Abstract
Acetate, propionate, and butyrate, collectively referred to as volatile fatty acids (VFA), are considered among the most important electron donors for sulfate-reducing bacteria (SRB) and heterotrophic nitrate-reducing bacteria (hNRB) in oil fields. Samples obtained from a field in the Neuquén Basin, western Argentina, had significant activity of mesophilic SRB, hNRB, and nitrate-reducing, sulfide-oxidizing bacteria (NR-SOB). In microcosms, containing VFA (3 mM each) and excess sulfate, SRB first used propionate and butyrate for the production of acetate, which reached concentrations of up to 12 mM prior to being used as an electron donor for sulfate reduction. In contrast, hNRB used all three organic acids with similar kinetics, while reducing nitrate to nitrite and nitrogen. Transient inhibition of VFA-utilizing SRB was observed with 0.5 mM nitrite and permanent inhibition with concentrations of 1 mM or more. The addition of nitrate to medium flowing into an upflow, packed-bed bioreactor with an established VFA-oxidizing SRB consortium led to a spike of nitrite up to 3 mM. The nitrite-mediated inhibition of SRB led, in turn, to the transient accumulation of up to 13 mM of acetate. The complete utilization of nitrate and the incomplete utilization of VFA, especially propionate, and sulfate indicated that SRB remained partially inhibited. Hence, in addition to lower sulfide concentrations, an increase in the concentration of acetate in the presence of sulfate in waters produced from an oil field subjected to nitrate injection may indicate whether the treatment is successful. The microbial community composition in the bioreactor, as determined by culturing and culture-independent techniques, indicated shifts with an increasing fraction of nitrate. With VFA and sulfate, the SRB genera Desulfobotulus, Desulfotignum, and Desulfobacter as well as the sulfur-reducing Desulfuromonas and the NR-SOB Arcobacter were detected. With VFA and nitrate, Pseudomonas spp. were present. hNRB/NR-SOB from the genus Sulfurospirillum were found under all conditions.
The injection of surface and produced waters into oil fields to sustain reservoir pressure (30, 32) is frequently accompanied by souring (increased sulfide concentrations) due to the activity of sulfate-reducing bacteria (SRB). SRB derive energy for growth by coupling the oxidation of oil organics in formation waters with the reduction of sulfate, present in the injection water, to sulfide. Approximately 70% of water-flooded reservoirs worldwide have turned sour, and the sulfur content of crude oils appears to have increased significantly over the past 10 to 20 years (11). Oil fields in Argentina conform to this worldwide trend, with H2S appearing in produced gas or following the breakthrough of injection water. This applies not only to offshore fields injected with seawater but also to reservoirs on land subjected to produced-water reinjection (PWRI), the process of injecting a mixture of produced water and fresh water (8). Increased H2S concentrations reduce the sales value of produced oil and gas and increase operating costs, as building H2S removal facilities or upgrading the field infrastructure to sour service can require substantial investment.
Although SRB can use a variety of organic electron donors, including low-molecular-weight aliphatic and aromatic hydrocarbons, alcohols, and carboxylic acids (5), volatile fatty acids (VFA; a mixture of acetate, propionate, and butyrate) are considered important electron donors in oil fields. VFA can originate from the oil phase and from biogeochemical processes. In diagenetic environments with temperatures below 85°C, formate, acetate, propionate, and butyrate are key intermediates in the anaerobic degradation of organic matter (22). Although their concentrations in low-temperature sediments are typically low (<15 μM) due to rapid rates of microbial consumption compared to their accumulation rates (7, 35), high concentrations of acetate (1,500 mg/liter) and of total carboxylic anions (4,000 mg/liter) have been reported in high-temperature sediments devoid of microbial activity (3, 7, 12). Therefore, restricted microbial activity in deeper hydrothermal metamorphic environments (28) allows the inflow of carboxylic acids into upper low-temperature horizons (4).
The sulfate used by SRB in oil fields often originates from the injection water. Sulfate limitation (when fresh water is injected), lack of other nutrients (e.g., phosphate), or poor physical conditions for growth (e.g., high temperature or the biocidal action of oil components) may all cause the zone of sulfide production to be located near the injection well bore (30). Established SRB activity can be controlled by the injection of nitrate (30), which stimulates resident heterotrophic nitrate-reducing bacteria (hNRB) and nitrate-reducing, sulfide-oxidizing bacteria (NR-SOB), collectively referred to as NRB. The mechanisms of control include the production of nitrite, which is a strong SRB inhibitor (14), and the competition of hNRB and SRB for the same oil organics (15, 18). The ability of the latter two groups to compete for VFA has not been examined in any detail. Previous bioreactor experiments have used lactate as the single electron donor (16, 17), in which case the two groups compete, by definition, for the same carbon and energy source. VFA consists of three components and is a physiologically more relevant electron donor for SRB and hNRB in oil fields. The time dependence of the use of and the competition for VFA components in microcosm and bioreactor configurations are evaluated in the present study.
MATERIALS AND METHODS
Source of samples.
Samples were obtained from a field in the Neuquén Basin, western Argentina (see Fig. S1 in the supplemental material). The field is composed of several structural blocks, which have gas caps of different sizes and oil legs in steeply dipping flanks. It was discovered in 1989, and production is mainly from siliciclastic sands from the Huitrin Formation (Mbr. Troncoso Inferior) deposited during the Cretaceous period. The porosity is 17% (vol/vol), and the mean permeability is 80 mD (approximately 0.079 μm2). The average production depths are 2,000 m below ground level from a net pay zone (the oil-containing layer) of 55 to 60 m. The in situ reservoir temperature of this zone is 65 to 70°C. An evaporitic deposit overlays the Troncoso Inferior, suggesting that the basin became isolated from Pacific waters and underwent desiccation in a semiarid or arid climate. Well-connected fluvial channel bodies trending northwestward provide a relatively homogeneous sheet-like sandstone in the layers of interest (9). Water injection started in 2000 and oil of 35° API gravity is currently produced by PWRI, by injecting a mixture of produced water and fresh water. An increase in the H2S content of produced gas has been detected, and this has been attributed to reservoir SRB. Samples (Table 1) were obtained in October 2005 for microbiological investigation to determine whether nitrate injection might be a possible technology for sulfide removal. The ion compositions of injection and produced waters are presented in Table 2. Samples were collected into 1,000-ml wide-mouth Nalgene high-density polyethylene bottles (Nalge Nunc Int.) and transferred to the laboratory within 2 weeks. Upon arrival, they were stored in an anaerobic hood (5% [vol/vol] H2, 10% CO2, and the balance N2) and used to start enrichment cultures.
TABLE 1.
Samples obtained from a field in the Neuquén Basin, western Argentina
| Sample | Source of sample | Temp (°C) | Type of sample or source |
|---|---|---|---|
| Ar1 | Injector | 28-35 | Clear water |
| Ar2 | Water treatment plant | 30-40 | Clear water |
| Ar3 | Produced water | 50-60 | Water/oil mixture |
| Ar4 | Injector | 28-35 | Clear water |
| SRB consortium | Combined Ar1, Ar2, Ar3, and A4 enrichment in MBSM-2 | 30 | This study |
TABLE 2.
