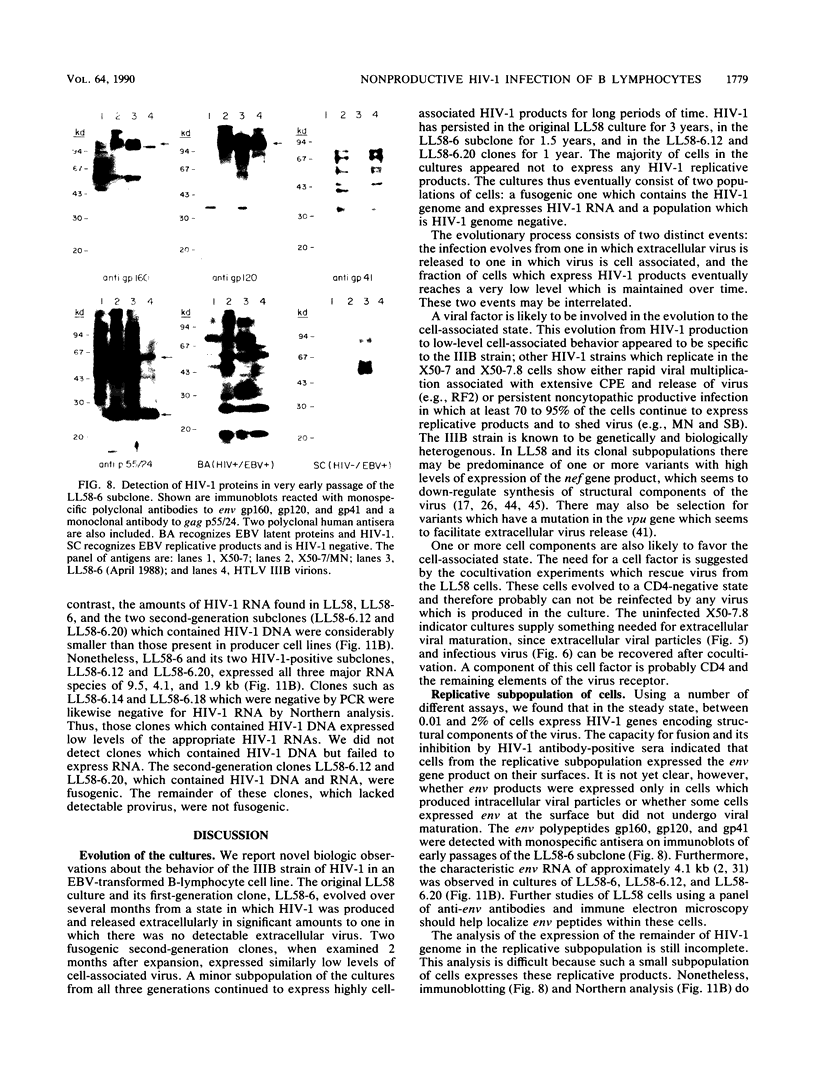
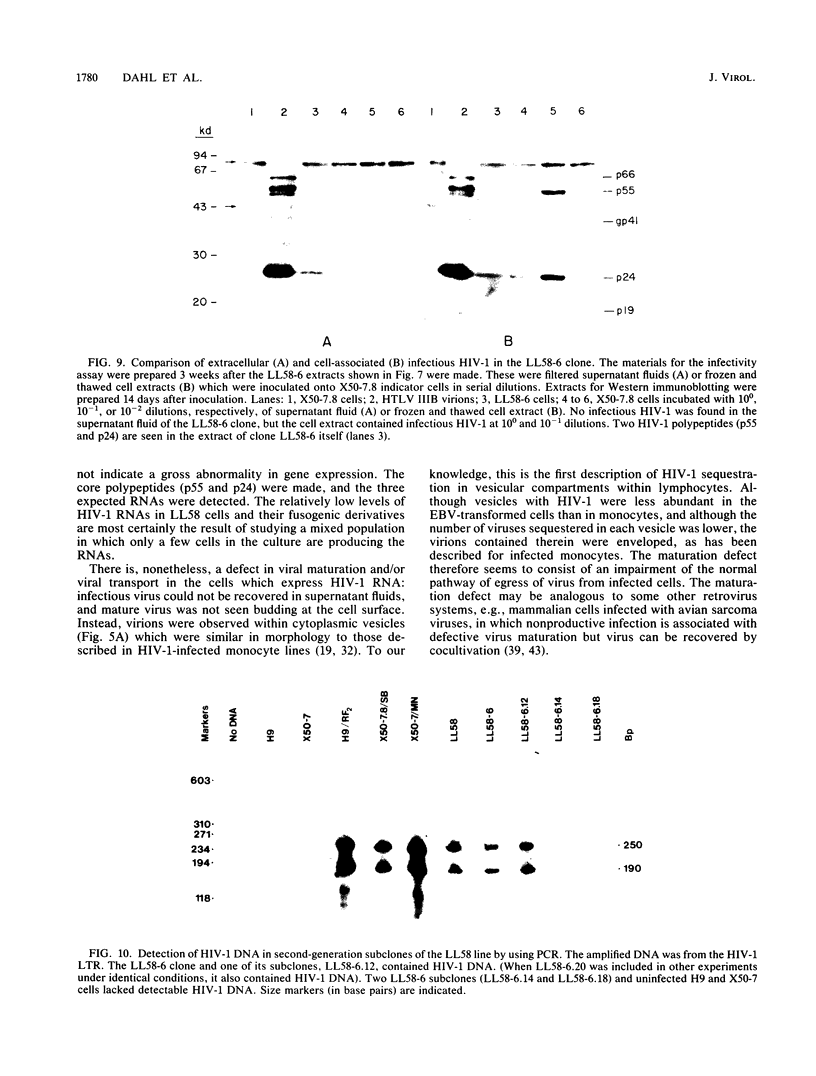
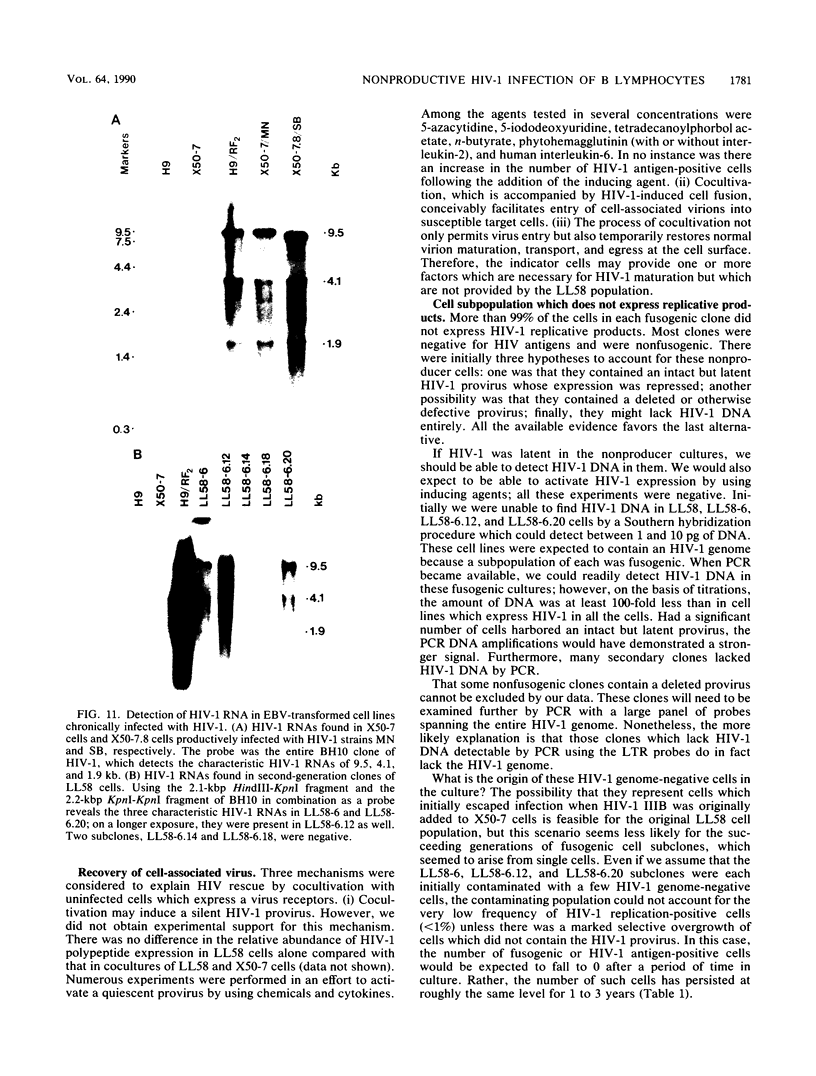
Abstract
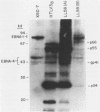
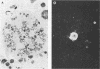
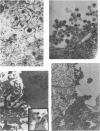
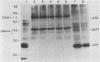
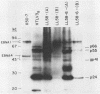
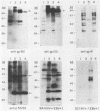
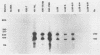
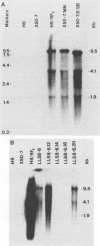
We have studied the interaction of different strains of human immunodeficiency virus type 1 (HIV-1) with an Epstein-Barr virus-transformed human B-lymphocyte line, X50-7. Previously we found that some HIV-1 strains replicated rapidly and were exclusively cytolytic; others induced persistent noncytopathic infection associated with continued shedding of extracellular virus (K. Dahl, K. Martin, and G. Miller, J. Virol. 61:1602-1608, 1987). We now describe a third form of cell-virus relationship in which infection by strain IIIB is maintained in a highly cell-associated state in a small subpopulation (less than 2%) of X50-7 cells. Neither viral subcomponents nor infectious virus was detectable in culture supernatants; however, the carrier lines were fusogenic and HIV-1 could be recovered following prolonged cocultivation with susceptible cells. In these chronic carrier cultures, virions were not seen budding at the cell surface, but a few were found within cytoplasmic vesicles. HIV-1 infection of first- and second-generation cell subclones of the carrier cell line rapidly evolved from a productive to a cell-associated state. There were low levels of HIV DNA, and RNA in the fusogenic secondary clones, but most clones lacked HIV-1 DNA, failed to express HIV-1 RNA, and exhibited no properties associated with HIV-1 infection. The experiments indicate that HIV-1 can be sequestered in human B lymphocytes. The cell cloning experiments introduce the possibility that the HIV-1 provirus may be lost from some lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiman W. A., Eastman R., Martin K., Katz B. Z., Rubinstein A., Pitt J., Pahwa S., Miller G. Opportunistic lymphoproliferations associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet. 1985 Dec 21;2(8469-70):1390–1393. doi: 10.1016/s0140-6736(85)92557-7. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Gallo R. C., Hahn B. H., Shaw G. M., Popovic M., Salahuddin S. Z., Wong-Staal F. Homology of genome of AIDS-associated virus with genomes of human T-cell leukemia viruses. Science. 1984 Aug 31;225(4665):927–930. doi: 10.1126/science.6089333. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tosato G. Defective regulation of Epstein-Barr virus infection in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related disorders. N Engl J Med. 1986 Apr 3;314(14):874–879. doi: 10.1056/NEJM198604033141403. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Chiodi F., Fuerstenberg S., Gidlund M., Asjö B., Fenyö E. M. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987 Apr;61(4):1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. H., Weller I., Iliescu V., Wara D. W. Epstein-Barr (EB) virus infection in homosexual men in London. Br J Vener Dis. 1984 Aug;60(4):258–264. doi: 10.1136/sti.60.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Bresser J., Stevenson M., Sakai K., Evinger-Hodges M. J., Volsky D. J. Susceptibility of human glial cells to infection with human immunodeficiency virus (HIV). FEBS Lett. 1987 Mar 9;213(1):138–143. doi: 10.1016/0014-5793(87)81479-5. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ratner L., Mitsuya H., Marselle L. M., Harper M. E., Broder S., Gallo R. C., Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3' region and markedly reduced cytopathic effects. Science. 1986 Aug 8;233(4764):655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Folks T., Powell D. M., Lightfoote M. M., Benn S., Martin M. A., Fauci A. S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986 Feb 7;231(4738):600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Grogan E. A., Enders J. F., Miller G. Trypsinized placental cell cultures for the propagation of viruses and as "feeder layers". J Virol. 1970 Mar;5(3):406–409. doi: 10.1128/jvi.5.3.406-409.