Abstract
Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) is a widely used explosive and a serious environmental pollutant. Nineteen strains of Rhodococcus spp. capable of utilizing RDX as the sole nitrogen source have been isolated. The cytochrome P450 system XplA-XplB, which is responsible for RDX breakdown, is present in 18 of these strains.
The explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) is a toxic anthropogenic compound that persists as an environmental pollutant as a result of decades of weapon manufacture, deployment, and decommissioning. Although RDX is a xenobiotic compound, bacteria have been shown to be able to degrade it. An unusual cytochrome P450 system comprising a flavodoxin domain fused to the N terminus of a cytochrome P450 (CYP177A1, XplA) and a partner flavodoxin reductase XplB (6, 7) is responsible for RDX degradation in Rhodococcus rhodochrous strain 11Y. The presence of oxygen influences which of two RDX degradation pathways is catalyzed by XplA (4).
Selective enrichments were performed with minimal medium containing RDX as the sole source of nitrogen, using previously reported methods (1). Samples were provided by the Defence Science and Technology Laboratory of the United Kingdom Ministry of Defence, taken from an explosive-contaminated Ministry of Defence site, and stored at 4°C prior to enrichment. Nineteen pure bacterial isolates were able to remove RDX at a concentration of 1 mM from growth medium in 5 days, as assayed by thin-layer chromatography performed using a previously described method (7). None of the isolates were able to utilize RDX supplied as the sole carbon and nitrogen source (data not shown).
The isolates were identified using the 16S rRNA gene, amplified from extracted genomic DNA using primers fD1 and rD1 (9) and PfuTurbo DNA polymerase (Stratagene). PCR (95°C for 2 min; 30 cycles of 95°C for 30 s, 55°C to 62°C for 30 s, and 72°C for 2 min; and 72°C for 10 min) yielded products of approximately 1.6 kb, which underwent sequencing. Each strain possesses a unique 16S rRNA sequence, differing from those of other strains by at least one nucleotide. BLAST searches showed that all bacteria belong to the genus Rhodococcus. These data, in conjunction with a phenotypic analysis of colony morphology on different media, determined that all strains were distinct, and strain names were assigned (Table 1).
TABLE 1.
RDX-degrading isolatesa
| Rhodococcus sp. strain | Location of isolationb | GenBank accession no. | Database match
|
||
|---|---|---|---|---|---|
| Rhodococcus erythropolis strain | GenBank accession no. | % Nucleotide identity | |||
| HS1 | A | AY168579 | EPWF | AY822047 | 99 |
| HS2 | A | AY168580 | AC76 | AJ717370 | 99 |
| HS3 | A | AY168581 | EPWF | AY822047 | 99 |
| HS4 | A | AY168582 | AC76 | AJ717370 | 99 |
| HS5 | A | AY168583 | AC76 | AJ717370 | 99 |
| HS6 | A | AY168584 | EPWF | AY822047 | 99 |
| HS7 | A | AY168585 | EPWF | AY822047 | 99 |
| HS8 | B | AY168586 | AC76 | AJ717370 | 99 |
| HS9 | B | AY168587 | AC76 | AJ717370 | 99 |
| HS10 | B | AY168588 | EPWF | AY822047 | 99 |
| HS11 | B | AY168589 | AC76 | AJ717370 | 99 |
| HS12 | C | AY168590 | AC76 | AJ717370 | 99 |
| HS13 | C | AY168591 | EPWF | AY822047 | 99 |
| HS14 | C | AY168592 | EPWF | AY822047 | 99 |
| HS15 | D | AY168593 | AC76 | AJ717370 | 99 |
| HS16 | D | AY168594 | AC76 | AJ717370 | 99 |
| HS17 | D | AY168595 | AC76 | AJ717370 | 99 |
| HS18 | D | AY168596 | AC76 | AJ717370 | 99 |
| HS19 | A | AY168597 | AC76 | AJ717370 | 99 |
Shown are the designated strain names, materials and areas from which the strains were isolated, 16S rRNA gene sequence accession numbers, and closest 16S rRNA matches.
A, soil sample adjacent to munitions building where floor dust sweepings are deposited; B, detritus from a munitions factory drain; C, detritus from a drain in an area where explosives are steamed out of shells; D, soil 3 m from an area where explosives are steamed out of shells.