Characteristics of injection and produced waters from a field in the Neuquén Basin, western Argentinaa
| Cation, anion, or characteristic | Injection water | Produced water |
|---|---|---|
| Cations | ||
| Na+ | 18,781 | 34,759 |
| Ca2+ | 951 | 1,766 |
| Mg2+ | 238 | 384 |
| Ba2+ | 0.00 | 0.00 |
| Fe2+ | 2.80 | 4.50 |
| K+ | 0.00 | 0.00 |
| Total cations | 19,973 | 36,915 |
| Anions | ||
| Cl− | 31,947 | 63,371 |
| SO42− | 1,833 | 2,749 |
| HCO3− | 503 | 892 |
| Total anions | 34,283 | 67,014 |
| Total sulfide | 4.40 | 4.50 |
| Total dissolved solids | 54,256 | 103,929 |
| Total hardness (as CaCO3) | 3,352 | 5,989 |
| pH | 7.1 | 7.3 |
| Temperature (°C) | 28-35 | 60-65 |
All values are given in mg/liter, except for the pH and temperature values.
Media, cultivation techniques, and growth conditions.
Marine basic salts medium-1 (MBSM-1) was specially formulated for the study of microbial activity in saline samples from oil reservoirs (Table 3). To avoid significant precipitation, solutions A and B were autoclaved separately and were mixed afterward in a dispensing system (Glasgerätebau Ochs GmbH, Germany) under a 90% (vol/vol) N2, 10% (vol/vol) CO2 atmosphere as described by Widdel and Bak (36). Prior to dispensing, bicarbonate, trace elements, and selenate/tungstate stock solutions were added (Table 3) and the pH was adjusted to 7.4. For microcosm studies, anoxic medium (100 ml) was dispensed aseptically into 120-ml serum bottles, which were closed with butyl rubber stoppers and crimped with aluminum rings. MBSM-2 (Table 3), which gave less precipitation, was designed for use in bioreactor experiments and in subcultures from the bioreactor. This medium was put together, as described for MBSM-1. SRB MBSM-1 contained 20 mM Na2SO4 and 3 mM VFA (3 mM each of sodium acetate, sodium propionate, and sodium butyrate). VFA were added from 1 M anoxic, sterile stock solutions of pH 7, prepared according to the method of Widdel and Bak (36). The SRB medium was prereduced with 1 mM Na2S, added from a 1 M stock solution filtered through a 0.2-μm Filtropur S filter (Sarstedt, Germany). hNRB MBSM-1 contained 20 to 35 mM NaNO3 and 3 mM VFA, whereas NR-SOB MBSM-1 contained 20 mM NaNO3 and 10 mM HS− (added from the 1 M Na2S stock). The medium was inoculated by adding 5 or 10% (vol/vol) inoculum. The experiments were performed in duplicate at room temperature (23°C) and at 60°C. Lactate (28 mM) was also used as an electron donor for the cultivation of SRB and hNRB in some experiments.
TABLE 3.
Composition of MBSM-1 and MBSM-2a
| Ingredient | Amount in:
|
|
|---|---|---|
| MBSM-1b | MBSM-2b | |
| Solution A | ||
| NaCl | 25.0 | 25.0 |
| MgCl2·6H2O | 1.8 | 0.4 |
| CaCl2·2H2O | 3.0 | 0.6 |
| Resazurine, 1% (wt/vol) | 3-4 drops | 3-4 drops |
| H2O | 570 ml | |
| Solution B | ||
| NH4Cl | 0.3 | 0.3 |
| KH2PO4 | 0.2 | 0.2 |
| KCl | 0.5 | 0.5 |
| H2O | 400 ml | 970 ml |
| Further additions | ||
| NaHCO3 (1 M) | 30 ml | 30 ml |
| Trace elementsc (1 ml) | ||
| Selenate/tungstatec (1 ml) | ||
All amounts are in g, except where stated otherwise.
For MBSM-1, solutions A and B were autoclaved separately and then combined; the further additions were then added. For MBSM-2, solution A-B was autoclaved; the further additions were then added.
See reference 36.
Effect of nitrite on sulfide production by SRB.
SRB enrichments of samples Ar1, Ar2, Ar3, and Ar4 in MBSM-2 with 20 mM sulfate and 3 mM VFA were transferred (5% inocula) several times and were then combined into a single bacterial consortium by transferring 2% (vol/vol) of each log-phase enrichment into the same medium. This SRB consortium was used to study the effect of nitrite on sulfide production by SRB and in bioreactor experiments. For the former, 5% (vol/vol) of the SRB consortium was inoculated into MBSM-2, and when cultures reached log phase, sterile NaNO2 was added to achieve final concentrations of 0.5 to 4 mM. Each experiment was repeated at least once. The concentrations of nitrite, sulfide, and VFA were monitored as a function of time.
Bioreactor setup and startup.
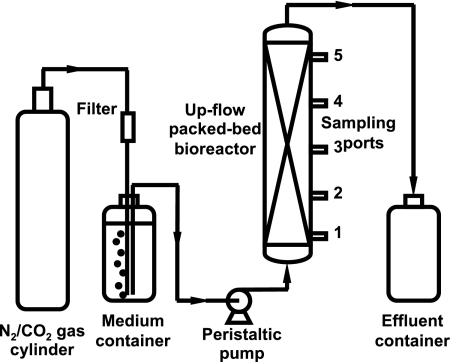
An upflow, packed-bed bioreactor, as described by Hubert et al. (16), was used (Fig. 1). The bioreactor was packed with white quartz sand with particle size 50 to 70 mesh (Sigma-Aldrich, Germany) to provide a matrix for biofilm establishment. The sand was washed once with 0.5 M hydrochloric acid and twice with deionized water and then dried at 105°C. To remove residual air from the column, nitrogen gas was introduced continuously from the bottom at a low flow rate during packing. A polymeric mesh pad was placed at the bottom of the bioreactor column to restrain the sand particles. Prior to the beginning of the experiments, the assembled bioreactor was autoclaved for 30 min at 120°C. The sterilized medium and effluent bottles were connected with preautoclaved, clear tubing (5/16 by 1/16 in; Tygon), and the bioreactor was filled with MBSM-2 (20 mM sulfate, no organics), using a P-1 peristaltic pump (Pharmacia Biotech). After 24 h at 8 ml/h, MBSM-2 with 20 mM sulfate and 3 mM VFA was introduced. The pump was then turned off, and the bioreactor was inoculated by injecting 4 ml of the SRB consortium into each port. SRB activity was monitored as the change in the concentrations of sulfide, sulfate, acetate, propionate, and butyrate as a function of time in samples taken from each port. Sulfate reduction was complete on day 16. MBSM-2 with 20 mM sulfate and 3 mM VFA was then pumped at 0.5 ml/h, with the flow rate being gradually increased to 8 ml/h until day 20. The concentrations of sulfide, sulfate, and carboxylic acids in the inflowing medium as well as in the bioreactor effluent were also periodically checked. The bioreactors were run at room temperature (23°C).
FIG. 1.
Schematic diagram of the bioreactor setup. The bioreactor consisted of a glass column (4.8 by 64 cm) with five sampling ports at 14-cm intervals. Reprinted with permission from reference 16. Copyright 2003 American Chemical Society.
Effects of nitrate addition on souring in the bioreactor.
Once the complete reduction of sulfate was reestablished at a flow rate of 8 ml/h on day 28, 5 mM nitrate was introduced in the inflowing medium. The nitrate concentration was increased to 7.5 and then to 10 mM on days 42 and 72, respectively. After 108 days, the experiment was discontinued. A new experiment was started in which a similarly inoculated bioreactor was eluted with MBSM-2 with 3 mM VFA and 10 mM sulfate (14 days), followed by 5 mM sulfate and 5 mM nitrate (14 days), followed by 10 mM nitrate (14 days). Samples from this experiment were used primarily to determine the effect of changing the electron acceptor on community composition as determined by denaturing gradient gel electrophoresis (DGGE).
Isolation of SRB, hNRB, and NR-SOB.