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Levine A. M., Gill P. S., Meyer P. R., Burkes R. L., Ross R., Dworsky R. D., Krailo M., Parker J. W., Lukes R. J., Rasheed S. Retrovirus and malignant lymphoma in homosexual men. JAMA. 1985 Oct 11;254(14):1921–1925. [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Cheng-Mayer C., Levy J. A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Monroe J. E., Calender A., Mulder C. Epstein-Barr virus-positive and -negative B-cell lines can be infected with human immunodeficiency virus types 1 and 2. J Virol. 1988 Sep;62(9):3497–3500. doi: 10.1128/jvi.62.9.3497-3500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Hunter E. A., Gonda M. A., Sturzenegger S., Markham P. D., Gallo R. C. HTLV-III infection of EBV-genome-positive B-lymphoid cells with or without detectable T4 antigens. Int J Cancer. 1987 Feb 15;39(2):198–202. doi: 10.1002/ijc.2910390213. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Sirianni M. C., Rossi P., Scarpati B., Ragona G., Seminara R., Bonomo G., Aiuti F. Immunological and virological investigation in patients with lymphoadenopathy syndrome and in a population at risk for acquired immunodeficiency syndrome (AIDS), with particular focus on the detection of antibodies to human T-lymphotropic retroviruses (HTLV III). J Clin Immunol. 1985 Jul;5(4):261–268. doi: 10.1007/BF00929461. [DOI] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Martin M. A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988 Sep 2;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Sumaya C. V., Boswell R. N., Ench Y., Kisner D. L., Hersh E. M., Reuben J. M., Mansell P. W. Enhanced serological and virological findings of Epstein-Barr virus in patients with AIDS and AIDS-related complex. J Infect Dis. 1986 Nov;154(5):864–870. doi: 10.1093/infdis/154.5.864. [DOI] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Miller G. Recovery of Epstein-Barr virus from nonproducer neonatal human lymphoid cell transformants. Virology. 1979 Jun;95(2):351–358. doi: 10.1016/0042-6822(79)90490-2. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L., Beckstead J. A., Volberding P. A., Abrams D. I., Levine A. M., Lukes R. J., Gill P. S., Burkes R. L., Meyer P. R., Metroka C. E. Non-Hodgkin's lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 30;311(9):565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]