis rDNA. Analysis of sequence data from PCR products amplified with the newly developed primers verified their specificity for C. cayetanensis DNA. A comparison of the consensus sequences from the five human isolates of the parasite amplified by our primers to the GenBank nucleotide collection using BLASTN (performed January 2008) showed a 95% similarity to C. cayetanensis AF301382 (13). No similarity to other Cyclospora species, coccidia, prokaryotes, or eukaryotes was found. The primers did not yield products when used with DNA template from E. vermiformis, E. ahsata, E. zvernii, E. falciformis, E. tenella, S. cruzi, C. parvum, T. gondii, G. lamblia, E. coli, T. spiralis, S. cerevisiae, or ovine fecal DNA. C. cayetanensis was also successfully amplified from within the mixed panel of DNA from the above organisms, as shown in Fig. 1.
FIG. 1.
Stained agarose gel with PCR products amplified using primers CCITS2-F and CCITS2-R. Lane 1, molecular weight marker; lanes 2 and 3, C. cayetanensis DNA; lanes 4 and 5, MP+; lanes 6 and 7, MP−; lanes 8 to 15, DNA extracted from 1,000, 100, 10, and 1 oocyst, respectively; lanes 16 and 17, water. For the 10- and 1-oocyst aliquots, DNA was extracted using the QIAamp DNA microkit (Qiagen). All other extractions were performed using the DNeasy blood and tissue kit (Qiagen).
Reliability and sensitivity.
Successful PCR amplification was achieved on all 15 replicates extracted from each of the three prepared stock solution aliquots containing 1,000 oocysts (Fig. 1). All 45 replicates of the 100-oocyst aliquots were amplified successfully, as were 43/45 and 41/45 of the 10- and 1-oocyst aliquots, respectively (Fig. 1). Use of the QIAamp DNA micro- kit for extraction from fewer than 100 oocysts improved PCR amplification, as shown by the greater band intensity for 1 oocyst compared to 100 (Fig. 1).
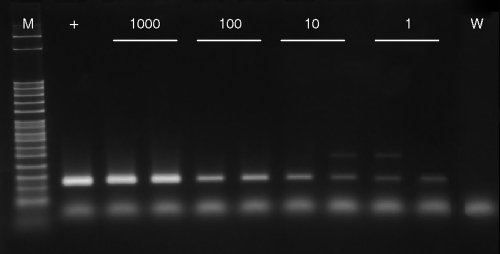
The PCR assay sensitivity was reduced when Cyclospora oocysts were present in concentrated basil wash sediment. Successful PCR amplification was achieved for all replicate samples of basil wash spiked with 1,000 (5/5) or 100 (5/5) oocysts (Fig. 2). When basil washes were spiked with 10 or 1 Cyclospora oocyst, PCR amplification was successful for 9/15 and 2/15 replicates, respectively (Fig. 2). Faint, spurious PCR products (Fig. 2) ranging in size from 200 to 400 bp were observed for 6 of the 40 spiked basil wash sediment samples. Sequencing and comparison of these products to the GenBank nucleotide collection using BLASTN did not produce any significant alignments.
FIG. 2.
Stained agarose gel with PCR products amplified from DNA extracted from 1,000, 100, 10, and 1 Cyclospora oocyst spiked into basil wash sediment using primers CCITS2-F and CCITS2-R. M, molecular weight marker; +, C. cayetanensis DNA; W, water.
DISCUSSION
Microscopic examination of concentrated produce wash preparations using UV fluorescence and modified acid-fast staining are likely the most commonly used approaches for routine detection of C. cayetanensis contamination in food. However, identification of oocysts requires sporulation, which can take one or more weeks, and an experienced microscopist to distinguish key morphological features of the sporulated oocyst (6, 11). Microscopic detection can be laborious and subjective. Furthermore, C. cayetanensis oocysts are usually present in low numbers, have no distinguishing species-specific morphological characteristics, and show variability in histological staining, which contribute to difficulties in their identification by microscopy (6). Here, we describe a simple method for isolation of C. cayetanensis oocysts from feces, the development and preliminary validation of an efficient and reliable DNA extraction procedure, and a sensitive PCR assay which overcomes many of the limitations of detection by microscopy.