A series of stoppered, anaerobic culture tubes (Bellco), containing 9 ml of anoxic MBSM-2 with appropriate additions for each specific group, was inoculated with liquid samples (1 ml) from the bioreactor in successive 10-fold dilutions. SRB and hNRB were grown in medium with 3 mM VFA and 20 mM sulfate or 20 mM nitrate, respectively. The tubes showing growth were used for further liquid culture enrichment. After two passages in the same medium, SRB cultures were then transferred into MBSM-2 with 20 mM sulfate and either 10 mM acetate, 5 mM propionate, or 2 mM butyrate. Samples from the hNRB dilution series were further enriched by repeated transfer in the same medium. NR-SOB were enriched in MBSM-2 with 20 mM nitrate and 5 mM sulfide. For colony purification, liquid culture enrichments of SRB, hNRB, or NR-SOB were streaked onto plates of the same medium solidified with 1.5% (wt/vol) Bacto-Agar (BD). The plates were incubated for 3 weeks at room temperature in steel jars (Oxoid) under a 90% (vol/vol) N2, 10% (vol/vol) CO2 atmosphere. Hydrogen sulfide for the growth of NR-SOB was also generated by injecting 3 ml of 2 M HCl in an open Falcon tube (BD), containing 3 ml of 1 M Na2S·9H2O, positioned in the jar. Single colonies were inoculated into stoppered, anaerobic tubes containing 10 ml MBSM-2 with 20 mM nitrate and 5 mM sulfide for NR-SOB and substrates as in the plating media for SRB and hNRB. Following growth, these were transferred periodically to maintain the cultures.
DNA extraction.
Enrichments of VFA-oxidizing SRB as well as of SRB pure cultures were centrifuged in 30-ml aliquots for 20 min at 4°C and 12,100 × g. The cell pellets were resuspended in 1 ml 0.15 M NaCl and 0.1 M EDTA (pH 8) and stored at −20°C. Genomic DNA was extracted and purified by methods described elsewhere (34) but modified to include one or more freeze-thaw steps. For the isolation of DNA from the bioreactor, 5-ml samples were taken from the sampling ports, or the effluent was collected on ice for 12 h. The samples were centrifuged and processed as described for the enrichments. Four replicate samples were taken approximately every 24 h for each of the bioreactor conditions (MBSM-2 with 3 mM VFA, containing 10 mM sulfate, 5 mM sulfate and 5 mM nitrate, or 10 mM nitrate). DNA extracted from these samples was pooled prior to PCR amplification.
PCR amplification of 16S rRNA genes.
Isolated DNA from SRB was amplified by PCR with universal bacterial primers 27F and 1389R (21) on a GeneAmp 2400 thermal cycler (Perkin-Elmer). The PCR mixture (50 μl) contained 2 pmol of reverse and forward primers, 1 unit of Taq polymerase (Qiagen), 25 ng deoxynucleoside triphosphates, 5 μl 10× buffer (Qiagen), and 10 μl Q-solution (Qiagen). The PCR included 20 cycles of 0.5 min at 95°C, 1 min at 50°C, and 4 min at 72°C, followed by a single cycle of 10 min at 72°C.
The 16S rRNA genes from hNRB and NR-SOB were amplified from whole cells using universal bacterial primers 27F and 1492R (21). Single colonies were picked from solid media and resuspended in 30 to 50 μl of TE (10 mM Tris, 0.1 mM EDTA, pH 7.4), and 10-μl aliquots were used for the PCR. Q-solution was excluded from the PCR mixture. The amplification protocol included 5 min at 94°C followed by 30 cycles of 0.75 min at 92°C, 1 min at 48°C, and 2 min at 72°C.
The PCR amplification of pooled DNA isolated from the bioreactor for DGGE was conducted with bacterial primers 27f-GC (5′-ccgcgccgcccggcggcggggcggggcgggggCAGAGTTTGATCCTGGCTCAG-3′; the lowercase letters indicate the GC clamp) and 534r (23). The PCR mixture (50 μl) was the same as described for hNRB. The amplification conditions were 5 min at 94°C, followed by 25 cycles of 1 min at 94°C, 1.5 min at 60°C, and 1 min at 72°C. Negative controls (PCR amplifications without added DNA) were performed routinely and did not yield amplified DNA.
DGGE analysis.
DGGE was performed with a Protean II xi cell (Bio-Rad) attached to a 60°C circulating water bath and placed within an 80-liter aquarium filled with deionized water and kept at 60°C with an immersion circulator. The PCR product (∼300 ng) was loaded onto a 6.5% (wt/vol) acrylamide gel in 1× Tris-acetate-EDTA, with a gradient of 40 to 60% denaturant (100% being 40% [vol/vol] formamide and 7 M urea). Electrophoresis was for 16 h at 60°C and 75 V. The gels were stained for 20 min with Sybr green I (Invitrogen), and bands were visualized by exposure to UV light for 0.1 s. The prominent bands were excised, and DNA was extracted in 50 μl TE overnight at room temperature. The extraction mixtures were then centrifuged for 5 min at 13,200 rpm, and 2 μl of the supernatant was removed for the PCR reamplification using primers 27f (lacking the GC clamp) and 534r. The PCR products were purified using the QIAquick PCR purification kit (Qiagen).
Phylogenetic analysis.
The sequencing of PCR products, obtained from SRB cultures, from hNRB or NR-SOB colonies, or from excised DGGE bands, was done using an ABI Prism 377 DNA sequencer (Applied Biosystems, Inc.) at University Core DNA Services at the University of Calgary. The sequences obtained were edited using Sequence Scanner software v1.0 (Applied Biosystems) and assembled using the Staden program GAP4 (29). Homologous sequences were retrieved from GenBank using BLAST software (1).
Analytical procedures.
Aqueous sulfide concentrations were determined colorimetrically with N,N-dimethyl-p-phenylenediamine (31). Sulfate was assayed by a turbidometric method, using BaCl2 (10), or by high-pressure liquid chromatography (HPLC), using a Waters 600E HPLC instrument equipped with a Waters 423 conductivity detector and a Waters column (IC-PAK Anion HC, 4.6- by 150-mm column; Waters, Japan) equilibrated with borate/gluconate eluent at 2 ml/min. Nitrate and nitrite concentrations were determined using the same Waters 600E HPLC instrument equipped with a Gilson Holochrome UV detector or a Gilson 151 UV/VIS detector, set at 200 nm. Nitrite concentrations were also determined with sulfanilamide/n-(naphthyl)-ethylenediamine reagent (2). The concentrations of lactate, acetate, propionate, and butyrate were determined using an HPLC instrument equipped with a Waters 600E system controller and a Waters 2487 UV detector at 210 nm, using a Prevail Organic Acids 5u column (250.0 by 4.6 mm; Alltech) with a mobile phase of 85% (vol/vol) 25 mM KH2PO4 (pH 2.5) and 15% (vol/vol) acetonitrile at 2.0 ml/min.
Nucleotide sequence accession numbers.
The sequences determined have been assigned GenBank accession numbers EU628133 to EU628158.
RESULTS
Microcosm experiments.