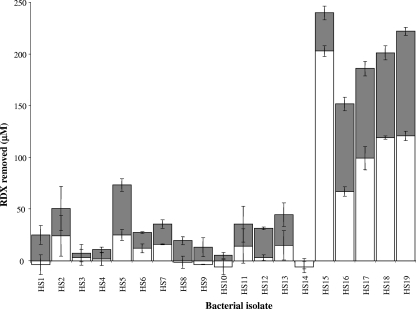
Resting-cell incubations were used to compare the RDX-degrading rates of the isolates. Cells were grown at 30°C in minimal medium containing 0.5 mM RDX as the sole nitrogen source and harvested 24 h after all the RDX had been removed from the growth medium, as assayed by high-performance liquid chromatography using a method previously described (7). Cells were rinsed twice in 40 mM potassium phosphate buffer, pH 7.5, and resuspended in the same buffer to a concentration of 500 mg (wet weight) per ml. Resting-cell incubations containing 250 μM RDX and 100 μl of cell suspension in 1 ml of potassium phosphate buffer, pH 7.5, were performed at 30°C with shaking at 110 rpm. The amounts of RDX removed by each strain over 30 min and 60 min were assayed by high-performance liquid chromatography (Fig. 1). The rates of removal of RDX varied significantly between the isolates, with five of the strains, HS15, HS16, HS17, HS18 and HS19, being significantly faster than the others.
FIG. 1.
RDX removal in resting-cell incubations. RDX was removed within 30 min (white bars) and 60 min (gray bars). Results shown are the averages from duplicate or triplicate samples ± one standard deviation as indicated by the error bars.
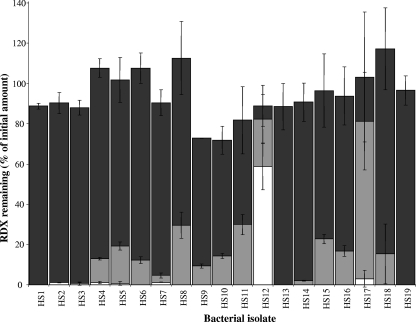
To determine whether the RDX degradation activity of these strains is based on a cytochrome P450 system, resting-cell incubations were performed, as described above, in the presence of metyrapone, a P450 inhibitor (8). Cells were harvested immediately after RDX removal from the culture, and the incubations were performed over 16 h. RDX-degrading activity was inhibited by 10 mM metyrapone for all strains, with over 70% of the original amount of RDX being retained with each of the incubations (Fig. 2). Metyrapone at 0.5 mM inhibited the ability of some of the strains to degrade RDX. Metabolic and permeability differences between strains are likely causes of the discrepancies. The cells used in this experiment were harvested at a different growth phase from those for Fig. 1; the differences in RDX removal indicate that the regulation of the expression of the RDX-degrading protein(s) may be strain specific.
FIG. 2.
Metyrapone inhibition of RDX removal in resting-cell incubations. RDX was removed over 16 h in the presence of 0 mM (white bars), 0.5 mM (pale gray bars), or 10 mM (dark gray bars) metyrapone. Results shown are the averages from duplicate or triplicate samples ± one standard deviation as indicated by the error bars.
To investigate the degree of homology of potential P450 components within these strains to xplA and xplB from R. rhodochrous strain 11Y, internal primers were designed for both genes: xplAf, 5′ACGTAACTGTCCTGTTCGGAA3′; xplAr, 5′ACGATCGGCAGTTTTCGGTA3′; xplBf, 5′ATGGACATCATGAGTGAAGTGG3′; and xplBr, 5′CTCAGCAGACCGATTC3′. PCR used Phusion DNA polymerase (New England BioLabs) with a PCR cycle of 94°C for 30 s and 30 cycles of 94°C for 30 s, 61°C for 45 s, and 72°C for 90 s. Products of the predicted sizes were found in all strains except strain HS4. Current work is directed toward determining whether strain HS4 carries a P450 system with more-limited homology to xplA and xplB or with a different P450 system altogether. To date, Southern blot hybridizations have not detected xplA or xplB homologues within HS4 (data not shown), but the metyrapone data suggest that a P450 system is involved. No PCR products were amplified from four negative control strains, R. rhodochrous CW25 (5), Rhodococcus sp. strain NCIMB 9784, Rhodococcus ruber DSM 44541, and Rhodococcus sp. strain CBS 717-73, which have never been exposed to explosives.