The developed DNA extraction method and PCR primers (CCITS2-F and CCITS2-R) provide a highly sensitive and reliable assay for the detection of C. cayetanensis. The high degree of sensitivity achieved here, where as little as a single purified oocyst can be repeatedly detected, is similar to or greater than what was previously reported by others using FTA filters (Whatman, Inc.) with nested PCR (15), quantitative real-time PCR (21), or PCR-RFLP (18). The cases in which DNA from the aliquots containing 10 or 1 purified oocyst was not detected by PCR were likely due to normal pipetting variability involving low numbers of organisms. Since the infective dose of C. cayetanensis is unknown but presumed to be low (6), the ability to detect a single oocyst would be critical for disease outbreak investigations and clinical diagnoses. For reliable detection of Cyclospora directly in concentrated produce wash sediments, duplicate or triplicate samples should be tested since the presence of plant material reduced PCR assay sensitivity.
To our knowledge, very few studies have reported validation of a C. cayetanensis DNA extraction and PCR detection technique for use with either fecal or produce samples. Steele et al. (19) evaluated the sensitivity of a method for PCR detection of C. cayetanensis isolated from raspberries, basil, and mesclun lettuce and reported consistent detection of 40 or fewer oocysts in raspberries and basil but only 1,000 oocysts in lettuce. Although several studies claim to detect DNA from 10 or fewer purified oocysts by PCR (15, 18, 21), data illustrating the reliability and repeatability of the described methods are lacking. Extraction of pure, high-quality DNA from coccidian oocysts is challenging due to their tough outer wall, which is resistant to both chemical lysis and physical lysis. The age, strain, and storage conditions of oocysts may also impact the effectiveness of DNA extraction (12). Thus, demonstration of the reliability of DNA extraction and PCR detection methods, as shown here, is essential prior to the method being implemented for diagnostic and food safety surveys and investigations.
Matrices typically presented for oocyst testing include feces, water, and fresh produce that are known to contain PCR inhibitors. An earlier study found that PCR detection of Cryptosporidium oocysts in environmental water samples is more sensitive if the oocysts are purified using IMS prior to DNA extraction (10). Since an IMS method for Cyclospora is not available, we have relied on simple flotation techniques to isolate and concentrate the oocysts from produce washes and fecal samples. While flotation techniques have been used with some success for isolation in samples with high numbers, they lack sensitivity and are not effective in completely separating the oocysts from the sample matrix, thus resulting in an impure sample. The DNA extraction method and PCR assay developed here are highly sensitive and reliable for detection of purified Cyclospora oocysts, but sensitivity was reduced in the presence of basil wash sediment. The successful routine use of IMS-fluorescent antibody and IMS-PCR methods for detection of Cryptosporidium in water suggests that IMS purification combined with our developed DNA extraction method and PCR assay would be highly suitable for sensitive Cyclospora detection in environmental samples.
The improved band intensity observed for PCR amplicons from 10- and 1-oocyst aliquots compared to the 100-oocyst aliquot is likely due to the lower volume of AE buffer (20 μl) used when eluting the DNA from the QIAamp DNA microkit columns, resulting in a higher concentration of DNA. According to the manufacturer, the microcolumns have a lower DNA binding capacity, thus allowing for improved elution efficiency when DNA concentrations are low. The DNeasy blood and tissue kit columns have a higher DNA binding capacity, and elution efficiency may be higher with larger elution volumes. Preliminary tests revealed that the QIAamp DNA microkit was more reliable for extraction of low numbers of oocysts: therefore, we recommend that the QIAamp DNA microkit be used when extracting DNA from fewer than 100 oocysts; the DNeasy blood and tissue kit can be used successfully for extracting from greater than 100 oocysts. Band intensity was not greater for PCR amplicons from 10- and 1-oocyst aliquots in basil wash sediment, which is likely due to the reduced sensitivity caused by the presence of inhibitors in plant material.