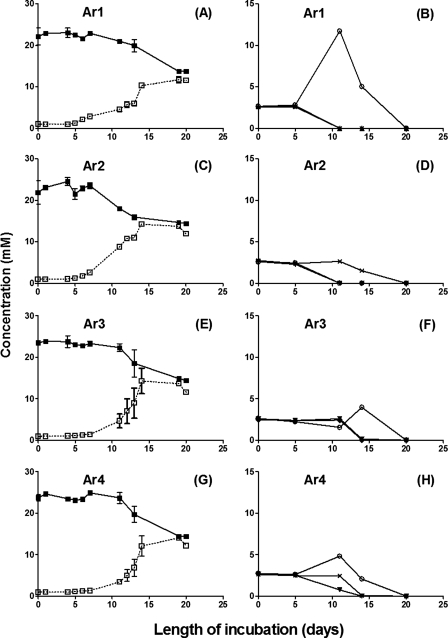
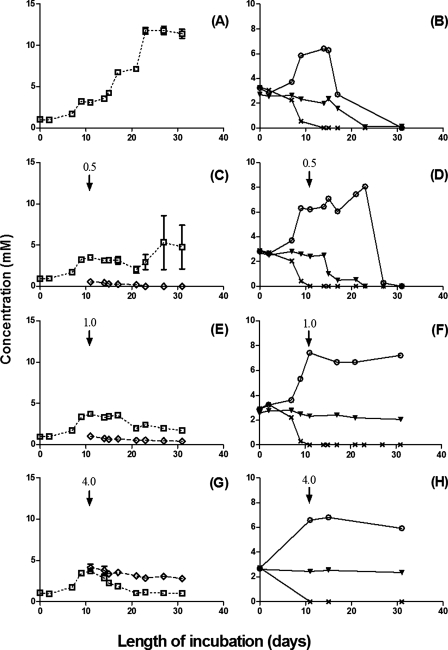
The inoculation of 10% (vol/vol) of a sample (Table 1) into MBSM-1 with supplements appropriate for each specific bacterial group did not reveal any microbial activity at 60°C after 4 weeks of incubation, indicating the absence of readily culturable thermophiles. However, the activities of mesophilic SRB, hNRB, and NR-SOB were found in all samples. The SRB in all four samples used both lactate (not shown) and VFA as electron donors for sulfate reduction. The use of VFA was complete in about 20 days (Fig. 2B, D, F, and H) and led to a transient accumulation of acetate in all incubations except those for Ar2. The strongest accumulation of acetate (up to 12 mM) was seen with Ar1 (Fig. 2B), indicating the use of VFA in two phases: (i) the oxidation of propionate and butyrate to acetate and CO2 (0 to 10 days) and (ii) the oxidation of acetate to CO2 (10 to 20 days).
FIG. 2.
Activities of VFA-oxidizing SRB in samples Ar1 (A, B), Ar2 (C, D), Ar3 (E, F), and Ar4 (G, H) from a field in the Neuquén Basin, western Argentina. Concentrations of sulfate (▪), sulfide (□), acetate (○), propionate (×), and butyrate (▾) are shown as a function of the length of incubation.
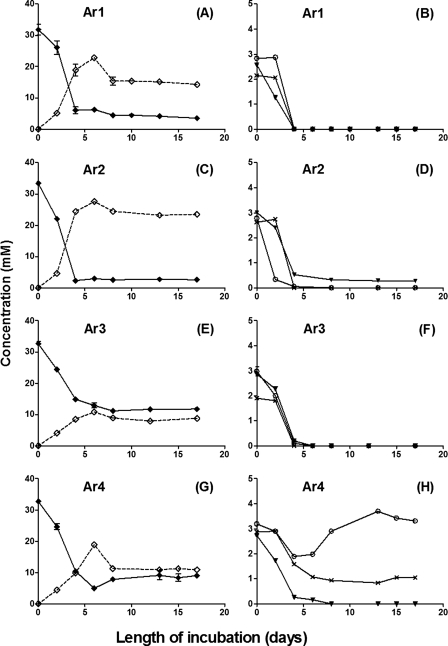
The oxidation of VFA by hNRB produced nitrite (Fig. 3A, C, E, and G) to a final concentration of 10 to 25 mM. The complete oxidation of 3 mM VFA to CO2 yields 126 mM of electrons (24 mM from 3 mM acetate, 42 mM from 3 mM propionate, and 60 mM from 3 mM butyrate), which could reduce 35 mM nitrate to 17 mM nitrite and 18 mM of N2-N (requiring 34 and 90 mM of electrons, respectively, i.e., a total of 124 mM). Hence, the high nitrite concentrations of 10 to 25 mM (Fig. 3) are caused by the high nitrate-to-VFA ratio. With the exception of sample Ar4 (Fig. 3H), the hNRB present in samples Ar1, Ar2, and Ar3 oxidized the three VFA components with very similar kinetics (Fig. 3B, D, and F). The accumulation of acetate, as found for SRB, was not seen.
FIG. 3.
Activities of VFA-oxidizing hNRB in samples Ar1 (A, B), Ar2 (C, D), Ar3 (E, F), and Ar4 (G, H) from a field in the Neuquén Basin, western Argentina. Concentrations of nitrate (⧫), nitrite (⋄), acetate (○), propionate (×), and butyrate (▾) are shown as a function of the length of incubation.
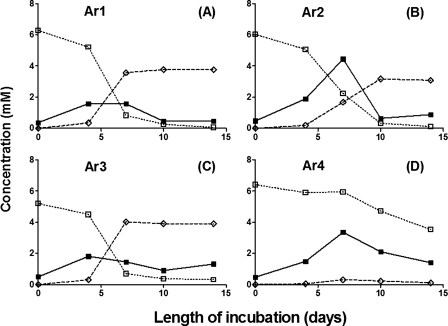
The activity of mesophilic NR-SOB was also observed in all samples. After 14 days of incubation, samples Ar1, Ar2, and Ar3 showed the complete oxidation of 6 mM of sulfide with the production of 3 to 4 mM nitrite (Fig. 4A to C). Sample Ar4 had less NR-SOB activity, oxidizing only 3 mM sulfide with the formation of 0.5 mM nitrite (Fig. 4D). Hence, sample Ar4 had similar SRB activity but lower hNRB and NR-SOB activities than samples Ar1, Ar2, and Ar3. The sulfate concentrations formed after 14 days were small relative to the concentration of sulfide oxidized, indicating the formation of sulfur and polysulfide.
FIG. 4.
Activities of NR-SOB in samples Ar1 (A), Ar2 (B), Ar3 (C), and Ar4 (D) from a field in the Neuquén Basin, western Argentina. Concentrations of sulfide (□), sulfate (▪), and nitrite (⋄) are shown as a function of the length of incubation.
Transferring 5% (vol/vol) hNRB enrichments of Ar1, Ar2, Ar3, and A4, grown in MBSM-1 with 3 mM VFA and 35 mM nitrate, into MBSM-1 with 3 mM VFA and 20 mM sulfate did not produce SRB activity (not shown). The high initial nitrite concentration of 1 to 2 mM may have prevented the growth of SRB to start. Transferring 5% (vol/vol) of the SRB enrichment into MBSM-1 with 35 mM nitrate and 3 mM VFA produced active nitrate reduction coupled with the oxidation of both residual sulfide and organic acids (not shown). Hence, the SRB enrichments retained all three microbial groups. The SRB consortium (obtained by combining SRB enrichments from Ar1, Ar2, Ar3, and Ar4) was therefore used throughout in further experiments.
Effect of nitrite on sulfide production by SRB.
The effect of adding 0.5 to 4 mM nitrite on sulfide production and VFA oxidation by the oil-field SRB consortium is shown in Fig. 5. In the absence of nitrite, the SRB consortium produced 14 mM sulfide in MBSM-2 with 3 mM VFA and 20 mM sulfate (Fig. 5A). VFA were oxidized entirely with the transient production of 6 mM acetate (Fig. 5B). The reduction of 14 mM sulfate to sulfide requires 112 mM of electrons, which can be provided by oxidizing 3 mM VFA to CO2 (126 mM of electrons). The addition of 0.5 mM nitrite inhibited sulfide production by about 50% (Fig. 5C) while slowing down the oxidation of especially acetate (Fig. 5D). Nitrite slowly disappeared in 14 weeks. After the addition of 1 or 4 mM nitrite, sulfide accumulation (Fig. 5E and G) and the consumption of butyrate and acetate (Fig. 5F and H) stopped. Propionate had already been metabolized prior to the addition of nitrite. The concentrations of sulfide and nitrite decreased slowly for 14 days after nitrite addition by an abiotic chemical reaction forming polysulfide, sulfur, and ammonia (19).