Partial sequences of the PCR products show >99% nucleotide identity to xplA and xplB (GenBank accession numbers DQ277702 to DQ277709 and DQ793172 to DQ793191, respectively). Homologues (>99%) of xplA and xplB (ABC17850 and ABI48986) were also amplified from the RDX-degrading Rhodococcus sp. strain DN22, isolated from an explosive-contaminated site in Australia (2). Homologues of xplA have also been described for the RDX-degrading strains Rhodococcus sp. strain YH1 from Israel (AAQ03207) and Gordonia sp. strain KTR9 and Williamsia sp. strain KTR4 from North America (3), further widening the known global distribution of xplA.
A comprehensive nucleotide BLAST search of the nucleotide and microbial genome databases found no further significant similarities to xplA and xplB. The best match for the amino acid sequences of XplA and XplB to translated microbial sequences is to a putative operon, ECA2071 to ECA2073, in the proteobacterium Erwinia carotovora subsp. atroseptica SCRI1043 (BX950851), with amino acid identities of 41% and 40% to the P450 domain of XplA and XplB, respectively. This operon comprises a putative oxidoreductase, P450, and a separate flavodoxin coding sequence.
In summary, a survey of aerobic RDX-degrading bacteria has shown that the vast majority possess systems with very high identity to the xplA-xplB RDX degradation system. XplA homologues have not been detected in environments that are not contaminated by explosives, despite the worldwide distribution of the gene. The highly conserved nature of xplA-xplB argues for a single evolutionary origin of this operon prior to its global distribution. We are investigating the possibility that xplA and xplB are located on a mobile genetic element and that RDX exerts a strong selection pressure for this element at contaminated sites.
Acknowledgments
This work was supported by funding from the Biotechnology and Biological Science Research Council, the Defence Science and Technology Laboratory of the United Kingdom Ministry of Defence, and the Strategic and Environmental Research and Development Program of the U.S. Department of Defense.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Binks, P. R., S. Nicklin, and N. C. Bruce. 1995. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl. Environ. Microbiol. 61:1318-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman, N. V., D. R. Nelson, and T. Duxbury. 1998. Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol. Biochem. 30:1159-1167. [Google Scholar]
- 3.Indest, K. J., F. H. Crocker, and R. Athow. 2007. A TaqMan polymerase chain reaction method for monitoring RDX-degrading bacteria based on the xplA functional gene. J. Microbiol. Methods 68:267-274. [DOI] [PubMed] [Google Scholar]
- 4.Jackson, R. G., E. L. Rylott, D. Fournier, J. Hawari, and N. C. Bruce. 2007. Exploring the biochemical properties and remediation applications of the unusual explosive-degrading P450 system XplA/B. Proc. Natl. Acad. Sci. USA 104:16822-16827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan, S., and E. R. Dabbs. 1993. Nocardioform arsenic resistance plasmid characterization and improved Rhodococcus cloning vectors. Plasmid 29:74-79. [DOI] [PubMed] [Google Scholar]
- 6.Rylott, E. L., R. G. Jackson, J. Edwards, G. L. Womack, H. M. Seth-Smith, D. A. Rathbone, S. E. Strand, and N. C. Bruce. 2006. An explosive-degrading cytochrome P450 activity and its targeted application for the phytoremediation of RDX. Nat. Biotechnol. 24:216-219. [DOI] [PubMed] [Google Scholar]
- 7.Seth-Smith, H. M. B., S. J. Rosser, A. Basran, E. R. Travis, E. R. Dabbs, S. Nicklin, and N. C. Bruce. 2002. Cloning, sequencing and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Appl. Environ. Microbiol. 68:4764-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testa, B., and P. Jenner. 1981. Inhibitors of cytochrome P-450s and their mechanisms of action. Drug Metab. Rev. 12:1-117. [DOI] [PubMed] [Google Scholar]
- 9.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]