The use of CCITS2-F and CCITS2-R primers resulted in a high degree of specificity for C. cayetanensis based on the species they were tested against. Since Cyclospora is an emerging parasite and cannot be cultured in vitro, there is a very limited amount of Cyclospora sp. sequence information available in GenBank, especially for the ITS-2 rDNA region. Also, isolates of non-human-derived Cyclospora are difficult to obtain. Therefore, further specificity testing is required to ensure that the primers are able to differentiate C. cayetanensis from the non-human-derived Cyclospora. However, the primers developed here showed no cross-reactivity when tested against DNA from five Eimeria species which are closely related to Cyclospora (17). Previously developed molecular methods for detection of Cyclospora have relied on RFLP (7), multiplex PCR (14), or sequencing to differentiate PCR amplicons generated from Eimeria DNA since they are similar in size to those of Cyclospora spp. We recommend that PCR products generated from diagnostic samples using the CCITS2-F and CCITS2-R primers be sequenced to verify the identity of the template DNA.
Environmental samples such as produce washes contain an unpredictable array of DNA templates that may potentially cross-react to generate spurious PCR products, despite efforts to design highly specific primers. We have demonstrated the specificity of the developed CCITS2-F/CCITS2-R primers for Cyclospora DNA when tested against DNA from other related coccidia. However, when DNA was extracted from Cyclospora oocysts spiked into concentrated basil wash sediment without any selective purification, such as IMS, spurious PCR products were sometimes produced. These products were easily distinguished from Cyclospora DNA based on amplicon size and by sequencing the excised 116-bp bands. In some cases, both Cyclospora PCR products (116 bp) and spurious environmental PCR products (ranging in size from 200 to 400 bp) were observed in the same sample, indicating that the spurious products are not likely being generated preferentially with a loss of sensitivity.
The methods described here for isolation and detection of C. cayetanensis oocysts are simple, reliable, efficient, specific, and sensitive and therefore have potential for application in laboratory diagnosis and food safety surveys and investigations, including those related to contaminated fresh produce. However, further validation using larger numbers and additional sample matrices is necessary.
Acknowledgments
We gratefully acknowledge Jeevan B. Sherchand (Department of Clinical Microbiology, Tribhuvan University, Maharajgunj Campus, Kathmandu, Nepal) for providing the fecal samples with Cyclospora oocysts, Janet E. Hill (Department of Veterinary Microbiology, Western College of Veterinary Medicine, University of Saskatchewan) for support with primer design and sequence analysis, Sarah Parker (Department of Large Animal Clinical Sciences, Western College of Veterinary Medicine, University of Saskatchewan) for assistance with the design of the validation plan, and Ian Patterson and Robyn Ostrander for valuable technical assistance in the laboratory.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Blans, M. C. A., B. U. Ridwan, J. J. Verweij, M. Rozenberg-Arska, and J. Verhoef. 2005. Cyclosporiasis outbreak, Indonesia. Emerg. Infect. Dis. 11:1453-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook, N., C. A. Paton, N. Wilkinson, R. A. B. Nichols, K. Barker, and H. V. Smith. 2006. Towards standard methods for the detection of Cryptosporidium parvum on lettuce and raspberries. Part 1. Development and optimization of methods. Int. J. Food Microbiol. 109:215-221. [DOI] [PubMed] [Google Scholar]
- 3.da Silva, A. J., F. J. Bornay-Llinares, I. N. S. Moura, S. B. Slemenda, J. L. Tuttle, and N. J. Pieniazek. 1999. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 4:57-64. [DOI] [PubMed] [Google Scholar]
- 4.Doller, P. C., K. Dietrich, N. Filipp, S. Brockmann, C. Dreweck, R. Vonthein, C. Wagner-Wiening, and A. Wiedenmann. 2002. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg. Infect. Dis. 8:992-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajadhar, A. A. 1994. Host specificity studies and oocyst description of a Cryptosporidium sp. isolated from ostriches. Parasitol. Res. 80:316-319. [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt, B. L. 2007. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 31:1040-1057. [DOI] [PubMed] [Google Scholar]
- 7.Jinneman, K. C., J. H. Wetherington, W. E. Hill, A. M. Adams, J. M. Johnson, B. J. Tenge, D.-L. Dang, R. L. Manger, and M. M. Wekell. 1998. Template preparation for PCR and RFLP of amplification products for the detection and identification of Cyclospora sp. and Eimeria sp. oocysts directly from raspberries. J. Food Prot. 61:1497-1503. [DOI] [PubMed] [Google Scholar]
- 8.Kansouzidou, A., C. Charitidou, T. Varnis, N. Vavatsi, and F. Kamaria. 2004. Cyclospora cayetanensis in a patient with travelers’ diarrhea: case report and review. J. Travel Med. 11:61-63. [DOI] [PubMed] [Google Scholar]
- 9.Levine, N. D. 1985. Laboratory diagnosis of protozoan infections, p. 365-386. In N. D. Levine (ed.), Veterinary protozoology. Iowa State University Press, Ames.