FIG. 5.
Effect of nitrite on sulfide production by an SRB consortium from a field in the Neuquén Basin, western Argentina. The concentrations of sulfide (□), nitrite (⋄), acetate (○), propionate (×), and butyrate (▾) are shown as a function of the length of incubation. The arrow (↓) indicates the time of nitrite addition. (A, B) No nitrite; (C, D) 0.5 mM nitrite; (E, F) 1 mM nitrite; (G, H) 4 mM nitrite.
Establishment of SRB consortium in the bioreactor.
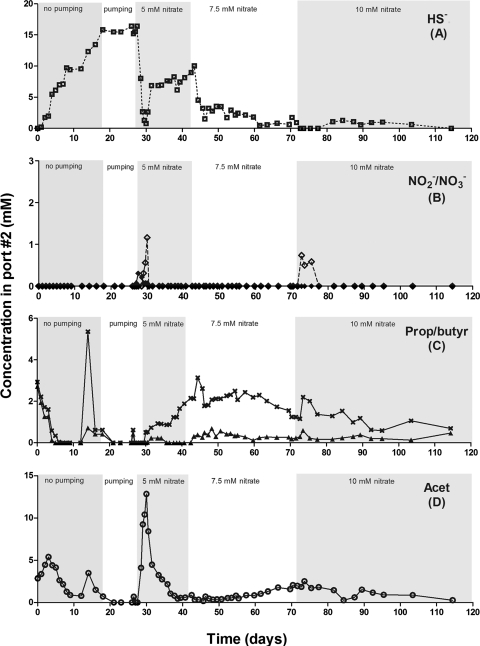
The production of sulfide started 1 day after inoculation, and within 8 days, half of the sulfate was reduced to yield 10 mM sulfide (Fig. 6, data for port 2; see also Fig. S2 [data for ports 1, 3, 4, and 5] and Fig. S3 [sulfate concentrations at all ports] in the supplemental material). Propionate and butyrate were metabolized within 5 days with the transient production of 6 to 7 mM acetate at all ports. When the flow was started and gradually increased to 8 ml/h, the reduction of 15 mM sulfate to 15 mM sulfide (75% of the added 20 mM) was achieved on day 28 from port 1 onward. The concentrations of all VFA components were zero at all ports under these conditions (Fig. 6C and D; see also Fig. S2C and D in the supplemental material), with CO2 being the expected product based on oxidation-reduction balance considerations.
FIG. 6.
Effect of nitrate addition on souring in an upflow bioreactor receiving VFA and sulfate. Concentrations at sampling port 2 are shown as a function of time for sulfide (□) (A), nitrite (⋄) and nitrate (⧫) (B), propionate (×) and butyrate (▴) (C), and acetate (○) (D). The inflowing medium contained 20 mM sulfate, 3 mM acetate, 3 mM propionate, and 3 mM butyrate and a changing nitrate concentration as indicated.
Effect of nitrate on SRB in the bioreactor.
The introduction of 5 mM nitrate led to a sharp spike of nitrite of 1 to 3 mM at most ports, with nitrate (2 mM) being observed at port 1 (Fig. 6B; see also Fig. S2B in the supplemental material). At day 30, residual sulfide concentrations of 0, 1, 5, 7, and 8 mM were observed at ports 1 to 5, respectively (from the bottom to the top of the bioreactor as shown in Fig. 1). This indicated that sulfide was being rapidly oxidized with nitrate by NR-SOB at ports 1 and 2 and that the produced nitrite inhibited sulfide production by SRB. This inhibition was apparent also from the profile of VFA concentrations, which showed the production of up to 13 mM acetate at ports 1 and 2 on day 30 with lower concentration increases to 7, 6, and 3 mM being observed at ports 3, 4, and 5, respectively (Fig. 6D; see also Fig. S2D in the supplemental material). The observed acetate production indicates that, at least initially, the conversion of propionate and butyrate to acetate was not inhibited, whereas the conversion of acetate to CO2 was strongly inhibited by nitrite (see the equations in the Discussion section). Apparently, these first conversions were less critically dependent on the reduction of sulfate to sulfide, whereas acetate oxidation was tightly linked to sulfate reduction, prior to the introduction of nitrate. The inhibition of SRB activity was never completely relieved in a subsequent 2-week period (days 30 to 42). This can be deduced from the fact that although all nitrate was permanently, completely reduced, some electron donor (especially propionate [2 mM]) remained despite the presence of significant residual sulfate concentrations throughout (see Fig. S3 in the supplemental material). The preferred use of acetate and butyrate over propionate, following nitrate addition, is consistent with the inhibition of SRB, which used acetate poorly both in the absence (Fig. 2B, F, and H and 5B) and in the presence of nitrite (Fig. 5D, F, and H).
Increasing the nitrate concentration to 7.5 mM on day 43 gave a decreased sulfide concentration of 3 mM initially to 1 mM on day 70 (Fig. 6A; see also Fig. S2A in the supplemental material). The remaining VFA concentrations were, on average, 2 mM propionate, 0.4 mM butyrate, and an increasing concentration of 0.5 to 3 mM acetate. These data do again indicate that SRB activity remained inhibited, as 10 to 15 mM sulfate was present throughout (see Fig. S3 in the supplemental material). A further increase to 10 mM nitrate on day 72 further lowered the sulfide concentrations (to 0 to 2 mM) as well as the VFA concentrations.
Microbial community analyses of bioreactor samples.
Several strains of SRB, hNRB, and NR-SOB were isolated into pure culture from bioreactor samples. Nearly complete 16S rRNA gene sequences, determined for five colony-purified hNRB (Table 4, entries 19 to 23), indicated that these belong to the genus Pseudomonas within the γ subclass of the Proteobacteria with 100% similarity to Pseudomonas putida and Pseudomonas stutzeri. An analysis of the partial 16S rRNA gene sequences for three colony-purified NR-SOB (Table 4, entries 24 to 26) indicated all to be most closely related to Sulfurospirillum sp. strain NO2B (97 to 98% similarity) within the ɛ subclass of the Proteobacteria. This strain had been previously isolated from an upflow, packed-bed bioreactor, receiving medium containing lactate, sulfate, and nitrate and inoculated with oil-field microbial consortia (16, 18). 16S rRNA gene sequencing of colony-purified SRB indicated that these belong to the genera Desulfobacter and Desulfotignum, both within the Deltaproteobacteria (Table 4, entries 11 and 12). Members of the genus Desulfobacter specialize in the use of acetate as an electron donor for sulfate reduction (5), whereas members of the genus Desulfotignum use aliphatic organic acids (20). Hence, these genera are expected in a VFA- and sulfate-containing medium.
TABLE 4.
Analysis of the bioreactor community composition by the sequencing of 16S rRNA genes isolated by cultivation or DGGE
| Entry no. | Inflowing medium or medium useda | Bioreactor sample source | Isolation methodc | Sequence length (bases)d | Organism IDe | GenBank IDe | Similarityf (%) |
|---|---|---|---|---|---|---|---|
| 1 | VFA, sulfate | port 1 | DGGE (1-1) | 432 | Desulfobotulus sp. | U85470.1 | 96 |
| 2 | VFA, sulfate | port 2 | DGGE (2-1) | 417 | Sulfurospirillum sp. strain C6 | DQ228139.1 | 99 |
| 3 | VFA, sulfate | port 2 | DGGE (2-2) | 352 | Sulfurospirillum sp. strain C6 | DQ228139.1 | 99 |
| 4 | VFA, sulfate | port 5 | DGGE (3-1) | 207 | Arcobacter sp. strain FWKO B | AY135396.1 | 96 |
| 5 | VFA, sulfate | port 5 | DGGE (3-2) | 475 | Desulfuromonas thiophila | Y11560.1 | 92 |
| 6 | VFA, sulfate | effluent | DGGE (6-1) | 461 | Desulfobotulus sp. | U85470.1 | 96 |
| 7 | VFA, sulfate | SRB consortiumb | DGGE | 485 | Desulfobacter halotolerans | Y14745.1 | 98 |
| 8 | VFA, sulfate | SRB consortiumb | DGGE | 514 | Desulfobacter latus | AJ441315.1 | 97 |
| 9 | VFA, sulfate | SRB consortiumb | DGGE | 380 | Sulfurospirillum sp. strain KW | DQ228139.1 | 98 |
| 10 | VFA, sulfate | SRB consortiumb | DGGE | 276 | Desulfobacter latus | AJ441315.1 | 96 |
| 11 | VFA, sulfate | ports | Plating medium (1-3a) | 1,354 | Desulfotignum balticum | AF418176.1 | 98 |
| 12 | VFA, sulfate | ports | Plating medium (1-1e) | 561 | Desulfobacter latus | AJ441315.1 | 98 |
| 13 | VFA, nitrate, sulfate | effluent | DGGE (9-1) | 428 | Sulfurospirillum sp. | DQ228139.1 | 99 |
| 14 | VFA, nitrate, sulfate | effluent | DGGE (9-2) | 238 | Sulfurospirillum sp. | DQ228139.1 | 100 |
| 15 | VFA, nitrate, sulfate | effluent | DGGE (10-1) | 236 | Sulfurospirillum sp. strain NO3A | AY135396.1 | 89 |
| 16 | VFA, nitrate | effluent | DGGE (13-1) | 456 | Pseudomonas sp. | DQ989211.2 | 100 |
| 17 | VFA, nitrate | effluent | DGGE (15-1) | 431 | Sulfurospirillum sp. | DQ228139.1 | 98 |
| 18 | VFA, nitrate | effluent | DGGE (15-2) | 468 | Sulfurospirillum sp. | DQ228139.1 | 99 |
| 19 | VFA, nitrate | ports | Plating medium (AG5) | 1,371 | Pseudomonas stutzeri | EU305565.1 | 100 |
| 20 | VFA, nitrate | ports | Plating medium (AG6) | 1,362 | Pseudomonas stutzeri | EU305565.1 | 100 |
| 21 | VFA, nitrate | ports | Plating medium (AG7) | 1,380 | Pseudomonas putida | DQ288952.1 | 100 |
| 22 | VFA, nitrate | ports | Plating medium (AG8) | 1,382 | Pseudomonas stutzeri | EF559249.1 | 100 |
| 23 | VFA, nitrate | ports | Plating medium (AG9) | 1,370 | Pseudomonas putida | EU305565.1 | 100 |
| 24 | VFA, nitrate | ports | Plating medium (L3-5) | 706 | Sulfurospirillum sp. strain NO2B | AY135395.1 | 97 |
| 25 | VFA, nitrate | ports | Plating medium (5-4F) | 919 | Sulfurospirillum sp. strain NO2B | AY135395.1 | 98 |
| 26 | VFA, nitrate | ports | Plating medium (L5-9) | 636 | Sulfurospirillum sp. strain NO2B | AY135395.1 | 97 |
Medium composition is described in the text.
The SRB consortium used for the inoculation of the bioreactor as described in Table 1.
The numbers in parentheses for the DGGE bands refer to the lanes and bands in Fig. 7, e.g., (13-1) refers to band 1 in lane 13. Isolate identification numbers are given in parentheses for the strains obtained by plating.
Length of the assembled sequence.
The GenBank ID numbers are the accession numbers of the closest cultivated relative or named clone identified by BLAST; the organism IDs are the names of these closest cultivated relatives or named clones.
Sequence similarity of the determined sequence and the GenBank ID.
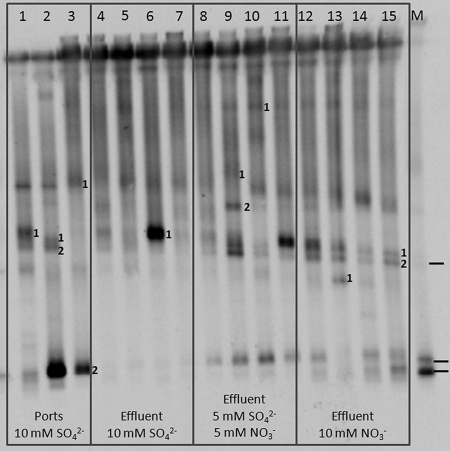
The bioreactor community was also analyzed directly by DGGE, without culturing. Banding patterns were obtained for community DNA isolated from bioreactor port and effluent samples. These were collected when the bioreactor received inflowing medium with 3 mM VFA, 10 mM SO42−, 5 mM SO42− and 5 mM NO3−, or 10 mM NO3− (Fig. 7). A separate analysis of the SRB consortium grown in MBSM-2 with 3 mM VFA and 20 mM SO42− was also done (Table 4, entries 7 to 10; gel not shown). The DGGE banding patterns changed when the electron acceptor was switched. The bands that were extracted and analyzed by PCR and sequencing are indicated in Fig. 7. Sequence identities derived for the marked bands are shown in Table 4. With just sulfate as the electron acceptor, a variety of SRB were detected, including members of the genera Desulfobotulus, Desulfuromonas, and Desulfobacter. Of these, Desulfobacter had also been identified by culturing. hNRB and NR-SOB, including Sulfurospirillum spp. and Arcobacter spp., were also detected under these conditions. Only Sulfurospirillum spp. were identified with both sulfate and nitrate as electron acceptors. However, bands in the same locations as those identified as Desulfuromonas (Fig. 7, lane 3, band 2) and Desulfobotulus (Fig. 7, lane 6, band 1) were still present at reduced intensity. When nitrate was the only electron acceptor present, Pseudomonas was readily detected (Fig. 7, lane 13, band 1), whereas this band was absent when only sulfate or sulfate and nitrate were the electron acceptors. Sulfurospirillum was also present under nitrate-only conditions (Fig. 7, lane 15, bands 1 and 2).
FIG. 7.
DGGE gel of samples from the bioreactor receiving 3 mM VFA and the following electron acceptors: 10 mM sulfate (lanes 1 to 7 [lane 1 represents port 1; lane 2, port 2; lane 3, port 5; and lanes 4 to 7, bioreactor effluent]), 5 mM sulfate and 5 mM nitrate (lanes 8 to 11, representing bioreactor effluent), or 10 mM nitrate (lanes 12 to 15, representing bioreactor effluent). The DGGE of markers (lane M) is also indicated. The markers are, from top to bottom, as follows: Thiomicrospira sp. strain CVO, Desulfovibrio vulgaris Hildenborough, and Thauera sp. strain N2. Bands tagged with a number were sequenced, as indicated in Table 4.