- 10.Lowery, C. J., J. E. Moore, B. C. Millar, D. P. Burke, K. A. J. McCorry, E. Crothers, and J. S. G. Dooley. 2000. Detection and speciation of Cryptosporidium spp. in environmental water samples by immunomagnetic separation, PCR, and endonuclease restriction. J. Med. Microbiol. 49:779-785. [DOI] [PubMed] [Google Scholar]
- 11.Mansfield, L. S., and A. A. Gajadhar. 2004. Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Vet. Parasitol. 126:73-90. [DOI] [PubMed] [Google Scholar]
- 12.Nichols, R. A. B., and H. V. Smith. 2004. Optimization of DNA extraction and molecular detection of Cryptosporidium oocysts in natural mineral water sources. J. Food Prot. 67:524-532. [DOI] [PubMed] [Google Scholar]
- 13.Olivier, C., S. van de Pas, P. W. Lepp, K. Yoder, and D. A. Relman. 2001. Sequence variability in the first internal transcribed spacer region within and among Cyclospora species is consistent with polyparasitism. Int. J. Parasitol. 31:1475-1487. [DOI] [PubMed] [Google Scholar]
- 14.Orlandi, P. A., L. Carter, A. M. Brinker, A. J. da Silva, D.-M. Chu, K. A. Lampel, and S. R. Monday. 2003. Targeting single-nucleotide polymorphisms in the 18S rRNA gene to differentiate Cyclospora species from Eimeria species by multiplex PCR. Appl. Environ. Microbiol. 69:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlandi, P. A., and K. A. Lampel. 2000. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J. Clin. Microbiol. 38:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pape, J. W., R. I. Verdier, M. Boncy, J. Boncy, and W. D. Johnson. 1994. Cyclospora infection in adults infected with HIV: clinical manifestations, treatment, and prophylaxis. Ann. Intern. Med. 121:654-657. [DOI] [PubMed] [Google Scholar]
- 17.Relman, D. A., T. M. Schmidt, A. A. Gajadhar, M. Sogin, J. Cross, K. Yoder, O. Sethabutr, and P. Echeverria. 1996. Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 173:440-445. [DOI] [PubMed] [Google Scholar]
- 18.Shields, J. M., and B. H. Olson. 2003. PCR-restriction fragment length polymorphism method for detection of Cyclospora cayetanensis in environmental waters without microscopic confirmation. Appl. Environ. Microbiol. 69:4662-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele, M., S. Unger, and J. Odumeru. 2003. Sensitivity of PCR detection of Cyclospora cayetanensis in raspberries, basil, and mesclun lettuce. J. Microbiol. Methods 54:277-280. [DOI] [PubMed] [Google Scholar]
- 20.Turk, M., M. Turker, M. Ak, B. Karaayak, and T. Kaya. 2004. Cyclosporiasis associated with diarrhoea in an immunocompetent patient in Turkey. J. Med. Microbiol. 53:255-257. [DOI] [PubMed] [Google Scholar]
- 21.Varma, M., J. D. Hester, F. W. Schaefer, M. W. Ware, and H. D. A. Lindquist. 2003. Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. J. Microbiol. Methods 53:27-36. [DOI] [PubMed] [Google Scholar]