DISCUSSION
Mesophilic SRB, hNRB, and NR-SOB are active and readily cultivated and identified in samples obtained from a field in the Neuquén Basin, western Argentina. We were not able to cultivate thermophiles from the samples obtained. This may have been due, in part, to the fact that most of the samples were from low-temperature facilities. Sulfide production in situ is thought to occur near the injection well bore (30), because high temperatures and nutrient limitations can prevent growth elsewhere in the reservoir. The near-well bore, active zone is cooled by contact with injection water. Hence, mesophilic SRB contribute to sulfide production in situ, even in fields with a resident temperature in the thermophilic range (22, 24).
Souring control by nitrate injection involves (i) direct sulfide oxidation by NR-SOB, (ii) the inhibition of SRB with nitrite, and (iii) the competition of hNRB with SRB for oil organics. All three of these factors apply in the current study in which VFA were used as the electron donors representing oil organics. VFA may be more relevant oil organics than lactate, used previously (16), as they are frequently found in oil-field produced waters. For example, produced water from a field operated by British Petroleum contained 100 mg/liter of acetate, 50 mg/liter of propionate, and 105 mg/liter of sulfate (32). Waters produced from Ekofisk, a high-temperature North Sea oil field (19), contained 136 to 152 mg/liter of acetate, up to 14 mg/liter of propionate, up to 27 mg/liter of butyrate, and 800 to 1,100 mg/liter of sulfate, the high concentration being due to seawater injection (6). Lower VFA concentrations were reported in produced water from the Draugen field in the Norwegian sector of the North Sea with a downhole temperature of 71°C with 3 to 10 mg/liter of acetate, 1 to 11 mg/liter of propionate, less than 2 mg/liter of butyrate, and 400 to 600 mg/liter of sulfate (33). Interestingly, the field from which the samples used in the current study were obtained also had very high sulfate concentrations (Table 2), despite not being injected with seawater. Knowing the concentration of microbially degradable oil organics (VFA, volatile organic compounds, and others) is important to estimate the nitrate dose required to prevent sulfide production. In work using lactate, the required nitrate (or nitrite) dose was found to be always proportional to the lactate concentration (16). In the current study, we found that nitrate was always completely reduced, irrespective of whether 5, 7.5, or 10 mM was added, with only transient spikes of nitrite (up to 3 mM) being observed (Fig. 6B; see also Fig. S2B in the supplemental material). However, sulfate coexisted with the remaining VFA. This indicates that, differently than with lactate, the activity of hNRB and NR-SOB caused SRB to remain partially inhibited. This caused VFA and sulfate to be present in eluent produced from the bioreactor, as is sometimes observed in actual oil fields. Assuming that nitrate is reduced to nitrogen, the complete oxidation of 3 mM VFA to CO2 would require 25 mM nitrate. The fact that 10 mM appeared to be sufficient (Fig. 6A; see also Fig. S2A in the supplemental material) again indicates that SRB inhibition contributed to the lack of sulfide production. Hence in the current study, the dose required to eliminate souring was not dictated by the VFA concentration, as was the case when lactate was used.
The significant drop in sulfide concentration following nitrate addition was caused by NR-SOB activity. The NR-SOB isolated and identified in this study were phylogenetically close to Sulfurospirillum sp. strain NO2B and Arcobacter sp. strain FWKO B. Both have been isolated previously from oil fields. Arcobacter sp. strain FWKO B couples the oxidation of sulfide to sulfur with the reduction of nitrate to nitrite (13), whereas Sulfurospirillum strains have been shown to have both NR-SOB and hNRB activity (18). Hence, our data confirm the suggestion that Sulfurospirillum spp. are widespread in the formation waters of different petroleum reservoirs (18).
Both NR-SOB activity (Fig. 4) and hNRB activity (Fig. 3) produced considerable concentrations of nitrite (4 to 25 mM). Nitrite concentrations as low as 1 mM caused the permanent inhibition of SRB activity (Fig. 5F). Hence, it is not surprising that the introduction of nitrate into the bioreactor caused a temporary upset, in which transient high acetate concentrations were produced (Fig. 6D; see also Fig. S2D in the supplemental material). The accumulation of acetate indicates that SRB oxidized VFA in two stages: (i) propionate + 2H2O → acetate + CO2 + 6H+ + 6e and butyrate + 2H2O → 2 acetate + 5H+ + 4e and (ii) acetate + 2H2O → 2CO2 + 7H+ + 8e, with the electrons being used for the reduction of sulfate to sulfide. When nitrate was introduced into the bioreactor, the inhibition of SRB with nitrite affected stage ii more than stage i, causing the transient accumulation of acetate. Hence, nitrite appeared to act preferentially with acetate-oxidizing SRB, like those of the genus Desulfobacter, which is strongly inhibited by nitrite (14). The mechanisms of VFA utilization by SRB have been reviewed elsewhere (26) and have suggested reasons for acetate accumulation by complete oxidizers (SRB that convert their organic substrates to CO2).
Although most of the SRB strains identified are well-known components of oil-field consortia, the presence of Desulfotignum species has only been reported recently (25). SRB of this genus may play a significant role in oil-field environments due to their capacity to respire sulfate with formate, acetate, butyrate, other fatty acids (with the exception of propionate), and aromatic compounds (20, 25). With respect to hNRB, Pseudomonas putida and P. stutzeri have been identified in petroleum reservoirs. Oil-field pseudomonads can reduce nitrate to nitrogen and metabolize a wide spectrum of oil organics (27).
The order of VFA component oxidation by SRB and hNRB from the field in the Neuquén Basin, western Argentina, differed. SRB oxidized propionate and butyrate first, followed by the oxidation of acetate, whereas hNRB oxidized all three components simultaneously. Hence, biocompetitive exclusion applied only partially to acetate, which appeared to be a poor SRB substrate but an excellent hNRB substrate. Control of souring caused by VFA-oxidizing SRB consortia involved (i) sulfide removal by NR-SOB, (ii) the inhibition of SRB with nitrite, which they were unable to overcome, and (iii) a little contribution of competitive exclusion. Nitrate addition to the inflowing medium of the bioreactor (the injection water) caused effluent concentrations of sulfide to be lower, while those of sulfate and VFA were higher. Nitrate and nitrite were not produced. Hence, our results indicate that in addition to lower sulfide concentrations, an increase in the concentrations of VFA in the presence of sulfate in waters produced from an oil field subjected to nitrate injection may indicate whether the treatment is successful.
Supplementary Material
Acknowledgments
This work was supported by a strategic grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to G.V. with financial contributions from ConocoPhillips Company and Baker Petrolite Corporation. G.V. holds an NSERC industrial research chair, which is also supported by Commercial Microbiology Limited, the Computer Modelling Group Limited, ConocoPhillips Company, Repsol YPF SA, Saudi Aramco, Shell Canada Limited, Suncor Energy Developments, Inc., and Yara International ASA, as well as by the Alberta Energy Research Institute.
Footnotes
Published ahead of print on 23 May 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.American Public Health Association. 1992. Standard methods for the examination of wastewater, p. 439-440. American Water Works Association and Water Pollution Control Federation, Washington, DC.
- 3.Barth, T. 1991. Organic acids and inorganic ions in waters from petroleum reservoirs, Norwegian continental shelf: a multivariate statistical analysis and comparison with American reservoir formation waters. Appl. Geochem. 6:1-15. [Google Scholar]
- 4.Barth, T., and M. Riis. 1992. Interactions between organic acid anions in formation waters and reservoir mineral phases. Org. Geochem. 19:455-482. [Google Scholar]
- 5.Birkeland, N.-K. 2005. Sulfate-reducing bacteria and archaea, p. 35-54. In B. Ollivier and M. Magot (ed.), Petroleum microbiology. ASM Press, Washington, DC.
- 6.Burger, E. D., A. Vedvik, G. E. Jenneman, O. Bache, and K. Voldum. 2007. Forecasting the effect of produced water reinjection on reservoir souring in the Ekofisk field, paper 06661. 61st NACE Annu. Conf. Expo., Houston, TX.
- 7.Carothers, W. W., and Y. K. Kharaka. 1978. Aliphatic acid anions in oil-field waters: implications for origin of natural gas. AAPG Bull. 62:2441-2453. [Google Scholar]
- 8.Cavallaro, A. N., M. I. Alberdi, and G. Galliano. 2007. Overview of H2S souring gases in Argentina reservoirs: origin and migration sceneries, paper SPE 107376. SPE Latin Am. Caribbean Petroleum Eng. Conf., Buenos Aires, Argentina.
- 9.Corbett, C., V. Blekhman, B. Coca, D. Diaz, G. Selva, M. Ayala, and L. Pina. 2001. A multidiscipline approach for the development of a waterflood in the Chihuido de la Salina field, paper SPE 69562. Society of Petroleum Engineers.
- 10.Cypionka, H., and N. Pfennig. 1986. Growth yield of Desulfotomaculum orientis with hydrogen in chemostat culture. Arch. Microbiol. 143:396-399. [Google Scholar]
- 11.Elshashawi, H., and M. Hashem. 2005. Accurate measurement of the hydrogen sulfide content in formation fluid samples—case studies. Paper SPE 94707. SPE Annu. Tech. Conf. Exhibit., Dallas, TX.
- 12.Fisher, J. B. 1987. Distribution and occurrence of aliphatic acid anions in deep subsurface waters. Geochim. Cosmochim. Acta 51:2459-2468. [Google Scholar]
- 13.Gevertz, D., A. J. Telang, G. Voordouw, and G. E. Jenneman. 2000. Isolation and characterization of strains CVO and FWKO B, two novel nitrate-reducing, sulfide-oxidizing bacteria isolated from oil field brine. Appl. Environ. Microbiol. 66:2491-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene, E. A., C. Hubert, M. Nemati, G. E. Jenneman, and G. Voordouw. 2003. Nitrite reductase activity of sulphate-reducing bacteria prevents their inhibition by nitrate-reducing, sulfide-oxidizing bacteria. Environ. Microbiol. 5:607-617. [DOI] [PubMed] [Google Scholar]
- 15.Hitzman, D. O., and D. M. Dennis. 1997. New technology for prevention of sour oil and gas, p. 406-411. In Proceedings of the SPE/DOE Exploration and Production Environmental Conference. Society of Petroleum Engineers, Dallas, TX.
- 16.Hubert, C., M. Nemati, G. E. Jenneman, and G. Voordouw. 2003. Containment of biogenic sulfide production in continuous packed-bed up-flow bioreactors with nitrate or nitrite. Biotechnol. Prog. 19:338-345. [DOI] [PubMed] [Google Scholar]
- 17.Hubert, C., M. Nemati, G. Jenneman, and G. Voordouw. 2005. Corrosion risk associated with microbial souring control using nitrate or nitrite. Appl. Microbiol. Biotechnol. 68:272-282. [DOI] [PubMed] [Google Scholar]
- 18.Hubert, C., and G. Voordouw. 2007. Oil field souring control by nitrate-reducing Sulfurospirillum spp. that outcompete sulfate-reducing bacteria for organic electron donors. Appl. Environ. Microbiol. 73:2644-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaster, K. M., A. Grigoriyan, G. Jenneman, and G. Voordouw. 2007. Effect of nitrate and nitrite on two thermophilic, sulfate-reducing enrichments from an oil field in the North Sea. Appl. Microbiol. Biotechnol. 75:195-203. [DOI] [PubMed] [Google Scholar]
- 20.Kuever, J., M. Könneke, A. Galushko, and O. Drzyzga. 2001. Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov. and description of strain SaxT as Desulfotignum balticum gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 51:171-177. [DOI] [PubMed] [Google Scholar]
- 21.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, United Kingdom.
- 22.Magot, M. 2005. Indigenous microbial communities in oil fields, p. 21-33. In B. Ollivier and M. Magot (ed.), Petroleum microbiology. ASM Press, Washington, DC.
- 23.Muyzer, G., E. C. De Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazina, T. N., A. A. Grigor'yan, N. M. Shestakova, T. L. Babich, V. S. Ivoilov, Q. Feng, F. Ni, J. Wang, Y. She, T. Xiang, Z. Luo, S. S. Belyaev, and M. V. Ivanov. 2007. Microbiological investigations of high-temperature horizons of the Kongdian petroleum reservoir in connection with field trial of a biotechnology for enhancement of oil recovery. Microbiology 76:287-296. [PubMed] [Google Scholar]
- 25.Ommedal, H., and T. Torsvik. 2007. Desulfotignum toluenicum sp. nov., a novel toluene-degrading, sulphate-reducing bacterium isolated from an oil-reservoir model column. Int. J. Syst. Evol. Microbiol. 57:2865-2869. [DOI] [PubMed] [Google Scholar]
- 26.Rabus, R., T. A. Hansen, and F. Widdel. 2006. Dissimilatory sulfate- and sulfur-reducing prokaryotes, p. 659-768. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 2. Springer, New York, NY. doi: 10.1007/0-387-30742-7_22. [DOI] [Google Scholar]
- 27.Shapleigh, J. P. 2006. The denitrifying prokaryotes, p. 769-792. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 2. Springer, New York, NY. doi: 10.1007/0-387-30742-7-23. [DOI] [Google Scholar]
- 28.Shock, E. L. 1988. Organic acid metastability in sedimentary basins. Geology 16:886-890. [Google Scholar]
- 29.Staden, R., K. F. Beal, and J. K. Bonfield. 1999. The Staden package, 1998. Methods Mol. Biol. 132:115-130. [DOI] [PubMed] [Google Scholar]
- 30.Sunde, E., and T. Torsvik. 2005. Microbial control of hydrogen sulfide production in oil reservoirs, p. 201-213. In B. Ollivier and M. Magot (ed.), Petroleum microbiology. ASM Press, Washington, DC.
- 31.Trüper, H. G., and H. G. Schlegel. 1964. Sulphur metabolism in Thiorhodeaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie van Leeuwenhoek 30:225-238. [DOI] [PubMed] [Google Scholar]
- 32.Vance, I., and D. R. Thrasher. 2005. Reservoir souring: mechanisms and prevention, p. 123-142. In B. Ollivier and M. Magot (ed.), Petroleum microbiology. ASM Press, Washington, DC.
- 33.Vik, E. A., A. O. Janbu, F. Garshol, L. B. Henninge, S. Engebretsen, C. Kuijvenhoven, D. Oilphant, and W. P. Hendriks. 2007. Nitrate-based souring mitigation of produced water-side effects and challenges from the Draugen produced-water reinjection pilot, paper SPE 106178. SPE Int. Symp. Oil Field Chem., Houston, TX.
- 34.Voordouw, G., V. Niviere, F. G. Ferris, P. M. Fedorak, and D. W. S. Westlake. 1990. The distribution of hydrogenase genes in Desulfovibrio and their use in identification of species from the oil-field environment. Appl. Environ. Microbiol. 56:3748-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellsbury, P., K. Goodman, T. Barth, B. A. Cragg, S. P. Barnes, and R. J. Parkes. 1997. Deep marine biosphere fueled by increasing organic matter availability during burial and heating. Nature 388:573-576. [Google Scholar]
- 36.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, vol. IV. Springer-Verlag, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.